This work aimed to investigate the prevalence of Trichinella infection in horses (Equus ferus caballus) handled by rural slaughterhouses across five distinctive socioeconomic regions in Mexico. Serum samples were obtained by non-probabilistic convenience sampling in the Eastern, Southern Central and Western regions (100 samples of each). Additionally, muscle tissue samples were collected from the East (n=45), Southeastern (n=88), Southern Central (n=39) and Southwestern (n=11) regions. Antibodies were determined by Western blot and the muscle tissue was examined by artificial digestion. A global antibody prevalence of 2% was obtained. Regionally, a prevalence of 5% was observed in the East and 1% in the Southern Central region. No antibodies were detected in the West region and no larvae were found in the muscle tissue samples. These findings support the low presence of Trichinella in Mexican horses, which can positively impact the Mexican horse meat trade.
Se investigó la prevalencia de Trichinella en caballos (Equus ferus caballus) de mataderos rurales en cinco regiones socioeconómicas de México. Los sueros se obtuvieron por muestreo de conveniencia no probabilístico en las regiones Este, Centrosur y Oeste (100 muestras de cada una). Además se colectaron muestras de tejido muscular de animales de las regiones Este (n=45), Centro Sur (n=39), Sudeste (n=88) y Sudoeste (n=11). Los anticuerpos se determinaron por Western blot y el tejido muscular se examinó por digestión artificial. Se obtuvo una prevalencia global de anticuerpos del 2%. A nivel regional se observó una prevalencia del 5% en el Este y del 1% en el Centrosur. No se detectaron anticuerpos en el Oeste y no se encontraron larvas en el tejido muscular. Los datos respaldan la baja presencia de Trichinella en caballos mexicanos, lo que puede impactar positivamente en su comercio.
Trichinellosis is a worldwide foodborne zoonosis transmitted through the consumption of raw or inadequately cooked meat containing viable muscle larvae of the nematode Trichinella. The parasite is one of the most important foodborne parasites in international trading. Although the Trichinella infection of horses is sporadic8, Boireau et al.2 reported that one of 13 Trichinella outbreaks recorded in Europe between 1975 and 1998 was associated with Mexican horse meat. Nowadays, Mexico has regulatory laws for inspection and export of pathogen-free meat; however, information about horse meat consumption in the country by human beings is scarce, even though, there are reports on the use of horse meat for making different sausages.
In Mexico, since 1990, the National System of Epidemiological Surveillance has been in charge of recording human trichinellosis clinical cases, while reports of swine infected with Trichinella have been documented in epidemiological studies since 190910,11. However, data about the prevalence of horses infected with Trichinella are scarce; indeed, the latest data on horses was published 10 years ago. Reports suggested an antibody prevalence between 7 and 17%13,15 and, a parasitological prevalence by artificial digestion between 1 and 5%1,7, while the prevalence by PCR was between 3 and 15%7,13.
Although artificial digestion is the standard assay for the detection of Trichinella5, antibody determination has proven to be a useful tool in epidemiological studies, due to the reliability of the excretory and secretory products of the muscle larvae and the versatility of the immunoenzymatic tests (ELISA, IIF and Western blot)14. By Western blot, serum samples of Trichinella patients and infected animals recognize a triplet of 45, 49 and 53kDa, which is the accepted diagnostic criterion6,12. In agreement with the International Commission on Trichinellosis, serological tests are suitable for the surveillance of domestic and wildlife animals to contribute to the knowledge on Trichinella circulation3. Thus, this work aimed to investigate the prevalence of Trichinella infection in horses (Equus ferus caballus) handled by rural slaughterhouses across five distinctive socioeconomic regions in Mexico.
Serum (n=300) and muscle tissue (n=183) samples of Equus ferus caballus were obtained from different rural slaughterhouses located in five of the eight socioeconomic regions of Mexico. Serum samples were obtained by non-probabilistic convenience sampling to obtain 100 samples per slaughterhouse. Consequently, this work was carried out in order to obtain basic and trend data that would allow us to assess the feasibility of a large-scale study that meets a powerful statistical rigor. The socioeconomic regions include 32 federative entities of Mexico, following indicators related to well-being such as education, occupation, health, housing, and employment. In the Eastern region, 100 serum samples were collected from two different federative entities, 55 from Hidalgo and 45 from Veracruz; these latter samples were paired with muscle tissue samples. In the Southeastern region, 88 samples of muscle tissue were collected (71 from Tabasco and 17 from Campeche). In the Southern Central region (State of Mexico), 100 serum and 39 muscle tissue samples were collected. In the Southwestern region (Guerrero), 11 samples of muscle tissue were collected and, in the Western region (Jalisco), 100 serum samples were collected. The muscle tissue samples consisted of 100g of tongue, diaphragm, or masseter, which were transported to the laboratory in a cooler at 4°C. Blood samples were collected from the jugular vein. An amount of 3–5ml of blood per horse was obtained and transported at 4°C to the laboratory. Blood was centrifuged at 3500rpm for 5min, and serum was recovered and packed in tubes of 1.5ml and frozen at −20°C until use.
Trichinella spiralis (MSUS/ME/92/CM-92) was maintained in female Wistar rats, 6 weeks old and 300±50g. Since 1991, the parasite strain has been maintained by serial passages in mice and rats at the Institute for Diagnostic and Epidemiological Reference (InDRE) in Mexico. Ethical approval of the experimental infection was obtained from Coordination of Immunological Research, InDRE. Muscular larvae were isolated by artificial digestion of animal carcasses with a solution of 0.5% pepsin (Sigma–Aldrich, MO, USA) in 0.2% hydrochloric acid. After larvae recovery, the excretory and secretory products (ESP) were prepared as previously reported12; the parasites were incubated in RPMI 1640 medium (Gibco BRL, Grand Island, NY) at 37°C for 48h in a humid atmosphere of 95% air and 5% CO2. The recovered medium was supplemented with enzymatic inhibitors and used as antigen.
The muscle tissue samples were analyzed by artificial digestion in accordance with previously described procedures and recommendations9; the digestion fluid was prepared with 0.5% pepsin (Sigma–Aldrich, MO, USA) in 0.2% hydrochloric acid and incubated for 30min at 44°C. Muscle larvae were identified at 10 magnifications in light field microscopy. Three samples of muscle tissue from each animal were observed.
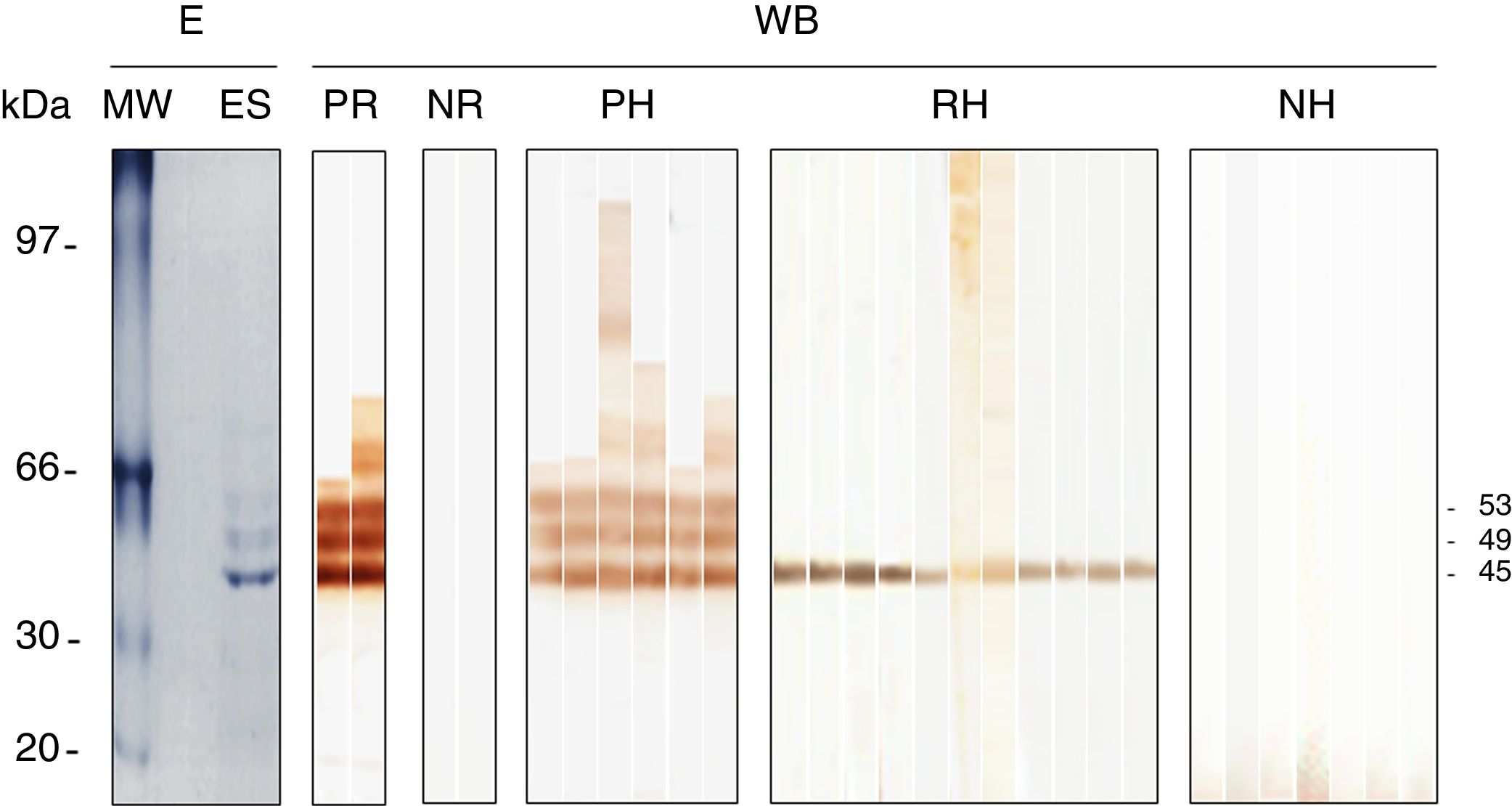
Antibody determination was conducted by a homemade Western blot previously standardized by our research group for the diagnosis of human trichinellosis12 and later used with appropriate modifications during experimental infection of rodents with Trichinella.4 Briefly, sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) was carried out using the mini-protean II electrophoresis system (Bio-Rad, CA, USA). The ESP (excretory–secretory products) were separated in 11% SDS–PAGE under reducing conditions (4μg/mm) and blotted onto a nitrocellulose membrane using a semi-dry vertical electro-transfer system (Bio-Rad). Strips of 0.3cm wide were incubated with the serum sample diluted 1:800, then incubated with anti-horse IgG peroxidase conjugate (Sigma–Aldrich, Saint Louis, Missouri, USA) diluted 1:2000. The positive control was a serum sample of an experimentally infected rat while the negative one, was a normal rat. These particular strips were incubated with anti-rat IgG peroxidase conjugate (Sigma–Aldrich). Afterwards, the substrate-chromogen solution of diaminobenzidine was added. Reactions were stopped with tap water. Serum samples that were reactive to the specific diagnostic antigens of 45, 49 and 53kDa were classified as positive.
A total of 183 muscle tissue samples (one per horse) were analyzed. Horses ranged from 2 to 8 years old, 98 were females and 85 males. All samples were negative by artificial digestion; however 6/300 (2%) serum samples were positive by Western blot. Moreover, 11/300 (3.7%) samples were reactive to the 45-kDa band; these samples were not considered positive. Table 1 shows the prevalence found in each socioeconomic region. In the Western region, no sample was positive; however, four samples were reactive to the 45kDa band. In the Southern Central region, one sample was positive (1%) and, four recognized the 45kDa band. In the Eastern region, 5/45 (11%) samples from Veracruz were positive; additionally, three serum samples were reactive to the 45kDa band. Figure 1 shows the different patterns obtained by Western blot (3 bands, one band and negative pattern).
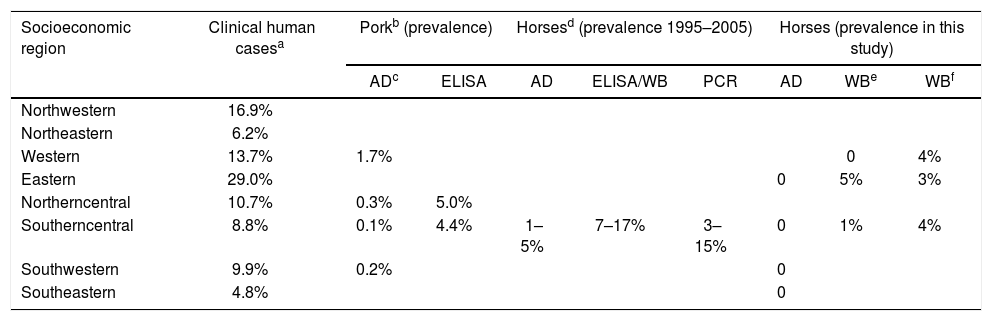
Epidemiological data of Trichinella in Mexico.
| Socioeconomic region | Clinical human casesa | Porkb (prevalence) | Horsesd (prevalence 1995–2005) | Horses (prevalence in this study) | |||||
|---|---|---|---|---|---|---|---|---|---|
| ADc | ELISA | AD | ELISA/WB | PCR | AD | WBe | WBf | ||
| Northwestern | 16.9% | ||||||||
| Northeastern | 6.2% | ||||||||
| Western | 13.7% | 1.7% | 0 | 4% | |||||
| Eastern | 29.0% | 0 | 5% | 3% | |||||
| Northerncentral | 10.7% | 0.3% | 5.0% | ||||||
| Southerncentral | 8.8% | 0.1% | 4.4% | 1–5% | 7–17% | 3–15% | 0 | 1% | 4% |
| Southwestern | 9.9% | 0.2% | 0 | ||||||
| Southeastern | 4.8% | 0 | |||||||
Western blot reactivity of serum samples obtained from slaughtered horses against the excretory and secretory products of Trichinella spiralis. The horses reacting by Western blot (WB) with the 45, 49 and 53kDa bands were considered positive (PH), while horses with reactivity to the 45kDa (RH) band or without (NH) reactivity were negative. The reactivity of the serum sample from positive (PR) and negative (NR) rats is also shown in addition to the electrophoretic (E) pattern of the excretory and secretory products (ES) of the muscle larvae. The molecular weight (MW) pattern is shown on the left of the panel.
Here, we studied the prevalence of Trichinella infection in horses handled by rural slaughterhouses across five distinctive socioeconomic regions in Mexico. We found that 6/300 (2%) serum samples recognized the triplet of 45, 49 and 53kDa in the ESP, fulfilling the diagnostic criteria to define a sample as positive by Western blot. These data suggest that horses had contact with the parasite but antibodies failed to show active parasitism because all the analyzed muscle tissue samples were negative to artificial digestion. Similar findings were reported by Viveros et al.13, where no Trichinella larvae were recovered after the artificial digestion of 170 diaphragm samples from horses; however, 20/170 (11.8%) serum samples were ELISA positive. The recurrent explanation to this controversy is that horses are not ideal hosts for Trichinella and, perhaps, the parasite could be eliminated rapidly; therefore, the finding of the parasite in horses is exceptional; however, contact between the parasite and host is evidenced by the presence of specific antibodies. Much has been discussed about the utility of antibody determination as method of diagnosis in horses, considering that antibodies disappear fast in the horses3; however, data of this work confirm that Trichinella is transmitted at low rate among horses.
In addition to the positive serum samples, here we found 11 (3.6%) reactive samples to the 45kDa band. Although the antigenic pattern of 45, 49 and 53kDa is the specific criterion for the diagnosis of Trichinella6,12, a cross-reaction cannot be denied; however, negligible cross-reactions have been demonstrated between the ESP and the serum samples of patients or animals with other infectious diseases. In contrast, evident false positive reactions have been documented when crude antigens of the muscular larvae are used in diagnostic techniques14. Actually, ESP are the only antigens recommended in the diagnostic tests for Trichinella infection3,14 and, hence the importance of serum samples from horses that recognized the 45kDa band in the ESP. If the reactivity to the 45kDa band is truly associated with Trichinella, the finding of these reactive samples is important, because in Mexico, T. spiralis is the only documented species. Furthermore, it is not known if there is another species circulating in Mexico. This fact could mask the true prevalence of Trichinella and depending on the point of view, this reactivity, could overestimate or underestimate the actual prevalence of the parasite.
Alternatively, the molecular techniques could be useful in epidemiological studies, considering that PCR has been much more sensitive and specific than routine laboratory techniques for different parasitic diagnoses. However, as far as it is known, for the diagnosis of Trichinella, the molecular methods have been useful in the genotyping of parasites recovered from animals or food. Nevertheless, the molecular techniques have the disadvantage that the sensitivity of the method depends on the amount of tissue that can be analyzed, without taking into account the infrastructure that the methodology needs. These disadvantages limit the use of the molecular techniques as a routine diagnostic method9. As an example, we can mention the work of Jiménez-Cardoso et al.7, who found no correlation between the muscle tissue samples with larvae and PCR positive samples; there were more positive samples by the molecular method than by artificial digestion. The possible interpretation of this finding, in the absence of larvae, is that the PCR assay was less specific than digestion. However, since artificial digestion is less sensitive than PCR, it is quite likely that PCR has the ability to detect parasitic loads that are not detectable by artificial digestion. This statement should be appropriately assessed in future studies.
A number of studies around the world have shown that the antibody prevalence in horses ranges between 3.5-7%2. In Mexico, the prevalence of horses infected with Trichinella varies depending on the methodology used. For example, antibody prevalence ranged between 7-17%; while, with the enzymatic digestion, the prevalence ranged from 1.25% to 5% and, with molecular methods from 3 to 15%1,7,13,15. The data here presented include samples from five socioeconomic regions of Mexico, which stand out as the main pig farmers. We find a global prevalence of 2%, which was associated to the Southern Central and Eastern regions. Interestingly, the main number of human trichinellosis reported between 1990 and 2018 in the national health system (Direccion General de Epidemiologia, https://www.gob.mx/salud/acciones-y-programas/direccion-general-de-epidemiologia-boletin-epidemiologico, accessed on January 25, 2019) corresponds to the Eastern Region with 340/1171 (29%) clinical cases, followed by 198/1171 (17%) clinical cases in the Northwestern Region. However, previous reports have shown that the principal prevalence of swine infected with Trichinella is located in the Western region10,11. In this study, no antibodies were found in the horse serum samples from the Western region. Although this study updates the prevalence and distribution of Trichinella in Mexico, data suggest that the epidemiological knowledge of the parasite is still insufficient.
In conclusion, we report a 2% prevalence of circulating antibodies to Trichinella in horses. Antibodies were detected in horses from the Eastern and Southern Central regions of Mexico. Further studies are needed to determine the prevalence of Trichinella in other socioeconomic regions of Mexico. These data support the low circulation of Trichinella in Mexican horses, which can positively impact on the Mexican international horse meat trade.
Conflict of interestThe authors declare that they have no conflict of interest.
The authors thank Rosa-María Reyes-Diaz for her outstanding technical assistance. Irma Valle and Martín Piñon for taking care of the animals at InDRE facilities. MSc Katia-Marleth Herrera-Aguirre and MSc Juan-Carlos Tapia-Cruz (native English speakers) for the English review of the manuscript. Jorge-Luis de-la-Rosa-Arana is a National System of Researchers (Consejo Nacional de Ciencia y Tecnología, México) fellow.