Bovine respiratory syncytial virus (BRSV) is one of the most relevant agents responsible for respiratory disease in cattle from both dairy and beef farms. BRSV is spread by horizontal contact causing a constant presence of seropositive animals that favors viral circulation throughout the year. Moreover, reinfections with BRSV are frequent between animals regardless of their age as BRSV does not confer long-lasting protective immunity. Several studies have demonstrated the circulation of BRSV in cattle from different regions of the world; however, little is known about the dynamics of BRSV infection in cows before and after they begin lactation. The aim of this work was to study the dynamics of BRSV neutralizing antibodies from birth up to 36 months of age in a closed dairy herd of Argentina specifically around the lactation period. Passive maternal antibodies against BRSV started to decrease monthly and became almost undetectable at 8 months of age. We detected two potential infection points at months 11 and 27 after birth, in which 30% and 45% of the animals showed seroconversion, respectively. Specifically, an increase in the proportion of seropositive cows after the start of lactation suggests that they became reinfected around the time they began lactating. We demonstrate the importance of understanding BRSV dynamics in a closed dairy herd to review the vaccination schedule of the animals to achieve protection against BRSV infection.
El virus respiratorio sincitial bovino (Bovine respiratory syncytial virus, [BRSV]) es uno de los principales agentes responsables de la enfermedad respiratoria en bovinos, tanto de tambos como de cría. El virus se transmite horizontalmente y causa la presencia constante de animales seropositivos, lo cual favorece la circulación viral a lo largo del año. A su vez, las reinfecciones por BRSV son frecuentes entre animales independientemente de su edad, dado que el virus no confiere inmunidad protectora a largo plazo. Numerosos estudios han demostrado la circulación de BRSV en bovinos de diferentes regiones del mundo, sin embargo, poco se conoce acerca de la dinámica de infección en vacas antes y después del inicio de la fase de lactancia. El objetivo de este trabajo fue estudiar la dinámica de anticuerpos neutralizantes anti- BRSV en vacas lecheras desde el nacimiento hasta los 36 meses de vida en un tambo cerrado de Argentina, específicamente, en el período de lactancia. Los anticuerpos pasivos específicos para BRSV comenzaron a declinar mensualmente hasta ser casi indetectables a los 6 meses. Detectamos dos potenciales puntos de infección a los meses 11 y 27 luego del nacimiento, momentos en los que el 30 y el 45% de los animales mostraron seroconversión, respectivamente. El incremento en la proporción de vacas seropositivas luego del comienzo de la lactancia sugiere que estas se reinfectaron en el inicio de dicha etapa. Demostramos la importancia de entender la dinámica de circulación del BRSV en un tambo cerrado, a fin de revisar el esquema de vacunación de los animales para que estén protegidos frente a la posible infección por este virus.
Bovine respiratory disease (BRD) is a group of infectious respiratory diseases that affect cattle and is responsible for significant economic losses to the farming industry worldwide15. Bovine respiratory syncytial virus (BRSV) plays a major role in this disease. BRSV belongs to the genus Orthopneumovirus, family Pneumoviridae, order Mononegavirales1. Morbidity can be as high as 60–80% and mortality can reach up to 20%15. After BRSV infection, pneumonia outbreaks are a frequent outcome given its tropism for the lower respiratory tract and its ability to predispose to secondary bacterial infection13. The virus is transmitted horizontally by direct contact between the animals, through aerosols and respiratory secretions; therefore, the infection is favored when there is close contact between the animals, as it happens during milking or in feedlots.
Antibodies against BRSV can be detected in animals of all ages, even in those with no respiratory signs. Infection with BRSV does not induce long-lasting immunity and calves can be reinfected throughout their lives, even with the same strain. Thus, antibody titers against BRSV are higher in older animals2. Reinfections are usually subclinical or cause mild clinical respiratory disease. Interestingly, it has been described that the presence of high titers of neutralizing antibodies (NAs) against BRSV significantly decreases the clinical severity of the disease15.
Hägglund et al.7 reported that BRSV is widely distributed in dairy farms in Sweden and that there is horizontal dispersion of the virus with a constant presence of seropositive animals, which indicates viral circulation throughout the year.
Although several reports have demonstrated that the virus is widespread all over the world, information about BRSV in Argentina is limited, especially in dairy herds. Odeón et al.8 reported the presence of antibodies against BRSV mainly in cattle under 12 months of age from Buenos Aires, Corrientes and La Rioja, with a greater spread of the virus in districts with larger movement of animals. Recently, we described the presence of NAs and risk factors associated with BRSV in feedlots from Santa Fe and Córdoba, Argentina5. In addition, we demonstrated evidence of the circulation of BRSV by antibody seroconversion in a longitudinal analysis of post-weaned animals from an Argentinian beef herd12. We have also reported the presence of antibodies against the virus in early and ultra-early weaned beef calves6.
Even though BRSV has been linked to decreased milk production in dairy cattle7, there is no information about the dynamics of BRSV infection in cows before and after they begin lactation.
In this work, we studied the dynamics of neutralizing antibodies against BRSV in a dairy herd from Santa Fe Province, Argentina for three years. Potential infection points based on seropositivity were evaluated.
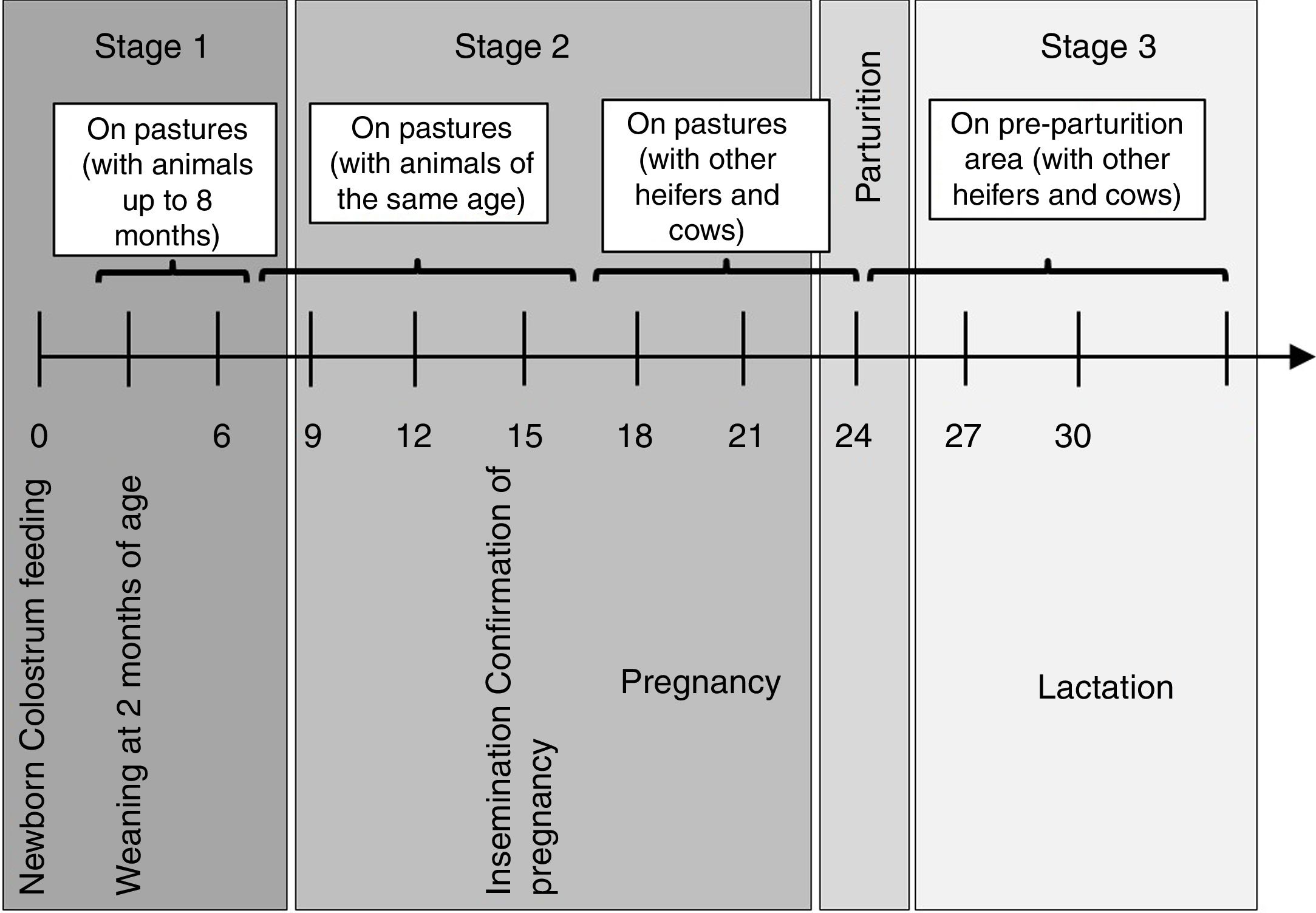
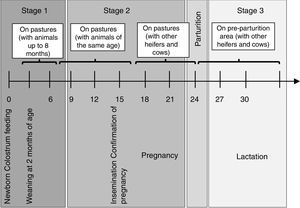
Materials and methodsThe herd was a large Holstein dairy herd from a farm in Santa Fe Province, consisting of 800 milking cows. The herd was closed; thus, there were no animals from other herds and no entry of animals from outside. Calves stayed with their dams for 2–5 days and they were individually tethered by a chain to a stake holding two buckets to feed bulk tank milk and balanced feed for 60 days, when they were weaned. After weaning, they were raised on pastures with animals of the same age group up to 8 months. Then, they were moved to pastures with animals of the same age and weight (end of first raising period). Animals were vaccinated against foot-and-mouth disease virus semiannually starting at 3 months of age. They also received 2 doses every 60 days against: Bovine herpes virus subtypes I and V (BoHV I and V), Bovine viral diarrhea virus (BVDV), Bovine parainfluenza-3 virus (bPI(3)V), Pasteurella multocida, Mannheimia haemolytica and Histophilus somni and clostridial agents, between 3 to 8 months of age. Regarding brucellosis, they were vaccinated once between 3 and 8 months. Rotavirus-Escherichia coli vaccine was administered at 60 and 30 days before parturition. Animals did not receive vaccination against BRSV during all the sampling period. Beginning at 15 months of age, heifers were artificially inseminated and placed in contact with other heifers and cows. Pregnant heifers were kept on pastures and 30 days before delivery were moved to a pre-parturition area. Stages of production management in the dairy herd are shown in Figure 1.
Serum samples were kindly provided by Geronimo Gutierrez (Laboratory of Adventitious Virus, INTA). Samples were collected from female calves born during July and August 2006 (n=60). Sampling took place from birth until 36 months of age. The first sample was collected within the first week of age and at months 2, 5, 8, 11, 15, 18, 21, 24, 27, 30 and 36. All the experimental proceedings were carried out following international recommendations (Guide for the Care and Use of Agricultural Animals in Research and Teaching) and the institutional manual of INTA (Guide for the care and use of experimental animals).
The viral neutralization assay of serum samples was carried out as described by Samal et al.11. Briefly, inactivated serum samples were four-fold diluted from 1:8 to 1:512. Serum dilutions were mixed with 100 TCID50 of A51908 BRSV strain and incubated for 1h at 37°C in a 5% CO2 atmosphere. This mixture was inoculated in duplicate onto MDBK cell monolayers (200 000cells/ml) in 96-well plates. Plates were incubated as mentioned above and CPE was observed at 5 DPI. Samples were considered positive when no CPE was observed. NA titers were expressed as the reciprocal of the maximum dilution in which no CPE was observed. Samples with titers lower than 4 were considered negative. This method was selected for its high sensitivity in seroprevalence studies. Seroconversion was defined as an increase in a base 2 four-fold dilution antibody titer.
Antibody titers to BRSV determined by NA were log10-transformed prior to the statistical analysis. Negative samples at a dilution of 1:4 were assigned an arbitrary antibody titer of 2 for the calculation of geometric mean titers (GMTs). Percentage of seropositive animals since birth until 36 months of age was tested using the Fisher's exact test for multiple comparison of proportions, p values were corrected by the Holm Method.
Group effects on the NA titers to BRSV were analyzed by a general linear mixed model (GLMM). The model included one main fixed factor: time (with twelve levels, as within-subjects factor). Animals were included in the model as a random factor. Heteroskedasticity of variance among time points was modeled using the var Indent option. The variance and covariance matrix included the assumption of an autoregressive effect (AR1) among the titers of the same bovine determined at different time points. The Akaike Information Criterion (AIC) was used for choosing the best-fitting model as a minimal adequate one. Thus, the model with the lowest AIC value was selected. The GLMM analysis was conducted by using the glmer function (lme4 package, R Development Core Team, 2014) through Infostat® statistical software connection to R. Statistical significance was assessed at p<0.05 for all comparisons.
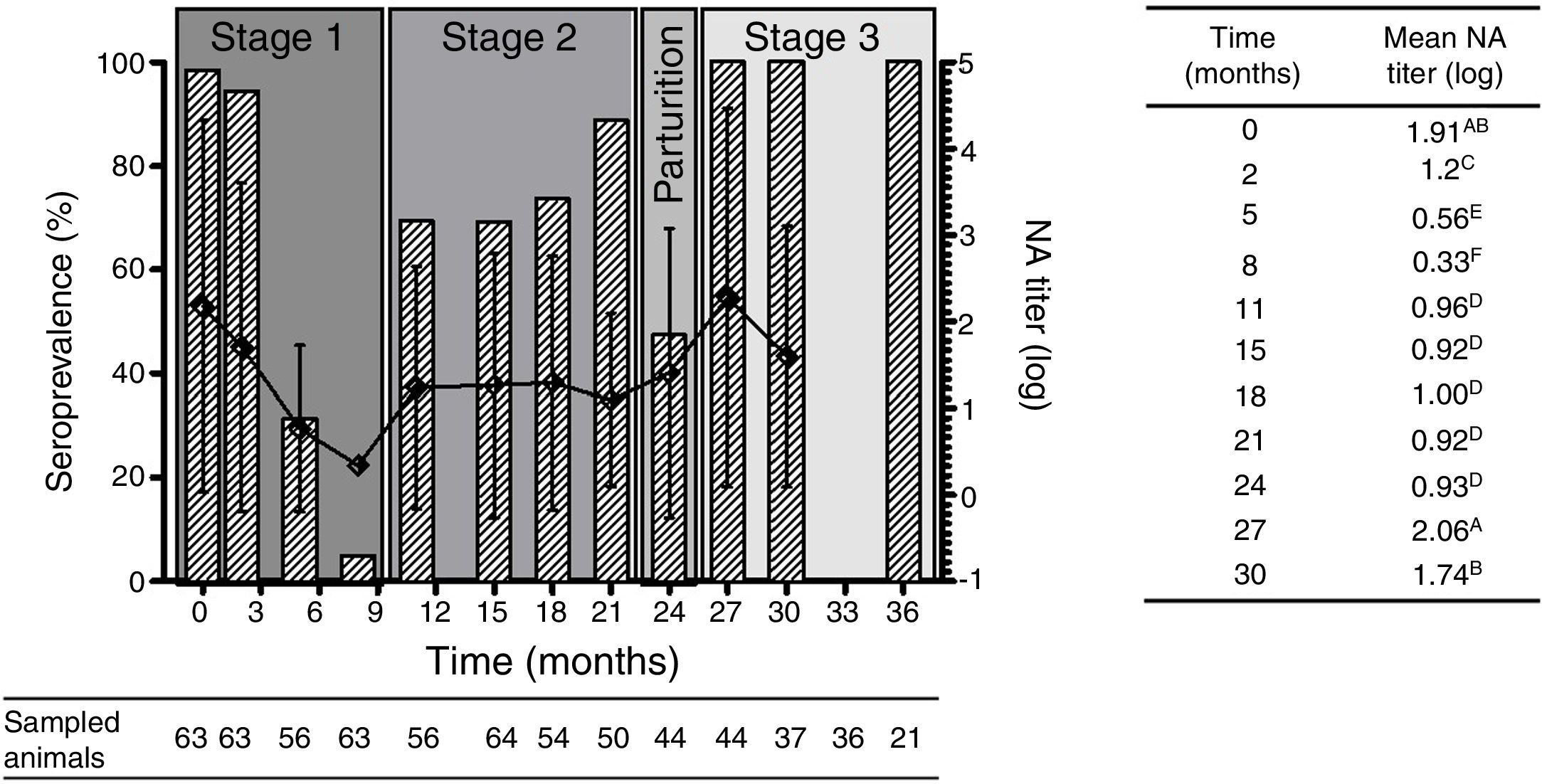
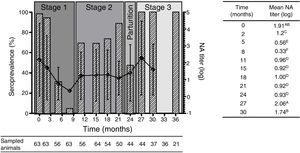
Results and discussionResults obtained from this study showed that at birth (stage 1), 98% of animals were seropositive for BRSV with high NA titers. These results are in concordance with those reported by Tuncer et al.13, who detected the presence of maternal antibodies in 100% of the calves sampled at one month of age in dairy herds from Turkey. Since the vaccines used in this dairy herd did not contain BRSV in their formulation, maternal antibodies must have derived from natural BRSV infections. In this study, passive maternal NA specific for BRSV started to decrease monthly according to IgG half-life. At two months of age a significant reduction in the mean NA titers was observed (Fig. 2). These NA levels remained low (almost undetectable) between months 5 and 8. In fact, 95% (60/63) of the animals were seronegative at 8 months of age (Fig. 2). These results are in agreement with those described by Uttenthal et al., who postulate that the level of BRSV NA significantly decreases during the first 6 months of age14.
(A) Seroprevalence mean of NA titers (log10) against BRSV since birth until 36 month old. The bars represent the percentage of seropositive animals at each time evaluated and the curve shows the average of NA titers of the seropositive animals at each time. Mean NA titer from month 36 were not included on statistical analysis due to the low number of samples obtained at that time point. (B) Mean of NA titers (log10) during time. Mean NA titer with same letter are not significantly different (p>0.05).
At 11 months of age (stage 2), when the first movement of the animals took place, (transference to pastures with other animals of the same age), primary infection was evidenced by a significant increase in NA titers in 60% (34/56) of the animals. This fact is in agreement with the first movement of animals to pastures where they took contact with other animals of the same age. These management practices, as well as the absence of specific NA against BRSV from month 5 to month 11, increases the susceptibility to infection by BRSV. Our results agree with what has been previously described by other authors, who stated that management practices within herds are predisposing risk factors for BRSV infection and that the first infection in dairy farms occurs mostly during the first two months mainly in autumn or winter2. NA titers remained stable until 18 months of age. Interestingly, at month 21, NA titers dropped slightly, while prevalence increased to 89% (44/50).
At parturition (24 months) a significant decline in the percentage of seropositive animals was observed (47%, 21/44). This could be associated, again, with management practices, since at that moment animals were isolated from the rest of the animals, explaining why there was no direct contact between them favoring infection with BRSV. Close contact between animals and crowding conditions are main risk factors that enhance the transmission of BRSV5. The concentration of serum antibodies in colostrum is another physiological process that must be considered, as it was documented that cows concentrate in colostrum 10 times the amount of IgG1 present in sera which can lead to a significant drop in serum IgG1.
An increase in seropositive animals (reaching 100% values) was observed at month 27 (after delivery, stage 3), when the animals re-entered the productive cycle (lactation cycle), coming back into close contact with other animals and under stress conditions, including recent calving and separation from their calves. In addition, the mean NA titers were significantly higher than those obtained during stage 2 (at that time the animals were in pastures with other heifers and cows, prior to parturition). This is in agreement with the study report from León et al.10 stating that reinfection is common in herds causing a significant increase in antibody levels in seropositive animals. After stage 3, NA titers began to decline.
Seroconversion percentages were analyzed at each stage. In this regard, two critical points of seroconversion were detected at months 11 and 27, with seroconversion rates of 30% and 45.7%, respectively.
Given that the dairy herd analyzed in this study was a closed one it seems unlikely that the virus could have been introduced from other sources, suggesting an intra-herd viral circulation of BRSV in this production system.
It is not clear if BRSV persists in the herd or if it is reintroduced from other sources before each outbreak10. Hypotheses about the way a virus remains in a herd or if it is reintroduced are controversial. It is possible that factors such as latency and reactivation, low level of circulation of the inter or intra-herd virus or hosts that act as a reservoir play a role in the maintenance of the BRSV9. Studies of the viral genome analysis from isolates indicated that during an outbreak the virus within the herds remains identical, but varies between outbreaks, suggesting that these are probably caused by “new” viruses rather than by latency or the existence of animals that act as carriers3. In our study, a possible hypothesis is that the virus was spread between animals over the years, which is accordance with previous results10. Nevertheless, further studies should be performed on isolates in order to assess the genetic diversity of the circulating virus.
There are several inactivated vaccines available in Argentina provided as part of multivalent products to prevent respiratory disease. At present, vaccination programs recommend two initial doses (2 weeks apart) to induce immunity. Protection induced by these vaccines and also after infection with the virus, tend to be short-lived (<4 months), especially in calves with colostral antibodies4. In this study at month 8 after birth, all calves were seronegative. This is a critical period since the animals are more susceptible to BRSV infection because of the low level of NA, and if an infection occurs, this could cause severe disease and even death.
A conventional vaccination schedule recommends two doses; the first one at weaning and a booster 21 days post-weaning. Our results demonstrate the need to review the vaccination schedule of animals in dairy farms in order to protect them against infection through vaccination against BRSV. In this regard, calves should be vaccinated with more effective vaccines in the face of maternally derived antibodies and also after 5 months of age where neutralizing antibodies significantly decrease and become almost undetectable at month 8 after birth. Strategic measures must be implemented to overcome economic losses due to respiratory disease caused by BRSV in dairy herds including the improvement of the vaccines used.
Conflict of interestThe authors declare that they have no conflicts of interest.
The authors wish to thank Osvaldo Zabal for his technical assistance and Dr Amanda Wooloms for reviewing and correcting this manuscript.
This work was supported by the Instituto Nacional de Tecnologia Agropecuaria PNSA-1115054, the MINCYT SECyT-BIDPICT-2011-0541 and CONICET.