Group A Rotavirus (RVA) remains a leading cause of severe diarrhea and child mortality. The variable domain of camelid heavy chain antibodies (VHH) display potent antigen-binding capacity, have low production costs and are suitable for oral therapies. Two sets of anti-RVA VHHs have been developed: ARP1-ARP3; 2KD1-3B2. Here, we explore the potential of both sets as a prevention strategy complementary to vaccination and a treatment option against RVA-associated diarrhea in endangered populations. Both sets have been expressed in multiple production systems, showing extensive neutralizing capacity against strains of RVA in vitro. They were also tested in the neonatal mouse model with various degrees of success in preventing or treating RVA-induced diarrhea. Interestingly, mitigation of the symptoms was also achieved with freeze-dried ARP1, so that it could be applied in areas where cold chains are difficult to maintain. 3B2 was tested in a pre-clinical trial involving gnotobiotic piglets where it conferred complete protection against RVA-induced diarrhea. ARP1 was used in the first clinical trial for anti-RVA VHHs, successfully reducing stool output in infants with RVA diarrhea, with no detected side effects.
Los rotavirus del grupo A (RVA) constituyen la principal causa de diarrea grave y mortalidad infantil. La porción variable de los anticuerpos de cadena pesada derivados de camélidos presentan una amplia capacidad de unión antigénica (reconocen sitios antigénicos no accesibles a los anticuerpos tradicionales, con elevada afinidad) tienen bajos costos de producción y resultan ideales para las terapias orales. A la fecha, se desarrollaron 2 pares de nanoanticuerpos VHH contra RVA: ARP1-ARP3 y 2KD1-3B2. En este trabajo, exploramos el potencial de ambos grupos de nanoanticuerpos como estrategias de prevención complementarias a la vacunación y como una opción de tratamiento frente a la diarrea asociada a RVA en poblaciones de riesgo. Ambos pares de nanoanticuerpos fueron expresados en diferentes sistemas de producción y mostraron amplia capacidad neutralizante contra diversas cepas de RVA in vitro. También fueron usados en el modelo de ratón lactante, en el que evidenciaron distintos grados de éxito en la prevención o el tratamiento de la diarrea inducida por RVA. Es interesante destacar que la mitigación de los síntomas también se logró con ARP1 liofilizado y conservado, por lo que podría ser utilizado en áreas donde es difícil mantener la cadena de frío. Asimismo, 3B2 fue testeado en una prueba preclínica utilizando como modelo al cerdo gnotobiótico, al cual confirió completa protección contra la diarrea inducida por RVA. ARP1 fue usado en la primera prueba clínica de nanoanticuerpos VHH contra RVA, donde redujo significativamente las deposiciones en pacientes pediátricos con diarrea positivos para RVA, sin evidenciar ninguna reacción adversa.
Group A Rotavirus (RVA) is the main source of severe diarrhea in young children, accounting for approximately 453,000 deaths every year, most of which occur in developing countries56. Globally, strains of four G-P combinations are responsible for about 90% of all RVA-associated diarrhea cases: G1[P8], G2[P4], G3[P8] and G4[P8]46. Although a similar distribution is found in Latin America, G9[P8] plays an important epidemiological role in this region, where the recent emergence of G12 strains has also been reported13,33.
Prevention of RVA-associated diarrhea: where do we stand today?Two vaccines, a single-strain attenuated human RVA (G1[P8]) vaccine (ROTARIX, GlaxoSmithKline Biologicals), and a multi-strain bovine-human reassortant RVA (G1-G4, P1A)8 vaccine (RotaTeq, Merck), are available and have shown high efficacy in preventing severe RVA gastroenteritis in industrialized14,43,63,64 and some developing countries3,18,43. However, studies show that RVA vaccines have significantly lower efficacy in countries with limited infrastructure and resources, where the burden of RVA-associated diarrhea is usually highest34,71. There are diverse factors that affect the performance of oral vaccines in impoverished settings including nutritional aspects such as malnutrition and zinc deficiency, the presence of competing enteropathogens, mucosal alterations in the gut due to persistent enteropathy and high levels of maternal antibodies (Abs) in breast milk5. Recently, several studies have determined that prenatal vitamin A deficiency alters the innate immune response against RVA vaccination31,65. Furthermore, children affected by severe combined immunodeficiency have suffered vaccine-acquired RVA infections and diarrhea40.
Future efforts should focus on optimizing the efficacy, safety, accessibility and delivery of vaccines among high risk populations30 as well as on developing new passive immunization strategies that could serve as a complement or alternative to vaccination. Recently, a new oral live attenuated RVA (G9 P[11]) vaccine (ROTAVAC) developed in India has passed clinical phase III9. ROTAVAC vaccine is cheaper than existing vaccines (1 USD per dose versus 15 USD for other commercial vaccines) and its licensure would substantially improve the access of developing countries to RVA vaccination9.
Treatment strategies for RVA-associated diarrheaTreatment strategies against RVA are non-specific and largely symptom-based. Clinical management of RVA-associated diarrhea is based on preventing dehydration through oral rehydration salts (ORS) administration, zinc supplementation, and continued feeding68. Several attempts to develop a specific treatment have been made, including the administration of RVA-specific bovine colostrum49, monoclonal Abs11 and egg yolk polyclonal immunoglobulin (Ig) YAbs47,50,61, probiotics25,41,72, drugs42,45,57 and natural herbal compounds6,32. Some of these studies showed efficacy in reducing diarrhea duration or fluid loss, but none of these treatments was adopted as a standardized procedure against RVA-associated diarrhea. Additionally, passive immunization strategies, including the use of animal colostrum or IgYAbs, have raised concern about possible allergic reactions and the presence of adventitious viruses.
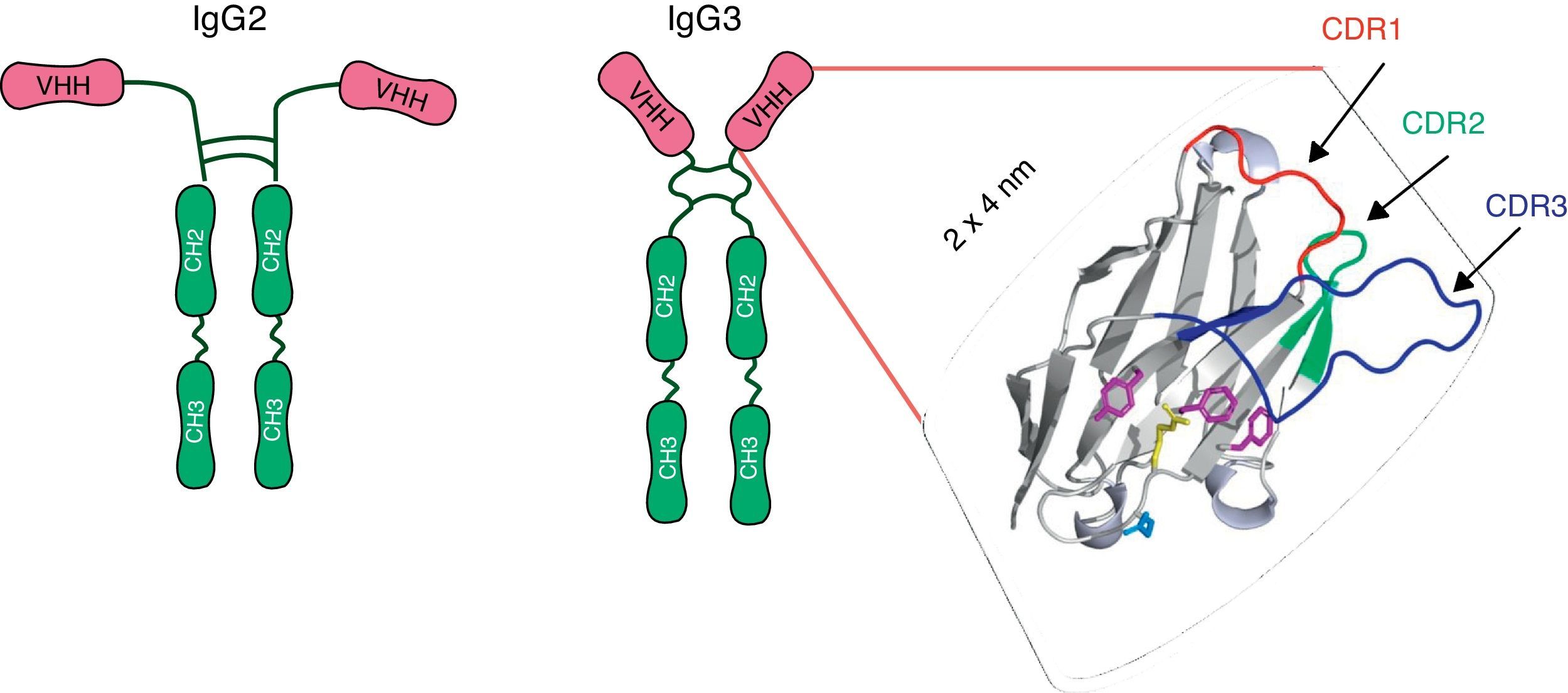
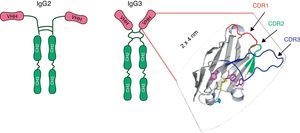
A new paradigm: camelid single-chain antibody fragments (VHHs)In 1993, Hamers-Casterman et al.27 discovered an IgG-like material in the serum of the camel (Camelius dromedarius). These molecules, which are present in all species of camelid, were composed of heavy-chain dimers and devoid of light chains, but had an extensive antigen-binding repertoire27 (Fig. 1). The variable domain of these heavy chain antibodies, known as VHH, consists of only one polypeptide chain and therefore may be cloned and expressed as a soluble protein constituting monoclonal recombinant antibody fragments. Because of their reduced size (15kDa) and the protruding shape of the paratope, VHHs display potent antigen-binding capacity and can interact with novel epitopes inaccessible to conventional Abs17,36,52. VHHs can be efficiently produced in various protein expression systems, including Escherichia coli23, yeasts22,60, insect cells and larvae infected with baculovirus24,62, and transgenic crops58. They entail low costs regarding the scaling up of production, purification and sterilization. Moreover, VHHs show exceptional resistance to high temperatures36 and extreme pH19,28, which makes them ideal candidates for developing an oral prevention strategy and a viable treatment option for RVA-associated diarrhea. Although nanobodies are susceptible to degradation by pancreatic enzymes in the small intestine, it has been proved that the co-administration of VHHs with milk or isolated proteins can protect them from proteolytic degradation (unpublished results). The small size, stable behavior, rapid clearance from blood, and a sequence sharing a high degree of identity with the human VH are all properties that predict low immunogenicity of a nanobody36, a feature that is highly desirable for antibody-based therapies. Indeed, no immune response against the VHH moiety was raised in mice or humans parenterally injected with nanobodies-containing constructs in the absence of adjuvants7,16. In the case of oral administration, no evidence was found concerning VHH translocation from the intestinal lumen into the bloodstream62.
Schematic representation of the heavy chains antibodies present in sera of the camelids: IgG2 and IgG3. Each heavy chain comprises a VHH, a hinge region and two constant domains. The VHH domain includes three Complementary Determining Regions (CDRs) which are depicted as colored loops: CDR1 red, CDR2 green and CDR3 blue. Adapted from Garaicochea L, Vega CG, Parreño V. VHH technology: A potential passive immune therapy to control Rotavirus infections in human infants. In Zeni CD, editor. Rotavirus Infections: Epidemology, clinical characteristics and treatment options. 1st edition. New York, Nova Publishers, 2014, p122. Copyright © 2014 by Nova Science Publishers, Inc.
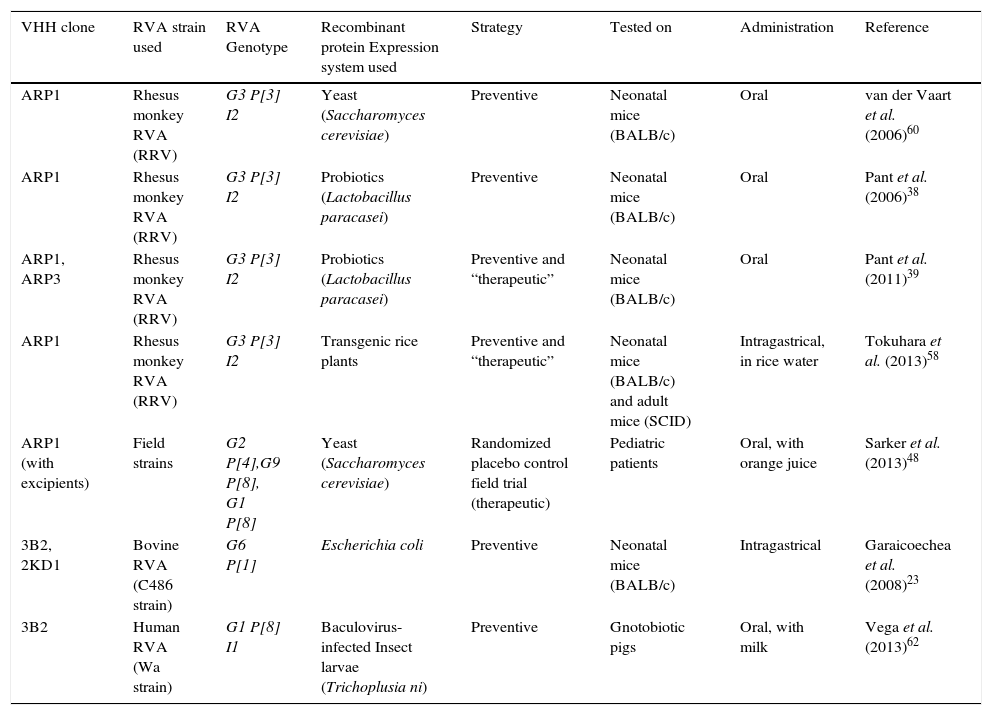
To the best of our knowledge, two sets of VHHs against RVA have been developed, ARP1 (also known as VHH1/2B10) and ARP3, both comprising the first set2,60, and 3B2 and 2KD1, comprising the second set23. ARP1/3 were isolated from an adult llama immunized with rhesus-monkey RVA (RRV) strain (G3P[3]I2)2,60 whereas 2KD1/3B2 were obtained from an adult llama previously immunized with recombinant VP6 protein derived from the bovine RVA C486 strain (G6P[1])23. Both the ARP1/ARP3 and 3B2/2KD1 sets have been used in previous studies including in vitro assays (Table 1) and protection/treatment experiments in different animal models (Table 2). ARP1 was recently tested in a clinical trial involving infants suffering from RVA diarrhea in Bangladesh48.
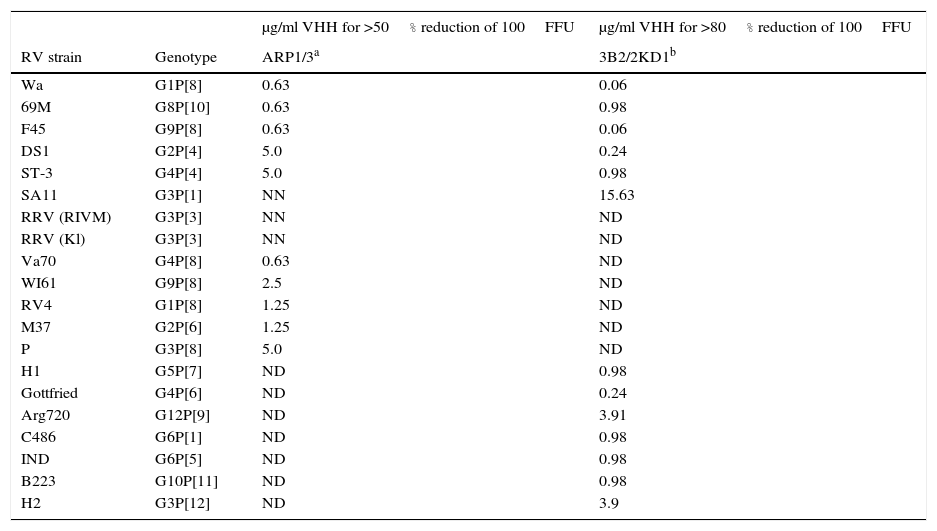
In vitro neutralizing capacity of ARP1/ARP3 and 2KD1/3B2 against different RVA strains by a fluorescent focus reduction assay
| μg/ml VHH for >50% reduction of 100FFU | μg/ml VHH for >80% reduction of 100FFU | ||
|---|---|---|---|
| RV strain | Genotype | ARP1/3a | 3B2/2KD1b |
| Wa | G1P[8] | 0.63 | 0.06 |
| 69M | G8P[10] | 0.63 | 0.98 |
| F45 | G9P[8] | 0.63 | 0.06 |
| DS1 | G2P[4] | 5.0 | 0.24 |
| ST-3 | G4P[4] | 5.0 | 0.98 |
| SA11 | G3P[1] | NN | 15.63 |
| RRV (RIVM) | G3P[3] | NN | ND |
| RRV (Kl) | G3P[3] | NN | ND |
| Va70 | G4P[8] | 0.63 | ND |
| WI61 | G9P[8] | 2.5 | ND |
| RV4 | G1P[8] | 1.25 | ND |
| M37 | G2P[6] | 1.25 | ND |
| P | G3P[8] | 5.0 | ND |
| H1 | G5P[7] | ND | 0.98 |
| Gottfried | G4P[6] | ND | 0.24 |
| Arg720 | G12P[9] | ND | 3.91 |
| C486 | G6P[1] | ND | 0.98 |
| IND | G6P[5] | ND | 0.98 |
| B223 | G10P[11] | ND | 0.98 |
| H2 | G3P[12] | ND | 3.9 |
Preventive and therapeutic strategies against Rotavirus-associated diarrhea using VHHs nanobodies
| VHH clone | RVA strain used | RVA Genotype | Recombinant protein Expression system used | Strategy | Tested on | Administration | Reference |
|---|---|---|---|---|---|---|---|
| ARP1 | Rhesus monkey RVA (RRV) | G3 P[3] I2 | Yeast (Saccharomyces cerevisiae) | Preventive | Neonatal mice (BALB/c) | Oral | van der Vaart et al. (2006)60 |
| ARP1 | Rhesus monkey RVA (RRV) | G3 P[3] I2 | Probiotics (Lactobacillus paracasei) | Preventive | Neonatal mice (BALB/c) | Oral | Pant et al. (2006)38 |
| ARP1, ARP3 | Rhesus monkey RVA (RRV) | G3 P[3] I2 | Probiotics (Lactobacillus paracasei) | Preventive and “therapeutic” | Neonatal mice (BALB/c) | Oral | Pant et al. (2011)39 |
| ARP1 | Rhesus monkey RVA (RRV) | G3 P[3] I2 | Transgenic rice plants | Preventive and “therapeutic” | Neonatal mice (BALB/c) and adult mice (SCID) | Intragastrical, in rice water | Tokuhara et al. (2013)58 |
| ARP1 (with excipients) | Field strains | G2 P[4],G9 P[8], G1 P[8] | Yeast (Saccharomyces cerevisiae) | Randomized placebo control field trial (therapeutic) | Pediatric patients | Oral, with orange juice | Sarker et al. (2013)48 |
| 3B2, 2KD1 | Bovine RVA (C486 strain) | G6 P[1] | Escherichia coli | Preventive | Neonatal mice (BALB/c) | Intragastrical | Garaicoechea et al. (2008)23 |
| 3B2 | Human RVA (Wa strain) | G1 P[8] I1 | Baculovirus-infected Insect larvae (Trichoplusia ni) | Preventive | Gnotobiotic pigs | Oral, with milk | Vega et al. (2013)62 |
Clones ARP1 and ARP3 successfully neutralized eleven RVA strains (Table 1): however, they surprisingly failed to neutralize strains SA11 (G3P[1]) and RRV (Kl variant, G3P[3]). The concentration of ARP1/ARP3 required a 50% reduction of the initial dose (100FFU) varied between strains and ranged from 0.63μg/ml to 5.0μg/ml2. Meanwhile, 3B2 and 2KD1 showed neutralizing activity against all thirteen strains tested, including SA11 (G3P[1]) (Table 1). In this case, a reduction of >80% of the virus dose (100FFU) was achieved with VHH concentrations ranging from 15.63μg VHH/ml for SA11 to 0.06μg VHH/ml for Wa and F45 human RVA strains, among others23,62 (Table 1). On the whole, both sets of anti-RVA VHHs successfully neutralized different RVA strains, although 2KD1/3B2 appeared to neutralize a wider range of strains requiring, overall, lower doses of VHH2,23.
Nevertheless, the precise mechanism involved in viral neutralization remains unknown for both sets of clones. 2KD1/3B2 recognize and bind specifically to the VP6 protein, as they were obtained from a llama immunized with RVA protein VP6. For ARP1 and ARP3, the binding site was uncertain as the llama had been immunized with complete virus particles. Recent studies indicated that ARP1 also recognizes and binds specifically to VP648. The authors further imply that attachment of ARP1 to VP6 might trigger a conformational change in outer layer proteins VP4 and/or VP7, thus preventing the viral particle from binding to or entering the cells48. Recent studies suggested that anti-RVA IgA Abs could neutralize viral particles by attaching to a negatively-charged patch on the surface of Type I channel and sterically blocking it1. Further experiments should assess if this mechanism could also be used by nanobodies.
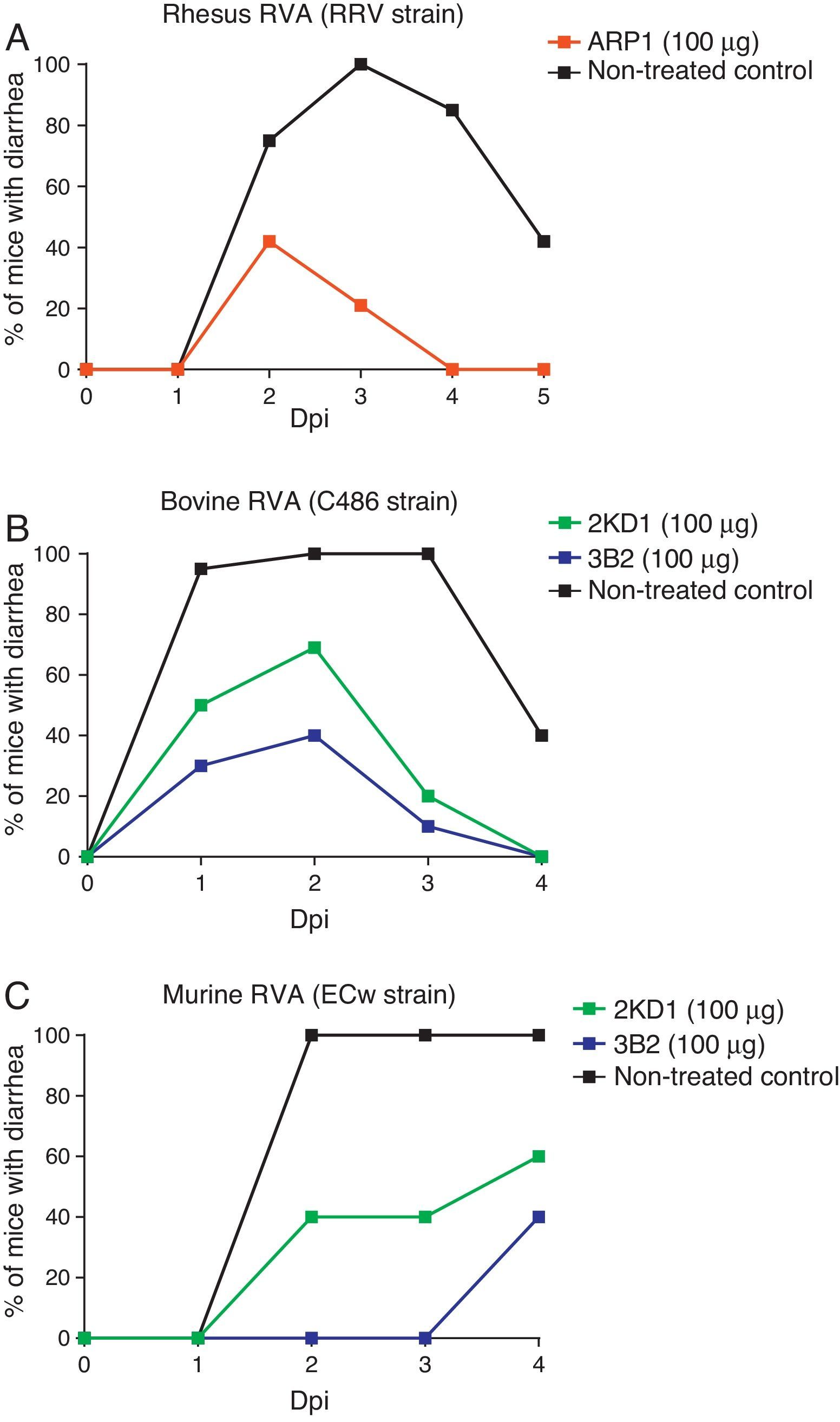
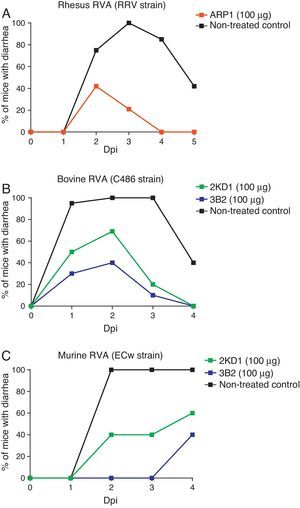
Prevention and treatment of RVA-induced diarrhea in neonatal miceBoth ARP1/ARP3 and 2KD1/3B2 were tested in a neonatal mouse model for protection against RVA-induced diarrhea23,60. The neonatal mouse model for RVA infection was developed using BALB/c mice in early studies searching for a reliable animal model mimicking RVA infection in human patients10. Mice are susceptible to murine RVA until 14 days of life but they can also be infected with some other RVA strains. However, homologous rotaviruses (RV) are, in general, more virulent and replicate more efficiently than heterologous RV in the intestine of the homologous host20. Moreover, RVA host restriction was proven to involve a multigenic nature in which efficient enteric replication requires a constellation of murine genes encoding VP3, NSP2, and NSP3 along with NSP120. These findings cast doubt on whether the neonatal mouse model infected with heterologous strains could be used in pre-clinical trials. Groups of four-day old suckling BALB/c mice received different clones of VHHs orally, prior to and after infection with a mixture of RRV strain and a VHH previously produced in yeast (Saccharomyces cerevisiae)60. Of these clones, however, only ARP1 was successful in reducing the prevalence of RVA-induced diarrhea and was consequently selected for further prophylactic experiments. Different amounts of the VHH were administered to four-day old mice from post inoculation days −1 to 4. On day 0, ARP1 was delivered two hours after infection with RRV (2×107PFU). While low daily doses (5 and 20μg) of the nanobody did not produce significant differences with respect to the non-treated control, higher amounts of the VHH (50 and 100μg) successfully reduced the number of days with diarrhea per pup60 (Fig. 2A).
Protection rate against diarrhea achieved by anti-RVA nanobodies in neonatal mice challenged with various RVA strains. Mouse pups were fed 100μg of each VHH from days 0 to 5 once a day by the intragastric route. On day 1, the pups were challenged intragastrically with RVA 2h after nanobody administration. Diarrhea was assessed by gentle abdominal palpation. (A) Protection rate achieved by ARP1 in mice challenged with 20 DD50 (2×107UFP) of rhesus RV. (B) Protection rate achieved by 2KD1 and 3B2 in mice challenged with 30 DD50 (6×105FFU) of bovine RV C486. (C) Protection rate achieved by 2KD1 and 3B2 in mice challenged with 316 DD50 of murine RV ECw.
Later on, ARP1 and ARP3, previously produced in yeast, were tested in the same mouse model and it was demonstrated that ARP1 and ARP3 target different epitopes using a competition ELISA50. If administered together, this combination could optimize viral neutralization and reduction of viral escape mutants. When tested in vivo, low doses of ARP1 and ARP3 partially protected mice infected with 2×107FFU of RRV strain (20 diarrhea doses [DD50]) from diarrhea. The same total amount (10μg) of the mix ARP1+ARP3 achieved a stronger protection than ARP1 or ARP3 alone, especially two days after inoculation with RVA (RRV strain); however, the difference was not significant. This could imply a synergistic effect of the clones that needs to be addressed.
Likewise, prophylactic experiments involving purified 2KD1 and 3B2 produced in E. coli were conducted23. Four day old BALB/c mice received a daily dose (100μg, 22.2mg/kg/day) of different clones of VHH from days post-inoculation −1 to 4 via intragastric gavage. On PID 0, mice were inoculated with either bovine RVA (C486 strain, G6P[1]) containing a total of 30 DD50 (6×105FFU/mouse) (experiment A) or with murine RVA (ECw strain, G16P[16]) containing 316 DD50 (experiment B). Two hours later the mice received the daily dose of VHH. A significant percentage (61%) of mice treated with 3B2 and infected with the bovine RVA strain (Experiment A) were protected against diarrhea until 96h post inoculation (Fig. 2A), at which point they were euthanized23. While 2KD1 was not capable of significantly reduce the rate of diarrhea at that time, mice treated with this clone significantly lowered the severity and duration of symptoms. When infected with the murine RVA (Experiment B), similar protection rates were obtained for pups treated with 3B2 (60%) before euthanasia (Fig. 2B). In contrast, all pups treated with 2KD1 subsequently developed diarrhea, although there was a delay in the onset of symptoms in comparison with the non-treated control. Mice infected with ECw murine RVA and treated with 3B2 significantly reduced viral shedding in intestine homogenates at 96h post challenge. The 2KD1-treated groups showed no detectable shedding by ELISA at that time23.
Protection against human RVA in gnotobiotic pigsThe ability of 3B2 to prevent the occurrence of RVA-induced diarrhea was also tested in gnotobiotic piglets62, an animal model that has been used widely to study human RVA pathogenesis4,66,69,70. In this case, 3B2 was produced using the Improved Baculovirus Expression System (IBES Technology), which employs baculovirus expression vectors with Trichoplusia ni larvae as living biofactories24. From the second day of life, gnotobiotic pigs were fed commercial bovine milk supplemented with VHH (ELISA Ab titer to RVA of 4096, 0.1mg/ml of milk, 44mg/pig/day or 20–40mg/kg/day) twice a day for 9 days. On day 3, the pigs were orally inoculated with 106,7 UFF of human RVA (Wa strain, G1P[8]I1) and were daily monitored for 21 days to assess the occurrence of diarrhea and viral shedding. Serum samples were collected weekly and were used to study immunological parameters. At 21 days post inoculation, the piglets were euthanized and their intestines were collected. Administration of 3B2 conferred complete protection against human RVA-induced diarrhea in all treated piglets62. The results of this study showed higher efficacy of 3B2 in preventing the occurrence of diarrhea than previous assays using this same VHH in mice models. Although viral shedding was detected in the 3B2-treated group, excretion duration was significantly lower than in the non-treated groups. Continued administration of 3B2 neither affected the host immune response to RVA infection nor elicited an immune response to the VHHs. This study is important regarding the use of VHHs for anti-RVA therapy in small infants62. Although, there are some anatomic differences between humans and pigs regarding the gastrointestinal tract, neonatal pigs resemble infants in several ways. Similar to infants, they are immunocompetent at birth, but immunologically immature and, as outbred animals, they are closer to the heterogeneity of human populations35. In addition, secretory IgA represents the dominant isotype in the intestine, milk and mucosal secretions35. For RVA infection, experimental studies in gnotobiotic piglets have been interpreted to indicate that some human RV strains can be adapted to replicate very efficiently in piglets, although a comparison to wild-type homologous porcine RV replication in piglets has not been performed. HRV-infected gnotobiotic pigs exhibit diarrhea, anorexia, dehydration, viremia and intestinal lesions mimicking those in children35. As the study was focused on prophylactic treatment, further research should analyze the efficacy of 3B2 when administered after inoculation or after the initial onset of diarrhea.
Probiotics expressing VHHsAs probiotics have been employed in the treatment of acute diarrhea55, their combined use with antiviral VHHs could generate a synergistic effect against RVA gastroenteritis38. In 2006, Pant et al.38 constructed Lactobacillus paracasei that expressed the anti-RVA VHH ARP1 in secreted and anchored forms. Contrary to previous in vitro assays, this time ARP1 in both types of lactobacilli was able to recognize and neutralize RVA (RRV strain) particles in vitro. For the secreted form, 125ng/ml reduced infectivity by 80% whereas for the anchored form 1000 bacteria (with approximately 1×104 VHH fragments/bacterium equaling approximately 100 ng VHH/ml) were necessary38. Expression of VHHs in the natural gut commensal bacteria surpasses degradation by gastric enzymes. Effectively, both VHHs were detected in the jejunum and ileum of neonatal mice 48h after treatment with a single dose38. The transformed lactobacilli were tested in the mouse model for rhesus RVA infection (2×107FFU) of RRV strain (20 DD50) as described previously60. ARP1-anchored lactobacilli successfully reduced the diarrhea rate in comparison to untreated mice or those treated with irrelevant VHHs. In addition, this treatment significantly reduced the duration and severity of the diarrhea and diminished intestinal inflammation and viral load. However, the ARP1-secreted lactobacilli group only displayed a mild reduction of the symptoms, probably because the expression levels were low. Interestingly, reconstituted freeze-dried ARP1-anchored lactobacilli were as protective against diarrhea as their fresh counterparts38, which is important regarding clinical administration in developing areas where cold chains are difficult to maintain.
In their study of 2011, Pant et al.39, administered yeast produced ARP1 and ARP3 (previously purified) both before and after inoculation with RRV RVA. When the mice were treated two hours after viral inoculation, 10μg of ARP1 successfully reduced diarrhea prevalence, whereas the same amount of ARP3 had no effect on the symptoms. Thus, the combination of ARP1+ARP3 was less efficient than when it was administered as a prophylactic strategy35. In 2011, Pant et al.39 went further and developed Lactobacillus paracasei that displayed anchored ARP1 and ARP3 on their surface both as monospecific or bispecific proteins: ARP1, ARP3, ARP1-ARP1, ARP3-ARP3, ARP1-ARP3. As ARP1 and ARP3 target different epitopes, these bispecific constructs might be more effective in preventing the selection of viral escape mutants to the therapy, a concern present among every antiviral strategy based on monoclonal Abs. Monospecific bivalent ARP compounds (ARP1-ARP1, ARP3-ARP3) did not show increased avidity for RVA, perhaps due to the limitation of effectively binding two proximal copies of the same epitope39.
Experiments were conducted with the multimeric VHH-anchored lactobacilli in the neonatal mouse model for simian RVA infection as was already described, using 2×107FFU of RRV strain (20 DD50) for inoculation. In contrast to previous results, monovalent anchored ARP1 was able to significantly diminish the prevalence and severity of the diarrhea but not the duration of the symptoms compared to the non-treated control. Anchored monovalent ARP3 showed a similar performance. Bivalent ARP3-ARP3-anchored lactobacilli reduced the prevalence and severity of the diarrhea; however, it was not more efficient that the monovalent form of ARP3. On the other hand, bivalent anchored ARP1 showed poor protection against RVA-induced diarrhea and was not able to significantly reduce disease prevalence or severity39. Lactobacilli expressing bispecific ARP1-ARP3 were more efficient in decreasing the prevalence and severity of the diarrhea with respect to the non-treated control and also in comparison to the mice treated with monovalent ARP1-producing anchored lactobacilli. This treatment also achieved a significant reduction in diarrhea duration, which was not observed with any of the other treatments.
Interestingly, a mixture of lactobacilli expressing monovalent ARP1 and ARP3 was only marginally protective against RVA-induced diarrhea, suggesting that when displayed in different bacterial cells, ARP1/ARP3 cannot access their epitopes simultaneously. This would further endorse the idea that the increased activity encountered for lactobacilli expressing ARP1-ARP3 is due to initial binding of ARP1 to its epitope, thus improving the targeting of a transient epitope by ARP339.
The therapeutic effect of ARP1 and ARP3 anchored-lactobacilli was also evaluated, administering the nanobodies two hours after infection and then daily. Lactobacilli expressing monovalent ARP1 significantly reduced the severity and duration of diarrhea, showing higher efficacy than when administered before infection. The administration of ARP3-producing bacteria did not reduce the symptoms, a result congruent with previous ones for yeast purified ARP339. The authors explain the differential performance of both clones by suggesting that ARP3 could only attach to a more concealed epitope if present during early stages of infection. However, it is also possible that ARP3 is less effective than ARP1 in neutralizing larger amounts of viral particles. Lactobacilli producing ARP1-ARP1 showed similar results to those of their monovalent form. However, the bispecific ARP1-ARP3 successfully diminished the severity and duration of diarrhea, showing significant differences with the non-treated control and the rest of the treated groups.
Viral load was also assessed by real time PCR in intestinal tissue, but not in feces. The copy numbers of the VP7 gene was significantly reduced only in mice treated prophylactically with lactobacilli producing monovalent anchored ARP1 or bispecific anchored ARP1-ARP3. When administered after infection, no treatment was able to decrease the viral load39.
Overall, probiotics expressing anti-RVA VHHs entail several advantages as an oral therapy in large-scale pediatric populations, such as biological safety, increased proteolytic resistance, and retention of activity after being freeze-dried or stored for long periods of time.
Transgenic crops expressing anti-RVA VHHsThe potential of plant cells to produce functional recombinant Abs has been demonstrated in different plant systems53. Previous studies reported a rice-based vaccine containing the cholera toxin B subunit effective against cholera in mice37,58. More recently, the same system, with the incorporation of RNAi technology, was used to create an anti-RVA product by concocting rice expressing ARP1 (MucoRice-ARP1) which could be used as rice powder or rice water58. RNAi technology was used to suppress the internal storage of rice protein production and enhance accumulation of ARP1 instead58. MucoRice-ARP1 successfully neutralized human RVA strains of different serotypes in vitro (F45, 69M, Va70, Wa, ST-3). For F45, 69M and Va70, the doses were similar to those of yeast purified ARP1 (Table 1). For ST-3 and Wa strains, 4 times the amount of MucoRice-ARP1 (10μg/ml) was needed.
When it was tested prophylactically – this time administering the first dose nine hours prior to infection – in the mouse model for RVV infection, 17μg of MucoRice-ARP1 significantly reduced the rate of mice with diarrhea and the severity of the symptoms in suckling mice infected with 2×107FFU of RRV. Mice pre-treated with MucoRice-ARP1 did not present histopathological changes in the small intestine and the viral load in that tissue was significantly lower. This reduction of the symptoms and of virus concentration was both achieved against a non-treated control and against a group of mice treated with wild type rice, although the latter showed some degree of improvement due to the astringent properties of common rice. Interestingly, no significant differences were found between fresh, heat-treated and long-term stored Mucorice-ARP1 when administered prophylactically to mouse pups58.
Furthermore, Mucorice-ARP1 significantly reduced the rate of mice with diarrhea, the severity of symptoms and the viral load in a neonatal mouse model when administered therapeutically (17μg) nine hours after infection with RRV RVA. Interestingly, MucoRice powder administered therapeutically also diminished the prevalence and severity of diarrhea CB-17 SCID/SCID neonatal mice58, which could represent a valid model for studying treatment response in immunocompromised infants. For what they called a therapeutic use of nanobodies, the authors of this study chose to administer the VHHs nine hours after infection. However, when discussing the stability of MucoRice-ARP1 in the neonatal mice gut they report that in up to 40% of the mice, MucoRice-ARP1 is detected even 9h after intragastric administration58. As in previous studies using ARP1 in the neonatal mouse model, this latter statement contradicts the experimental design if a therapeutic effect of the VHHs is what the authors seek to analyze.
Overall, the expression of VHHs in transgenic crops could represent an attractive strategy to generate large amounts of ready-to-use nanobodies utilizing limited resources. In contrast to previous systems such as tobacco leaves, the use of comestible seeds implies that there is no need for purification. Furthermore, the final product was a highly soluble rice powder that can be maintained at room temperature and showed high stability for up to a year under these conditions58. Since the primary target for any RVA prevention or treatment strategy would be pediatric populations from developing countries, high stability of the product without the need for maintaining cold chain and low productions costs, all represent crucial features for its effective implementation. Given that groups receiving wild type rice powder showed mild improvement in relation to the control group treated with PBS, the expression of anti-RVA in rice has the additional advantage of this synergistic effect against RVA-associated diarrhea.
Treatment against RVA-associated diarrhea in children from Bangladesh: a randomized placebo-controlled trialRecently, a proof of concept clinical trial was performed in male infants with RVA infection in Bangladesh48. The design of the study implied a single center, randomized, double-blind, placebo-controlled parallel group clinical trial to assess the efficacy of ARP1 in lowering the severity and duration of symptoms compared to placebo, in male children presenting acute onset dehydrating diarrhea.
Briefly, 176 male children aged 6–24 months suffering from acute watery diarrhea for 48h or less and who tested positive for RVA in stool by ELISA, but negative for Vibrio cholerae, were randomly assigned to the ARP1 or placebo group. ARP1 was produced in S. cerevisiae yeast as previously described and the treatment dose was calculated from studies in the neonatal mouse models60, with a maximum daily dose of 35mg/kg of ARP1 per day. The nanobody was administered in single dose sachets, each containing 165mg of the yeast preparation expressing 35% of ARP1, 835mg maltodextrin and 5mg caramel color (57.7mg ARP1/sachet) whereas the placebo consisted of 1000mg of maltodextrin with 5mg caramel color. Each dose was suspended in 10ml of oral rehydration solution and administered orally, starting on day 0 and every eight hours for 4–5 days. Frequency, consistency and amount/volume of stool were recorded periodically and stool consistency scores established the duration of symptoms. Samples of fresh stools were collected daily to determine the presence of RVA by ELISA and to identify the RVA serotype (G and P genotypes) by PCR; RVA-specific IgA was assessed in serum samples.
When only RVA infection was present, the total cumulative stool output was significantly lower in the ARP1-treated groups compared with the placebo group, showing an overall reduction of 22.5%48. However, when other concomitant infections occurred, there were no significant differences regarding stool output, which is important to consider as RVA-associated diarrhea can often present with other infections, mostly of bacterial etiology. ARP1 did not significantly reduce diarrhea duration, in contrast with previous studies in mice60, although the latter were mostly based on a preventive use of ARP1. No differences were found concerning viral clearance by ELISA between treated and placebo groups. These results are in accordance with previous data from mice experiments wherein administration of ARP1 did not reduce viral shedding, even when administered prior to infection60 and in contrast with mice experiments for 2KD1/3B2 where the RVA shedding significantly diminished after administration of the 2KD1/3B223. The ARP1 and placebo groups showed different frequencies of RVA serotypes, although statistical analyses indicated that this finding did not affect the trialoutcomel48. However, because previous assays showed that ARP1 had a different neutralization capacity against different strains of RVA2, this matter should be considered more thoroughly.
Although the results of the first field trial for anti-RVA VHHs are promising, there is still room for improvement. The design of the study fails to account for the effect of S. cerevisiae against RVA-associated diarrhea because the placebo group only received colored maltodextrin. Numerous previous studies have addressed the use of another yeast species and its effect against RVA-induced diarrhea with dissimilar results8,12,15. Future studies comprising the use of ARP1 as an oral therapy against RVA should elucidate the role of yeast in the obtained results, given that previous experiments in the neonatal mouse model only used purified VHHs60.
Future prospectsThe discovery of heavy-chain antibodies in 1993 was a groundbreaking event in the field of immunology and antibody-based therapies. In particular, llama-derived single domain antibody fragments (VHHs) have proven to be powerful viral neutralizers21,51,60,62 and have been studied for their therapeutic use against respiratory syncytial virus29, rabies virus29, influenza virus29,67 and human immunodeficiency virus21,54 among others. Up to date, diarrhea remains a critical health issue worldwide. In children from impoverished areas, the recurrence of diarrhea during their first 2 years of life might cause, on average, an 8cm growth shortfall and 10 IQ point decrease by the time they are 7–9 years old26. The use of VHHs as a prevention or treatment agent against RVA offers a wide range of possibilities regarding the control of RVA-associated diarrhea and the related deaths of children under five years old.
Here, we have surveyed different production strategies for anti-RVA VHHs: E. coli, yeasts, insect larvae infected with baculovirus, probiotics and transgenic crops. Regarding the latter, we believe that transgenic crops such as rice plants constitute a highly eligible option. Anti-RVA VHHs expressed in rice plants do not require purification prior to administration and they entail a synergistic effect against diarrhea due to the astringent properties of the rice. They can also be stored at room temperature for months without compromising anti-viral activity58, which represents a considerable advantage for the administration of anti-RVA VHHs in impoverished areas where cold chains are difficult to maintain.
However, we have previously seen that stability and proteolytic resistance of 3B2/2KD1 are highly increased by the addition of milk (unpublished results). Moreover, milk is distributed by governments of many developing countries as part of their social network programs. The addition of anti-RVA VHHs to milk that is massively delivered in high risk populations might be a profitable and successful prevention strategy for RVA that has not yet been implemented. Given the previous success in developing transgenic cattle that expresses various molecules of therapeutic interest in its milk44,59, development of transgenic cattle expressing anti-RVA VHHs within mammary glands could represent another eligible strategy for VHH production that excludes the need of thorough purification techniques otherwise required and could entail higher social acceptance than traditional immunization. Regardless of which production, administration or clinical strategy is elected, for VHHs to succeed as standardized prophylaxis and treatment strategy against RVA, their production and clinical management must be within long-term public policies allowing impoverished populations to access higher health standards.
Clarification of the molecular interactions that mediate the neutralization of RVA particles by VHH clones needs to be addressed in the immediate future. A complete understanding of the molecular mechanisms involved could optimize prevention and therapeutic uses of these molecules. Previous studies with either set of clones have not approached the possible rise of viral escape mutants to anti-RVA VHH, which is a substantial concern for any antiviral treatment or passive immunization strategy, especially in the case of a highly transmissible and extensive disease such as RVA.
FundingPICT 328/10, Agencia Nacional de Promoción Científica y Tecnológica, MINCyT, Argentina.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare that they have no conflicts of interest.