The aim of the present study was to analyze the chemical composition of Curcuma longa, Pimenta dioica, Rosmarinus officinalis, and Syzygium aromaticum essential oils (EOs) and their antifungal and anti-conidiogenic activity against Fusarium verticillioides. The chemical profile of the EOs was determined by GC/MS. The antifungal and anti-conidiogenic activities were evaluated by the agar dilution method. The tested concentrations were 1000ppm, 500ppm, 250ppm and 125ppm. S. aromaticum EO exhibited the highest antifungal effect, followed by P. dioica and to a lesser extent C. longa. The major compounds of these EOs were eugenol (88.70% in S. aromaticum and 16.70% in P. dioica), methyl eugenol (53.09% in P. dioica), and α-turmerone (44.70%), β-turmerone (20.67%), and Ar-turmerone (17.27%) in C. longa. Rosmarinus officinalis poorly inhibited fungal growth; however, it was the only EO that inhibited conidial production, with its major components being 1,8-cineole (53.48%), α-pinene (15.65%), and (−)-camphor (9.57%). Our results showed that some compounds are capable of decreasing mycelial growth without affecting sporulation, and vice versa. However, not all the compounds of an EO are responsible for its bioactivity. In the present work, we were able to identify different major compounds or mixtures of major compounds that were responsible for antifungal and anti-conidiogenic effects. Further experiments combining these pure components are necessary in order to achieve a highly bioactive natural formulation against the phytopathogenic fungus F. verticillioides.
El objetivo del presente estudio fue analizar la composición química de los aceites esenciales (AE) de Curcuma longa, Pimenta dioica, Rosmarinus officinalis y Syzygium aromaticum, y su actividad antifúngica y anti-esporuladora contra Fusarium verticillioides. La composición de los AE se analizó por CG-EM. La actividad antifúngica y anti-esporuladora se evaluó a través del método de dilución en agar usando las siguientes concentraciones: 1.000, 500, 250 y 125ppm. El AE de S. aromaticum demostró el mayor efecto antifúngico, seguido del de P. dioica y, en menor medida, del C. longa. Los compuestos principales de estos AE fueron eugenol (88,70% en S. aromaticum y 16,70% en P. dioica), metil eugenol (53,09% en P. dioica) y α-turmerona (44,70%), β-turmerona (20,67%) y Ar-turmerona (17,27%) en C. longa. El AE de R. officinalis fue el que menor efecto inhibitorio presentó sobre el crecimiento fúngico, pero fue el único que inhibió la producción de conidias; sus principales componentes fueron 1,8-cineol (53,48%), α-pineno (15,65%) y (−)-alcanfor (9,57%). Nuestros resultados demostraron que algunos compuestos son capaces de disminuir el crecimiento del micelio de F. verticillioides sin afectar la esporulación, y vice versa. Sin embargo, no todos los compuestos de un AE son responsables de su bioactividad. En el presente trabajo, pudimos identificar diferentes compuestos o mezclas de compuestos que fueron responsables de los efectos antifúngicos y anti-esporuladores. Se necesitan nuevos experimentos que evalúen la combinación de estos compuestos puros para lograr una formulación bioactiva y de origen natural para el control de F. verticillioides.
Fusarium verticilloides (Sacc.) is a fungal pathogen of maize which is economically important in global agriculture for being the major causal agent of stalk and ear rot worldwide, thus causing great yield reductions. Moreover, F. verticillioides is a producer of highly toxic mycotoxins both in the field and in stored grains, which has been associated with harmful effects in humans and farm animals. Great efforts have been made in order to control food deterioration by fungi, with the application of synthetic fungicides being the most commonly used storage strategy. However, despite the efficacy of these chemical substances, a significant number of them have led to the development of fungal resistance, proved to be toxic for the environment, and caused residual toxicity on grains33. The latter issue represents a major problem because of the regulatory standards for pesticide tolerance levels in agricultural products, which has important implications for their exportation34. Therefore, there is an increasing public demand for the development of new and safer antifungal agents for grain preservation. In recent years, many natural compounds have attracted the attention of scientists, such as plant extracts or essential oils (EOs)41.
Essential oils are hydrophobic substances of complex mixtures of volatile organic compounds, which are obtained from different parts of aromatic plants. Some of the EO volatile constituents include terpenoids, alcoholic compounds, phenylpropanoids, aldehydes, acidic compounds, among others13. Because of their strong odor, EOs have long been used as spices and additives in the food flavoring industry. Furthermore, many of these volatile constituents are bioactive against several bacteria, yeasts as well as filamentous fungi, being potentially useful as food preservatives41. In this regard, previous works have investigated the practical applicability of EOs either in their pure form or in formulations to protect stored grains from deterioration caused by pests, using different types of storage containers, and increasing the shelf-life of food commodities13,22,42,43,49,56. Indeed, a number of EO-based commercial formulations are being currently marketed as Talent® (Netherlands), Fungastop™, and Armorex™ (Soil Technology Corp, USA) for the control of various foodborne pathogens.
The antifungal activity of EOs has been extensively studied against Fusarium sp., Aspergillus sp., Penicillium sp., Alternaria sp., and Verticillium sp., among other economically important fungal genera6,21,32,46,56,64. However, the effectiveness of a particular EO as antifungal is determined not only by its fungitoxic effect, but also by its ability to decrease or inhibit conidial production. Conidia are asexually-produced spores that are released in large amounts, promoting fungal dispersion and secondary infection processes. Previous studies have reported strongly reduced sporulation in Aspergillus terreus and Penicillium expansum exposed to oregano EO, and in Fusarium oxysporum and P. expansum using lavender EO. On the other hand, sage EO promoted conidial production in a dose-dependent manner, which is an undesirable effect of any EO23. In either case, the effect on sporulation is not addressed by most studies evaluating the bioactivity of EOs.
In the present work, we aimed to study the chemical composition of the EOs from turmeric (Curcuma longa L.), allspice (Pimenta dioica L. Merr), rosemary (Rosmarinus officinalis L.), and clove (Syzygium aromaticum L. Merr & Perry). These aromatic plants are highly used as spices and flavors worldwide and their antifungal activities have been reported in Aspergillus sp., Fusarium sp., Penicillium sp., Botrytis sp., and Alternaria sp.2,3,20,25,40,53 We evaluated the effect of the selected EOs on fungal growth and conidial production in Fusarium verticillioides. Furthermore, we conducted a Principal Component Analysis, to study the relationships between EO constituents and EO bioactivity.
Materials and methodsEssential oilsThe EOs from Curcuma longa L., Pimenta dioica L. (Merr), Rosmarinus officinalis L., and Syzygium aromaticum (L.) Merr & Perry were obtained from Santo Domingo's local market (Santo Domingo, Dominican Republic).
Composition of the essential oilsQuantitative and qualitative analyses of the EOs were performed using a Perkin Elmer Clarus 580 chromatograph-mass spectrometer equipped with a DB5 column (30m×0.25mm, film thickness 0.25μm; Elite 5 MS Perkin Elmer). The temperature of the injector was 200°C. The oven temperature was programmed as follows: 60°C for 5min, ramped up to 170°C at 4°C/min, and then raised to 250°C at 20°C/min. Helium was used as the carrier gas and the flow rate was maintained at 1ml/s. The GC/MS interface temperature was 200°C. Electron impact mode on mass spectrometer was set at 70eV with a mass scan range of 40–300 atomic mass units (amu). Diluted samples (1/100 v/v in n-heptane) of 1μl of each EO were manually injected in the split-less mode. Kovats retention indices (KI) were calculated after an analysis of C8–C21 alkane series (Sigma-Aldrich), under the same chromatographic conditions. The identification of EO compounds was based on the comparison of their mass spectrum and KI with those from the NIST-08 Mass Spectral Library (US National Institute of Standards and Technology) and literature data. The amount of each EO constituent was expressed as a relative percentage by peak area normalization8.
Fungal strain and inoculum preparationThe fungal strain Fusarium verticillioides M3125 (supplied by Dr. Robert Proctor, United States Department of Agriculture, Agricultural Research Service, National Center for Agricultural Utilization Research, Peoria, IL, USA), was used in all the experiments. This fungal strain was originally isolated from corn in California and is a prolific producer of fumonisins28. In addition, strain M3125 (also known as strain 7600) has been genomically characterized (Genbank Accession AAIM00000000.2; PRJNA15553).
For inoculum preparation, sterile distilled water was added to a F. verticillioides culture grown in potato dextrose agar (PDA) medium at 28°C for 7 days. Conidia were dislodged by a gentle agitation of the plate. Conidial final concentration was adjusted to 1×106conidia/ml with a Neubauer chamber.
Antifungal activityThe inhibitory effect of the EOs on mycelial radial growth was evaluated by the agar dilution method, using Czapek Dox Agar modified (OXOID; CDA; 2g NaNO3, 0.5g C3H7MgO6P, 0.5g KCl, 0.01g FeSO4, 0.35g K2HPO4, 15g agar and 30g sucrose per liter)1,7,37,46. Different aliquots of each EO were diluted in 20ml of autoclaved (121°C for 15min) CDA culture medium melted and tempered at 45°C, to achieve the following concentrations: 1000ppm, 500ppm, 250ppm and 125ppm. The Petri dishes containing the solidified medium were aseptically point-inoculated in the center of the plate adding 10μl of the conidial suspension (1×106conidia/ml). Incubation was carried out at 28°C in the dark for 8 days, the time at which fungal growth of the control reached the edge of the plate. Five replicates were performed for each EO and concentration, and the experiment was repeated twice. Petri dishes containing CDA culture medium without the addition of any EO were used as control.
The antifungal activity was calculated as the percent of inhibition of radial growth relative to the control at day 8, according to the following formula:%inhibition=(RC−RT)/RC×100, where RC represents the average growth radius of the colonies from the control plates and RT, the average growth radius of the colonies from the treatment Plates3,21,37,55,56. The radiuses of the colonies were measured in two directions at right angles to each other using a caliper.
Conidial productionThe effect of EOs on conidial production was evaluated at day 8 of fungal growth, using the same plates that were employed to calculate the antifungal activity. Conidia were harvested by adding 10ml of sterile distilled water per plate and gently scraping the surface of the colonies with a Drigalsky spatula. The resulting suspension was removed and filtered using Miracloth (Calbiochem®; Sigma–Aldrich; USA) that retained mycelial debris, but permitted the passage of conidia. An aliquot of 100μl of the filtrate was diluted with 900μl of sterile distilled water. The quantification of conidia was performed with a Neaubauer chamber using an optical microscope (10×; Zeuss Primo Star)1. Conidial concentration was expressed as number of conidia per area (cm2) of the colonies grown in the plates. The area of the colonies was calculated using the following formula A=πxr2, where A represents the area and r is the average of the radius of the colonies, which was obtained as explained above. The experiment was conducted twice, each having five replicates per EO and concentration.
Statistical analysesData from the antifungal activity and conidial production were analyzed by one-way analysis of variance (ANOVA) followed by an LSD Fisher Multiple Comparison test, using Infostat Professional Software (Facultad de Ciencias Agropecuarias, Universidad Nacional de Córdoba). Results giving p<0.05 were considered significantly different.
Multivariate analysisA principal component analysis (PCA) was performed to study the relationship between the EO constituents and the bioactivity of the EOs. In the present study, the PCA was used to reveal the grouping among the EOs in a multidimensional space (the score plot) by using the EO constituents and their bioactivity as variables. Those compounds with a relative percentage ≤1.0 were not considered in the analysis. A total of 25 variables were included in the analysis (23 volatile compounds+antifungal effect+anti-conidiogenic effect). To obtain the anti-conidiogenic values, data from conidial production were transformed by using the inverse function (y=1/x). The PCA was conducted using the Infostat Professional Software.
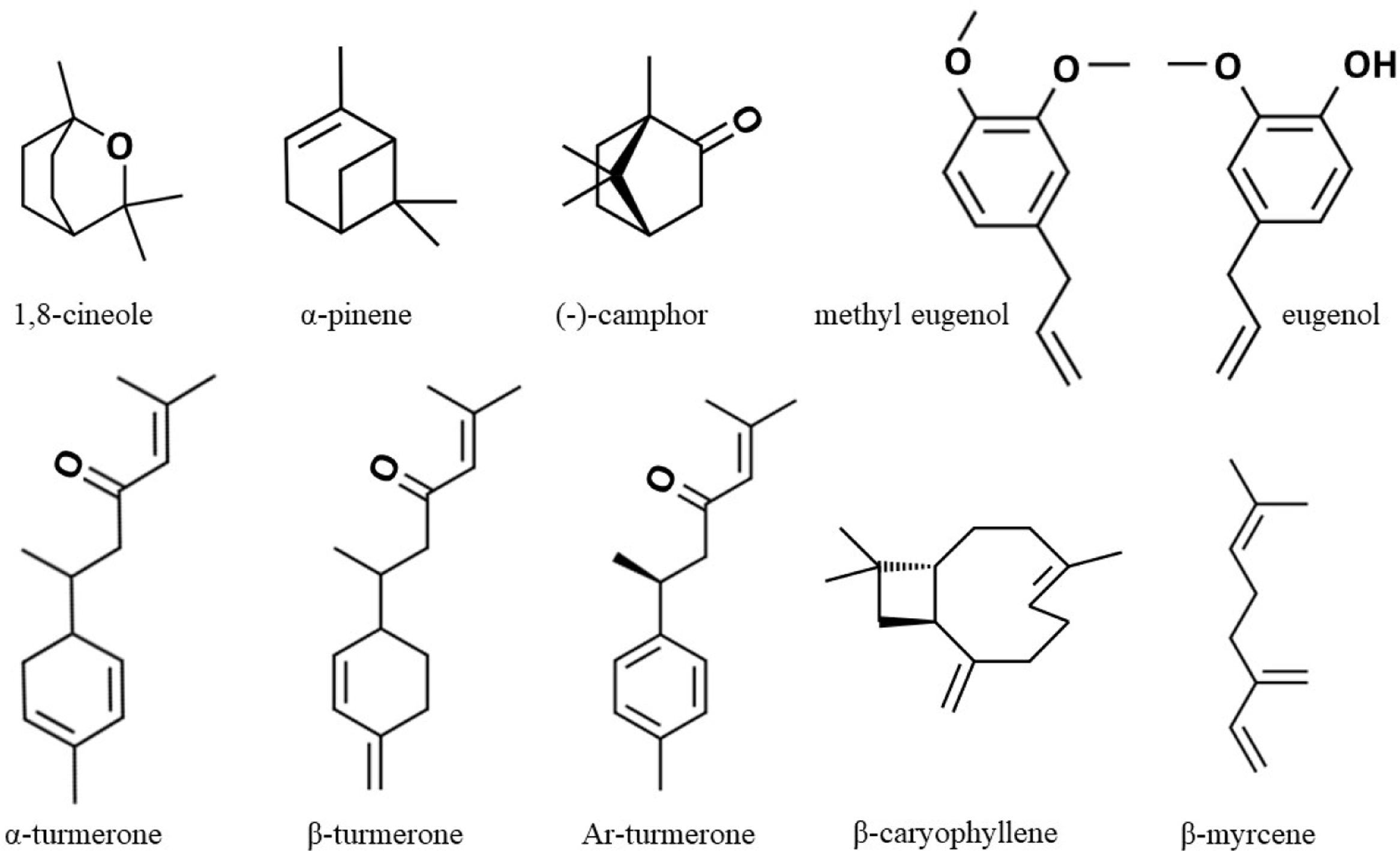
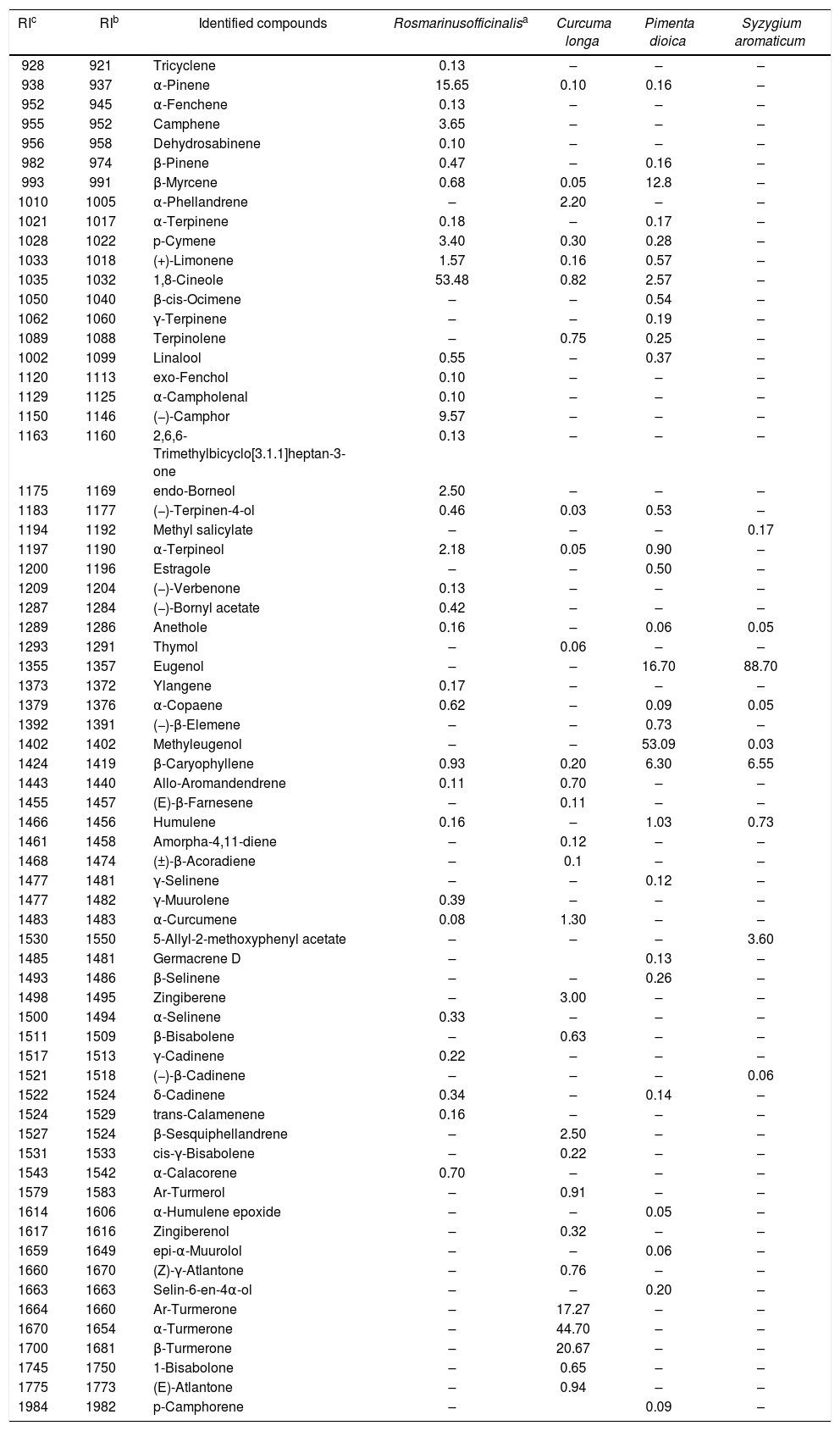
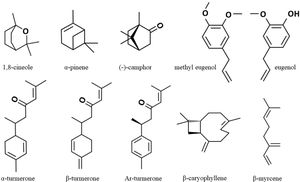
ResultsChemical composition of the essential oilsThe chemical composition of Rosmarinus officinalis, Curcuma longa, Pimenta dioica, and Syzygium aromaticum EOs are shown in Table 1 according to their elution order in a DB-5 capillary column. Only those components with relative percentage ≥0.05 are reported. In R. officinalis EO, 34 compounds were identified, whose major components were 1,8-cineole (53.48%), α-pinene (15.65%), and (−)-camphor (9.57%). For C. longa, 30 compounds were identified with α-turmerone (44.70%), β-turmerone (20.67%), and Ar-turmerone (17.27%) being the prevalent compounds. The profile of P. dioica EO was characterized by 30 compounds with methyl eugenol (53.09%), eugenol (16.70%), and β-myrcene (12.80%) being the major constituents. Finally, S. aromaticum EO presented only 9 constituents, with eugenol as the main component (88.70%), followed by β-caryophyllene (6.55%) (Fig. 1). As shown in Figure 1, there are clear differences in the chemical structure of these components. The compounds α-pinene and β-myrcene are bicyclic and acyclic monoterpene hydrocarbons, respectively. β-Caryophyllene is a sesquiterpene hydrocarbon with two rings in its structure. The remaining compounds are oxygenated, but with different functional groups. The monoterpenes 1,8-cineole and (−)-camphor have an epoxide and a carbonyl group, respectively. Both methyl eugenol and eugenol are phenylpropanoids; methyl eugenol has two methoxy groups and eugenol has one methoxy and one hydroxy group. On the other hand, turmerones are sesquiterpenoid α,β-unsaturated ketones, which are characterized by the presence of the carbonyl group.
Chemical profile of C. longa, P. dioica, R. officinalis, and S. aromaticum essential oils.
| RIc | RIb | Identified compounds | Rosmarinusofficinalisa | Curcuma longa | Pimenta dioica | Syzygium aromaticum |
|---|---|---|---|---|---|---|
| 928 | 921 | Tricyclene | 0.13 | – | – | – |
| 938 | 937 | α-Pinene | 15.65 | 0.10 | 0.16 | – |
| 952 | 945 | α-Fenchene | 0.13 | – | – | – |
| 955 | 952 | Camphene | 3.65 | – | – | – |
| 956 | 958 | Dehydrosabinene | 0.10 | – | – | – |
| 982 | 974 | β-Pinene | 0.47 | – | 0.16 | – |
| 993 | 991 | β-Myrcene | 0.68 | 0.05 | 12.8 | – |
| 1010 | 1005 | α-Phellandrene | – | 2.20 | – | – |
| 1021 | 1017 | α-Terpinene | 0.18 | – | 0.17 | – |
| 1028 | 1022 | p-Cymene | 3.40 | 0.30 | 0.28 | – |
| 1033 | 1018 | (+)-Limonene | 1.57 | 0.16 | 0.57 | – |
| 1035 | 1032 | 1,8-Cineole | 53.48 | 0.82 | 2.57 | – |
| 1050 | 1040 | β-cis-Ocimene | – | – | 0.54 | – |
| 1062 | 1060 | γ-Terpinene | – | – | 0.19 | – |
| 1089 | 1088 | Terpinolene | – | 0.75 | 0.25 | – |
| 1002 | 1099 | Linalool | 0.55 | – | 0.37 | – |
| 1120 | 1113 | exo-Fenchol | 0.10 | – | – | – |
| 1129 | 1125 | α-Campholenal | 0.10 | – | – | – |
| 1150 | 1146 | (−)-Camphor | 9.57 | – | – | – |
| 1163 | 1160 | 2,6,6-Trimethylbicyclo[3.1.1]heptan-3-one | 0.13 | – | – | – |
| 1175 | 1169 | endo-Borneol | 2.50 | – | – | – |
| 1183 | 1177 | (−)-Terpinen-4-ol | 0.46 | 0.03 | 0.53 | – |
| 1194 | 1192 | Methyl salicylate | – | – | – | 0.17 |
| 1197 | 1190 | α-Terpineol | 2.18 | 0.05 | 0.90 | – |
| 1200 | 1196 | Estragole | – | – | 0.50 | – |
| 1209 | 1204 | (−)-Verbenone | 0.13 | – | – | – |
| 1287 | 1284 | (−)-Bornyl acetate | 0.42 | – | – | – |
| 1289 | 1286 | Anethole | 0.16 | – | 0.06 | 0.05 |
| 1293 | 1291 | Thymol | – | 0.06 | – | – |
| 1355 | 1357 | Eugenol | – | – | 16.70 | 88.70 |
| 1373 | 1372 | Ylangene | 0.17 | – | – | – |
| 1379 | 1376 | α-Copaene | 0.62 | – | 0.09 | 0.05 |
| 1392 | 1391 | (−)-β-Elemene | – | – | 0.73 | – |
| 1402 | 1402 | Methyleugenol | – | – | 53.09 | 0.03 |
| 1424 | 1419 | β-Caryophyllene | 0.93 | 0.20 | 6.30 | 6.55 |
| 1443 | 1440 | Allo-Aromandendrene | 0.11 | 0.70 | – | – |
| 1455 | 1457 | (E)-β-Farnesene | – | 0.11 | – | – |
| 1466 | 1456 | Humulene | 0.16 | – | 1.03 | 0.73 |
| 1461 | 1458 | Amorpha-4,11-diene | – | 0.12 | – | – |
| 1468 | 1474 | (±)-β-Acoradiene | – | 0.1 | – | – |
| 1477 | 1481 | γ-Selinene | – | – | 0.12 | – |
| 1477 | 1482 | γ-Muurolene | 0.39 | – | – | – |
| 1483 | 1483 | α-Curcumene | 0.08 | 1.30 | – | – |
| 1530 | 1550 | 5-Allyl-2-methoxyphenyl acetate | – | – | – | 3.60 |
| 1485 | 1481 | Germacrene D | – | 0.13 | – | |
| 1493 | 1486 | β-Selinene | – | – | 0.26 | – |
| 1498 | 1495 | Zingiberene | – | 3.00 | – | – |
| 1500 | 1494 | α-Selinene | 0.33 | – | – | – |
| 1511 | 1509 | β-Bisabolene | – | 0.63 | – | – |
| 1517 | 1513 | γ-Cadinene | 0.22 | – | – | – |
| 1521 | 1518 | (−)-β-Cadinene | – | – | – | 0.06 |
| 1522 | 1524 | δ-Cadinene | 0.34 | – | 0.14 | – |
| 1524 | 1529 | trans-Calamenene | 0.16 | – | – | – |
| 1527 | 1524 | β-Sesquiphellandrene | – | 2.50 | – | – |
| 1531 | 1533 | cis-γ-Bisabolene | – | 0.22 | – | – |
| 1543 | 1542 | α-Calacorene | 0.70 | – | – | – |
| 1579 | 1583 | Ar-Turmerol | – | 0.91 | – | – |
| 1614 | 1606 | α-Humulene epoxide | – | – | 0.05 | – |
| 1617 | 1616 | Zingiberenol | – | 0.32 | – | – |
| 1659 | 1649 | epi-α-Muurolol | – | – | 0.06 | – |
| 1660 | 1670 | (Z)-γ-Atlantone | – | 0.76 | – | – |
| 1663 | 1663 | Selin-6-en-4α-ol | – | – | 0.20 | – |
| 1664 | 1660 | Ar-Turmerone | – | 17.27 | – | – |
| 1670 | 1654 | α-Turmerone | – | 44.70 | – | – |
| 1700 | 1681 | β-Turmerone | – | 20.67 | – | – |
| 1745 | 1750 | 1-Bisabolone | – | 0.65 | – | – |
| 1775 | 1773 | (E)-Atlantone | – | 0.94 | – | – |
| 1984 | 1982 | p-Camphorene | – | 0.09 | – |
RIc: calculated Kovats retention indices; RIb: retention indices from bibliography. The volatile content of each EO is expressed as relative percentage (%) by peak area normalization.
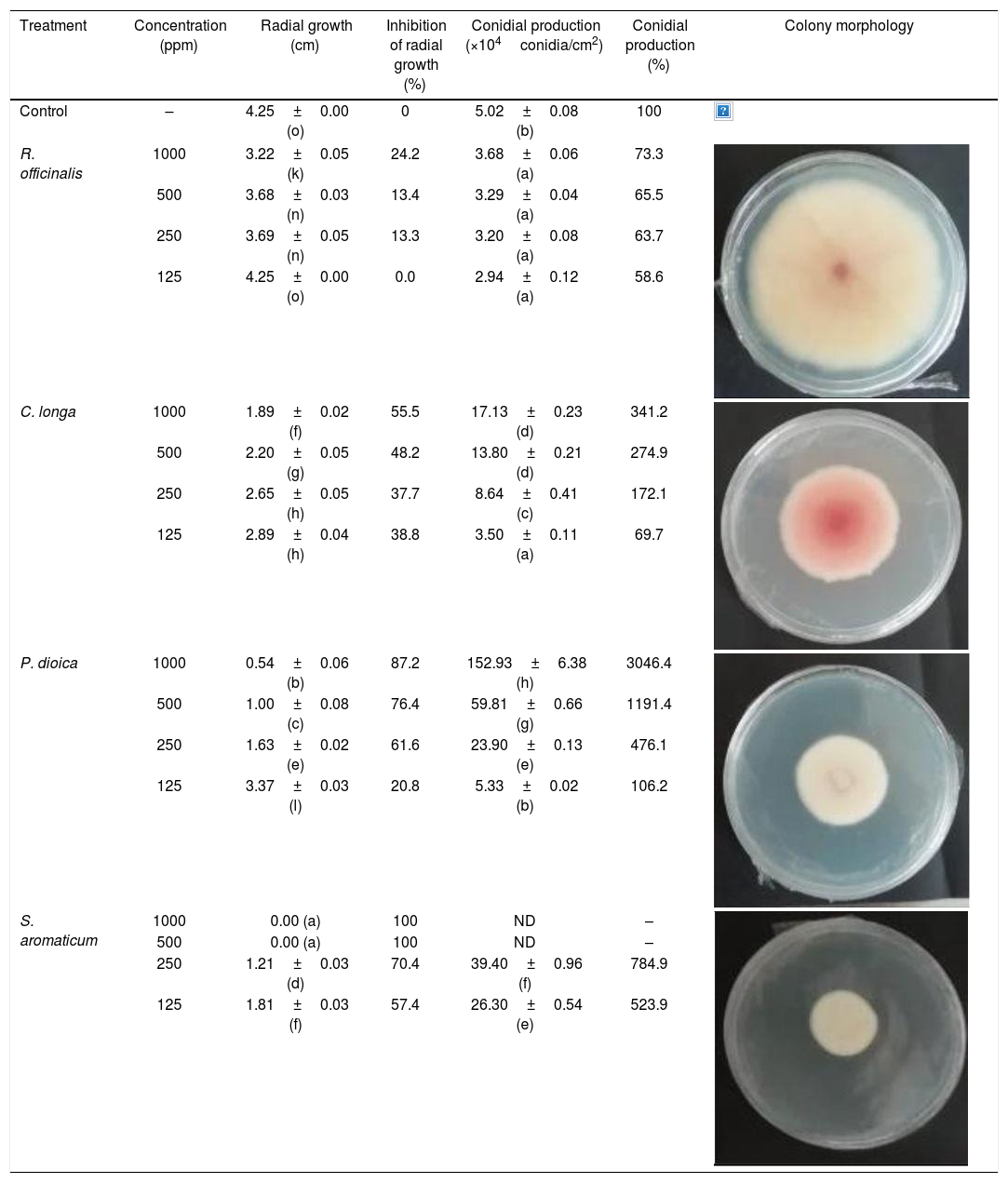
The antifungal activity of the four EOs was evaluated against F. verticillioides. Except for R. officinalis at 125ppm, all EOs showed significant differences compared to the control at all the tested concentrations (Table 2). The EO from S. aromaticum completely inhibited fungal growth at 1000ppm and 500ppm. Furthermore, this oil showed higher inhibition percent (70.4% and 57.4%) at lower concentrations (250ppm and 125ppm) compared to the remaining EOs at the same concentrations. The next more effective EO against F. verticillioides was P. dioica, followed by C. longa, with an inhibition percent of 87.2% and 55.5% at 1000ppm, respectively. In contrast, the EO from R. officinalis showed a lower antifungal effect with 24.2% inhibition at 1000ppm.
Bioactivity of R. officinalis, C. longa, P. dioica, and S. aromaticum against F. verticillioides M3125.
| Treatment | Concentration (ppm) | Radial growth (cm) | Inhibition of radial growth (%) | Conidial production (×104conidia/cm2) | Conidial production (%) | Colony morphology |
|---|---|---|---|---|---|---|
| Control | – | 4.25±0.00 (o) | 0 | 5.02±0.08 (b) | 100 | |
| R. officinalis | 1000 | 3.22±0.05 (k) | 24.2 | 3.68±0.06 (a) | 73.3 | |
| 500 | 3.68±0.03 (n) | 13.4 | 3.29±0.04 (a) | 65.5 | ||
| 250 | 3.69±0.05 (n) | 13.3 | 3.20±0.08 (a) | 63.7 | ||
| 125 | 4.25±0.00 (o) | 0.0 | 2.94±0.12 (a) | 58.6 | ||
| C. longa | 1000 | 1.89±0.02 (f) | 55.5 | 17.13±0.23 (d) | 341.2 | |
| 500 | 2.20±0.05 (g) | 48.2 | 13.80±0.21 (d) | 274.9 | ||
| 250 | 2.65±0.05 (h) | 37.7 | 8.64±0.41 (c) | 172.1 | ||
| 125 | 2.89±0.04 (h) | 38.8 | 3.50±0.11 (a) | 69.7 | ||
| P. dioica | 1000 | 0.54±0.06 (b) | 87.2 | 152.93±6.38 (h) | 3046.4 | |
| 500 | 1.00±0.08 (c) | 76.4 | 59.81±0.66 (g) | 1191.4 | ||
| 250 | 1.63±0.02 (e) | 61.6 | 23.90±0.13 (e) | 476.1 | ||
| 125 | 3.37±0.03 (l) | 20.8 | 5.33±0.02 (b) | 106.2 | ||
| S. aromaticum | 1000 | 0.00 (a) | 100 | ND | – | |
| 500 | 0.00 (a) | 100 | ND | – | ||
| 250 | 1.21±0.03 (d) | 70.4 | 39.40±0.96 (f) | 784.9 | ||
| 125 | 1.81±0.03 (f) | 57.4 | 26.30±0.54 (e) | 523.9 | ||
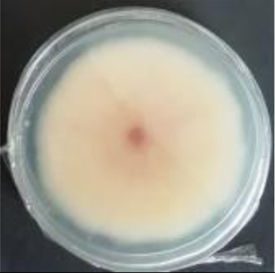
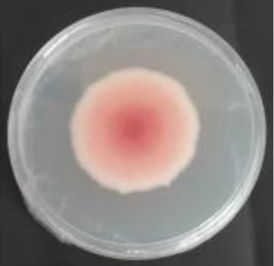
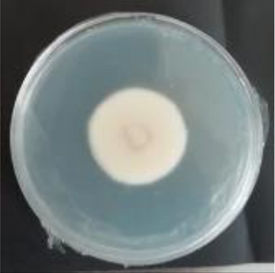
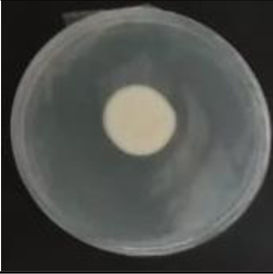
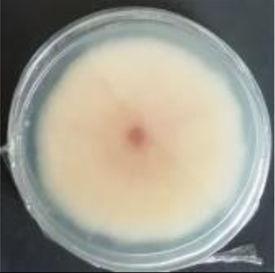
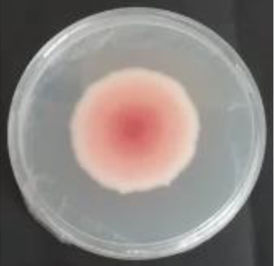
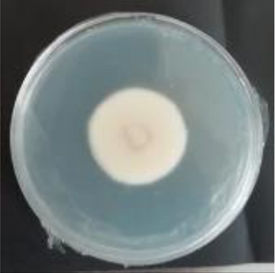
Values are expressed as mean±SE. Mean values (within the same column) with the same letter are not significantly different according to the DGC multiple comparison test (p<0.05). ND indicates that no data could be taken due to complete mycelial growth inhibition. The images correspond to F. verticillioides 8-day reverse colonies grown on CDA at 250ppm of each treatment.
On the other hand, the only effective EO against fungal sporulation was R. officinalis. Conidial production was around 30–40% lower than the control, without significant differences among the tested concentrations (Table 2). A statistically similar amount of conidia was observed in C. longa at 125ppm. Moreover, the remaining EOs at all concentrations promoted fungal sporulation in a dose-dependent manner. The stimulation effect is particularly strong in P. dioica where conidial production ranges from 5 to 30 times higher than the control at 250ppm and 1000ppm, respectively (Table 2).
An interesting result to notice is that both aerial mycelial formation and colony coloration were different among the tested EOs. Colonies with C. longa EO acquired a reddish pigmentation, with lax aerial mycelium. In contrast, fungal colonies grown in the presence of P. dioica and S. aromaticum showed an unpigmented, dense aerial mycelium. On the other hand, colonies grown with R. officinalis acquired an orange pigmentation with lax aerial mycelium, similar to those from the control (Table 2).
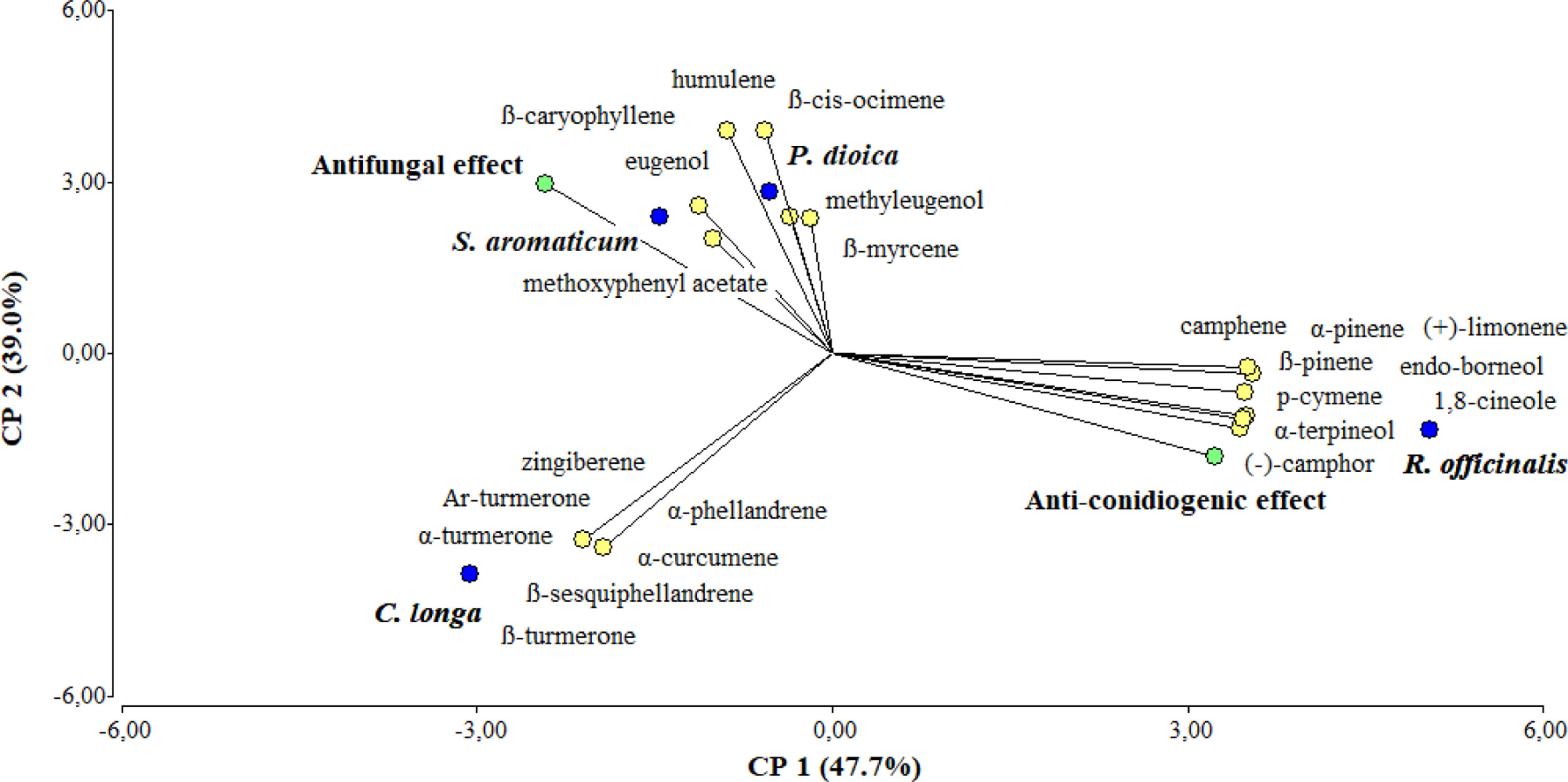
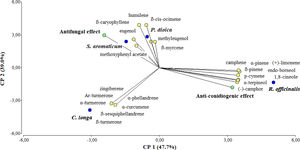
Multivariate analysisThe PCA analysis was performed to better understand the differences among the EOs, in terms of composition and bioactivity (Fig. 2). The three principal components of this analysis accounted for 100% of the differentiation. The first and second component explained 47.7% and 39.0% of the total variance, respectively.
Principal component analysis (PCA). The first two principal components account for 47.7% and 39.0% of the total variation, respectively. A total of 25 variables were included in the analysis: 23 volatile compounds (yellow circles)+antifungal effect+anti-conidiogenic effect (green circles). The tested EOs are shown in blue circles. Only those compounds with a relative percentage >1.0 were considered in the analysis.
It was evidenced in the PCA that the EOs (blue circles) were well-separated in the score plot. This segregation was achieved by the first and second principal components (PC1 and PC2) and was strongly influenced by the chemical profile of each EO and their bioactivity. It was observed in the loading plot that R. officinalis was segregated from C. longa, P. dioica, and S. aromaticum by the first principal component (PC1) and C. longa was discriminated from P. dioica and S. aromaticum by the second principal component (PC2). In PCA, the orientation of vectors, as well as the angles between them reflect the correlation among EO constituents, bioactivity and EOs. For example, in our study the compounds eugenol and 5-allyl-2-methoxyphenyl acetate were positioned close to S. aromaticum, indicating that those compounds were found in higher amounts in S. aromaticum oil. Likewise, the compounds α-phellandrene, α-curcumene, zingiberene, β-sesquiphellandrene, Ar-turmerone, α-turmerone, and β-turmerone were near C. longa, the compounds β-myrcene, β-cis-ocimene, methyl eugenol, β-caryophyllene, and humulene were plotted near P. dioica, while the compounds α-pinene, camphene, β-pinene, p-cymene, (+)-limonene, 1,8-cineole, (−)-camphor, endo-borneol, and α-terpineol were positioned close to R. officinalis. The pattern observed in the score plot was consistent with the results from Table 1. It was also observed that the variable “Antifungal effect” was plotted very close to S. aromaticum, and to a lesser extent, to P. dioica, while the variable “Anti-conidiogenic effect” was positioned near R. officinalis, indicating that these EOs were the ones that showed higher inhibition of mycelial growth and conidial production, respectively, as was also shown in Table 2. In fact, despite the differences in their volatile constituents, EOs from C. longa, S. aromaticum and P. dioica were discriminated from R. officinalis, the EO that showed the lowest antifungal effect (Table 2).
DiscussionThe results obtained in the present study show the effect of four commercial EOs to inhibit radial growth and conidial production in Fusarum verticillioides. Although the antifungal activity of EOs against filamentous fungi has been extensively studied6,21,32,46,56,64, the reports about inhibition of conidia production are considerably less abundant. Based on our results, the effect of EOs on mycelial growth was independent from their effect on conidial production. Indeed, some EOs were capable of decreasing mycelial growth without affecting conidiation, and vice versa, which was related to the EO concentration. For example, Rosmarinus officinalis at 125ppm did not show significant differences in growth, but sporulation was highly inhibited compared to the control. In contrast, P. dioica at 125ppm inhibited mycelial growth, but did not show any significant differences in conidial production, compared to the control. Moreover, there were no significant differences in fungal growth between 125ppm and 250ppm in C. longa, but conidiation was more than 2 times lower at 125ppm compared to 250ppm.
Our results showed that S. aromaticum EO was the most bioactive oil against F. verticillioides, which fully suppressed fungal growth at 1000ppm and 500ppm. As was shown in the PCA score plot, the second most bioactive EO against F. verticillioides growth was P. dioica. The most prevalent compounds of these EOs are eugenol (88.70% in S. aromaticum and 16.70% in P. dioica) and methyl eugenol (53.09% in P. dioica). The antifungal activity of phenolic compounds and other cyclic terpenes has previously been reported11,16,47. In general, the bioactivity of these compounds has been correlated with their lipophilic character that gives them the ability to penetrate cell walls41. Furthermore, previous studies that compared the antifungal properties of phenylpropanoids, concluded that eugenol has stronger inhibitory effect than methyl eugenol, due to the free –OH group available to form hydrogen bonds with the active sites of different enzymes, thus disrupting their activity5,26. It has also been reported that the –OH group in the structure of aromatic terpenoids act as the hydrophilic portion that increases its solubility. Therefore, eugenol would be retained in the membrane, inducing changes that lead to significant consequences in ion homeostasis. The –OH group associated with a system of delocalized electrons (6-membered aromatic ring) would permit the –OH to lose its proton easily and act as a proton exchanger, causing higher membrane damage and thus, stronger antifungal effect compared to its O-methyl derivative, methyl eugenol27. Furthermore, the interactions with other compounds present in lower amounts, such as β-myrcene (12.80% in P. dioica) and β-caryophyllene (6.55% in A. aromaticum) may contribute to the antifungal effect of these EOs. For example, it was reported that the aliphatic monoterpene β-myrcene decreases the stability and changes the morphology of lipid membranes48. Additionally, β-caryophyllene, together with other sesquiterpenes, showed an inhibitory effect against fungi and bacteria58.
As shown in the PCA score plot, C. longa EO profile was characterized by a set of major compounds that were absent in the other tested EOs. The presence of high amounts of sesquiterpenoid α-turmerone (44.70%), β-turmerone (20.67%), and Ar-turmerone (17.27%) segregated this EO to the left side of the score plot. C. longa EO showed a moderate antifungal activity (55.5% at 1000ppm), compared to S. aromaticum and P. dioica. Previous studies have reported an inhibitory effect of C. longa EO against different filamentous fungi3,20,38. According to the authors, the antifungal effect of C. longa EO was attributable to turmerones. These sesquiterpenoid α,β-unsaturated ketones penetrate the plasma membrane inducing changes in the fatty acid composition and alteration in permeability of the cell membrane38. A previous study revealed that among terpenoids, aldehydes and ketones showed stronger inhibitory effects, whereas, pure hydrocarbons were less active at similar concentrations26. Furthermore, ketones with an extra double bond between the alpha and beta carbons showed stronger inhibitory activity against F. verticillioides compared to saturated ketones45. The α,β-unsaturation increase the polarizability of the molecule, which is associated with stronger intermolecular attractive forces such as the London dispersion type. As a consequence α,β-unsaturated ketones can bind with amino acids and nucleic acids, affecting different fungal metabolic pathways14,19,30.
According to the PCA score plot, the segregation of R. officinalis EO to the right side of the plot was achieved by the first principal component. This EO showed weak antifungal activity, but it was the only EO that inhibited conidial production at all tested concentrations. Three major components characterized R. officinalis EO: 1,8-cineole (53.48%), α-pinene (15.65%), and (−)-camphor (9.57%)8. Lucini et al.31, reported weak inhibitory activity of 1,8-cineole and (−)-camphor against Sclerotium cepivorum compared to other monoterpenes. Morover, the inhibitory activities of 1,8-cineole and α-pinene were described by Kadoglidou et al.23, against A. terreus, F. oxysporum, P. expansum, and Verticillium dahliae with a dose-dependent effect. It was stated that compounds with epoxide groups in their structure (such as 1,8-cineole) exert their antifungal effect by causing damage cell membranes35. However, different parameters of the molecule must be considered to explain the effectiveness of a pure compound. Regardless of the epoxide group, the weak antifungal effect of 1,8-cineole and (−)-camphor might be due to their low solubility in the membrane (determined by its log p-octanol/water partition coefficient), which may cause a delay in their toxic effects31. In addition, conidiation was partially inhibited in Aspergillus sp., Ulocladium sp., Coprinellus sp., and two isolates of Penicillium sp., when treated with Eucalyptus sp. EO, with 1,8-cineole being its major constituent52. On the other hand, except for C. longa at 125ppm, conidiation was stimulated with C. longa, P. dioica and S. aromaticum at all evaluated concentrations in a dose-dependent trend. In fact, the concentrations that showed stronger inhibitory effect on fungal growth were also the ones that produced higher conidiation. For instance, fungal growth with P. dioica at 1000ppm was reduced more than 8 times, but conidial production was 30 times higher than the control. Lower concentrations of this EO led to weak growth inhibition effects, but also, to less sporulation. The increased conidial production observed from 125ppm to 1000ppm could be a fungal response to growing dosages of toxic stressful compounds. It was reported that abiotic stresses, such as light or the presence of certain chemical compounds in the growth medium, trigger fungal conidiation. This would indicate that hyphal cells stop normal growth and initiate conidiophore formation suggesting that conidial production might act as a defense mechanism23.
Our results showed distinct morphological changes among the tested EOs in both the formation of aerial mycelium and colony coloration. A visible variation of morphological features was reported in Aspergillus flavus, Aspergillus fumigatus, Aspergillus niger, F. oxysporum and Trichophyton rubrum when they were exposed to different EOs18,37,40,58,61. Either changes in mycelium color or a completely loss of pigmentation were observed in these fungal species, along with morphological changes in the hyphae17,36,39,54,57,59. These changes could be related to the interference of the EO components on enzymatic reactions of wall synthesis, which affects fungal morphogenesis and growth. However, the effect of EOs on pigment biosynthesis has been poorly addressed by most studies evaluating EOs as antifungal agents. In contrast, their influence on several mycotoxin biosynthesis has been widely reported in Fusarium sp.10,15,44 and other fungal species18,24,50. Mycotoxins, as well as pigments, are biosynthetically related fungal secondary metabolites. In fact, the genes responsible for their production are typically located adjacent to each other and show similar patterns of expression9. Our results suggest that EO constituents interfered with polyketide pathways, such as those leading to pigment biosynthesis62. Additionally, EOs from turmeric, allspice, rosemary and clove proved to be effective as inhibitors of mycotoxin production in different fungal species, such as Aspergillus sp., Fusarium sp., Penicillium sp., among others4,20,38,55,61. As was stated above, F. verticillioides is a producer of a group of mycotoxins called fumonisins with fumonisin B1 being the one with higher toxicity and incidence in stored grains29. Previous investigations have addressed the inhibitory effect of certain EOs on fumonisin B1 biosynthesis by F.verticillioides12,60,63. In those studies, EOs exerted their inhibitory effect through two different modes of action; as a consequence of decreasing fungal growth or by reacting with the active sites of target enzymes responsible for the biosynthesis of fumonisin B1.
ConclusionIn general, EOs are considered potentially effective substances against microorganisms affecting stored products, such as filamentous fungi. However, not all the compounds of an EO are responsible for its bioactivity. In addition to this, there is a large variation in the chemical composition of EOs according to the origin of cultivars, the maturity stage, the nutritional status of the plants, and the geographical area51. In this context, studying the antifungal activity of EOs is the first step toward the identification of highly bioactive pure compounds or mixtures of pure compounds. In the present work, we were able to identify different major compounds or mixtures of major compounds that were responsible for the antifungal and anti-conidiogenic effects on F. verticillioides. Such is the case of eugenol, methyl eugenol, β-myrcene, turmerones, 1,8-cineole, α-pinene, and (−)-camphor. It has also been stated that the antifungal activity of EOs depends on their major components. However, it is noteworthy that the antifungal activity of EOs is not always strictly correlated with major components because the presence of minor constituents may lead to additive, synergistic or antagonistic effects. Further experiments combining these pure components are needed in order to develop a highly bioactive formulation of natural compounds against the phytopathogenic fungus F. verticillioides. Then, we would achieve the same or higher bioactivity as the crude EO, but applying lower amounts of pure compounds. In addition, the ability of these EOs and their pure compounds to inhibit or decrease fumonisin B1 production by F. verticillioides, as well as their effect on conidial germination should be included in future studies.
FundingThis work was supported by the National Research Council of Argentina (CONICET), National Ministry of Science and Technology (FONCYT-PICT 2016-2496 and FONCYT-PICT 2018-3697) and Universidad Nacional de Córdoba (SECYT).
Conflict of interestThe authors declare that they have no conflicts of interest.
The authors would like to thank to Dr. Pablo Cortina, Dr. Marcela Palacios and Mtr. Damián Barrionuevo for technical support.