The aim of this study was to identify entomopathogenic fungi infecting spiders (Araneae) in a protected area of Buenos Aires province, Argentina. The Araneae species identified was Stenoterommata platensis. The pathogens identified were Lecanicillium aphanocladii Zare & W. Gams, Purpureocillium lilacinum (Thom) Luangsa-ard, Houbraken, Hywel Jones & Samson and Ophiocordyceps caloceroides (Berk & M.A. Curtis). This study constitutes the southernmost records in the world and contributes to expanding the knowledge of the biodiversity of pathogenic fungi of spiders in Argentina.
El objetivo de este estudio fue identificar hongos entomopatógenos de arañas en un área protegida de la provincia de Buenos Aires, Argentina. La especie de araña identificada fue Stenoterommata platensis. Los patógenos identificados fueron Lecanicillium aphanocladii Zare y W. Gams, Purpureocillium lilacinum (Thom) Luangsa-ard, Houbraken, Hywel Jones y Samson y Ophiocordyceps caloceroides (Berk y M.A. Curtis). Este estudio constituye el registro más austral del mundo y contribuye a ampliar el conocimiento de la biodiversidad de hongos patógenos de arañas en Argentina.
Entomopathogenic fungal species parasitizing spiders have been reported worldwide2,4,7,10,11. Commonly, the fungal genera recorded include Akanthomyces, Cordyceps, Gibellula, Nomuraea, Ophiocordyceps and Torrubiella. Gibellula mainsii, G. brunnea and G. clavata have been reported in South America9, whereas Gibellula clavulifera var. major has been recorded in Mexico6. However, no attempts have been made to identify the host spider species. In Argentina, the only record of fungal pathogens of spiders is Nomuraea atypicola infecting Actinopus spp.3. Thus, the aim of this study was to identify entomopathogenic fungi of Araneae in “El Destino”, a protected area of Buenos Aires province, Argentina.
Araneae specimens were collected during 2012, 2013 and 2014 from the protected area known as “El Destino”, located in Pearson (33°39′09″S and 60°53′25″W), Magdalena, Buenos Aires province, 3km away from the Río de La Plata River. The environments are principally forests of Celtis tala Gillet ex Planchon (Ulmaceae), associated with Jodina rombifolia Hook et Arn. – (Santalaceae) Acacia caven (Mol.) Mol. (Leguminosae). These areas have soils containing sedimentary shells and are subject to seasonal flooding. Spiders were hand-collected individually mainly from under trees (leaf litter) and under stones close to vegetation, and from soil samples and holes, and deposited with fine forceps into small clean capped plastic tubes that were identified with the site, date, and collector's name. Fungi were recovered from freshly collected specimens using direct isolation techniques1,5. For this purpose, the spiders were collected with fine forceps sterilized by flame and their surface was superficially disinfected by submerging them first in ethanol 70° for 20–30s and then washed in sterile distilled water. Mycelia or fungal spores were taken with a sterile needle or looper and inoculated into 60mm sterilized Petri dishes. The culture medium used was Sabouraud dextrose agar (SDYA)+antibiotics (gentamicin+chloramphenicol) and SDYA with crystal violet and dodine. The dishes were then sealed with Parafilm® and cultures were incubated in an incubator in darkness at 25°C for 2 weeks.
Microscopic and macroscopic descriptions were made from SDYA and from malt extract agar for Lecanicillium aphanocladii and Purpureocillium lilacinum, respectively. Mycelia were mounted in lactophenol/cotton blue (0.01%, w/v) and observed by phase contrast under an Olympus CH3 microscope. Fungal preparations were photographed using a Nikon Optiphot microscope equipped with differential interference contrast fitted with a Canon PowerShot A80 camera. The length and width of fungal structures (conidia, conidiogenous cells and mycelia) were measured to enable species identification. Fungal species were identified according to taxonomic keys and monographs in Samson et al.8, and Zare and Gams12.
The Araneae species identified was Stenoterommata platensis Holmberg 1881 (Mygalomorphae, Nemesiidae) and 13 specimens (nine females, two males and two juveniles) were collected.
Fungal identification and taxonomic observations:
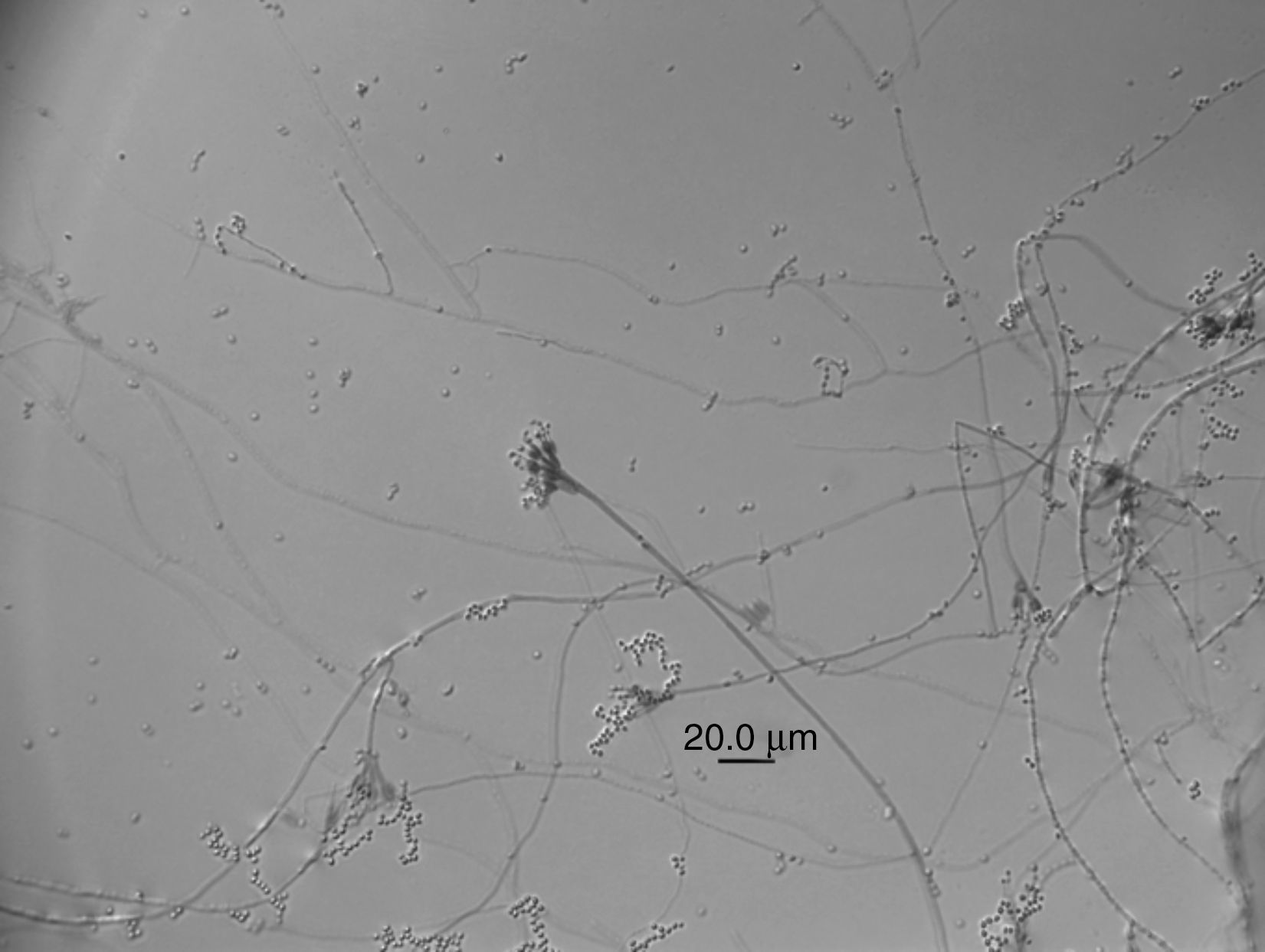
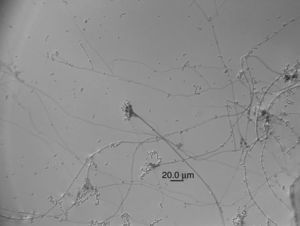
Lecanicillium aphanocladii Zare & Gams Nova Hedwigia 73 (1–2): 27 (2001) (Fig. 1).
=Synonymy:
Acremonium aranearum Petch, Transactions of the British Mycological Society 16 (4): 242 (1931).
Aphanocladium aranearum (Petch) W. Gams, Cephalosporium-artige Schimmelpilze: 198 (1971).
Classification: Ascomycota, Pezizomycotina, Sordariomycetes, Hypocreomycetidae, Hypocreales, Cordycipitaceae.
Date: June 20, 2014.
Culture collection access number: CEP 556
Collector: Barneche, J.
Leg. & det.: López Lastra, C.C.
Description:
Macroscopic characterization: colonies growing up to 50mm in diameter after 10 days at 25°C. Color white cream to lightly orange and colony reverse white cream, mycelia sticky.
Microscopic characterization: conidiogenous cells 4.5–9μm in length (average 48.5)×2μm in width. Globose to slightly ovoid conidia 4.03–5.72μm in length (average 4.82μm)×1.90–3.09μm (average 2.60μm) in width.
Purpureocillium lilacinum (Thom) Luangsa-ard, Houbraken, Hywel-Jones & Samson, FEMS Microbiology Letters 321: 144 (2011) (Fig. 2).
=Synonymy
Penicillium lilacinum Thom, Bull. Bur. Anim. Ind. U.S. Dep. Agric.: 73 (1910)
Paecilomyces lilacinus (Thom) Samson, Studies in Mycology 6: 58 (1974)
Penicillium amethystinum Wehmer
Spicaria rubidopurpurea Aoki, Bull. Imp. Seri cult. Exp. Sta. Japan: 419–441 (1941)
Classification: Ascomycota, Pezizomycotina, Sordariomycetes, Hypocreomycetidae, Hypocreales, Ophiocordycipitaceae.
Date: June 20, 2014.
Culture collection access number: CEP 555
Collector: Barneche, J.
Leg. & det.: López Lastra, C.C.
Description:
Macroscopic characterization: colonies growing up to 35–40mm in diameter after 7 days of incubation at 25°C. Cottony and powdery mycelia presenting white color at the beginning, turning to pink or purple with increasing time of culture, reverse is purple.
Microscopic characterization: phialides 4.45–5.00μm (average 4.78μm) in length and 1.39–1.79μm (average 1.64μm) in width. Globose conidia 1.79–2.27μm (average 2.0μm) in diameter.
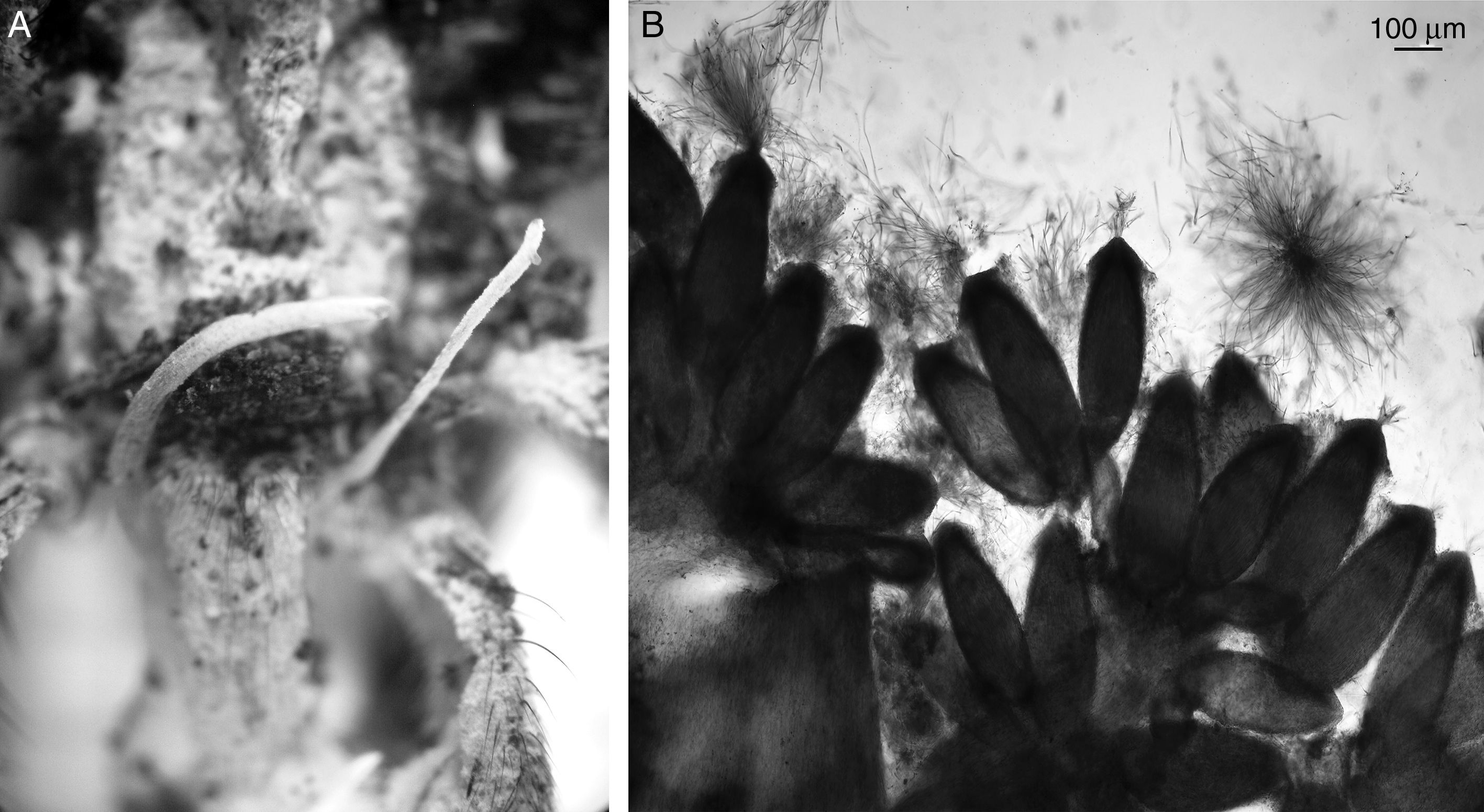
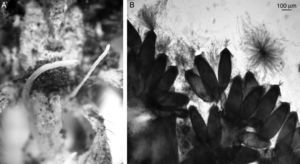
Ophiocordyceps caloceroides (Berk. & M.A. Curtis) Petch, Transactions of the British Mycological Society 18 (1): 63 (1933) (Fig. 3A and B).
=Synonymy
Cordyceps caloceroides Berk. & M.A. Curtis, Botanical Journal of the Linnean Society 10: 375 (1869).
Classification: Ascomycota, Pezizomycotina, Sordariomycetes, Hypocreomycetidae, Hypocreales, Cordycipitaceae.
Date: December 02, 2014.
Herbarium access number: CEP 28.
Collector: Manfrino, R. and Barneche, J.
Leg. & det.: López Lastra, C.C. and Hywel-Jones, N.
Description:
Microscopic characterization: mature stroma with perithecia 90–190μm (x¯:134 μm) red color when mature. Asci 50–90μm in length (average 13μm) and 4–5μm in width (average 4.8μm), presenting filiform septate ascospores 10–25μm in length (average 13μm) and 4–5μm in width (average 4.8μm).
This study contributes to further increasing the knowledge of species of entomopathogenic fungi that infect spiders and of their biodiversity range. This is the second report of fungal species infecting spiders in Argentina and the southernmost record for O. caloceroides (which had never been reported in Argentina before). L. aphanocladii and C. polyarthra constitute the first reports of fungi infecting spiders in Argentina.
Ethical disclosuresProtection of human and animal subjectsThe authors state that no experiments have been performed on humans or animals for this research.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Conflict of interestThe authors declare that they have no conflicts of interest.
Ethical responsibilitiesNone.
To CONICET (National Research Council of Argentina) for partially financial support trough doctoral fellowships to R. Manfrino and A. Gutiérrez, to UNLP National University of La Plata (Universidad Nacional de La Plata) for financial support through Grant N° 760, “Programa de Incentivos” (UNLP).