We report the case of a twenty-year-old immunocompetent male patient presenting to the emergency room with pharyngitis and fever. Blood cultures were drawn and Arcanobacterium haemolyticum (rough biotype) was recovered. The presence of the arcanolysin gene was investigated at the molecular level and the upstream region was amplified and sequenced in order to correlate it with the smooth or rough biotype. Although the isolate was susceptible to penicillin, vancomycin and gentamicin, empirical treatments first with amoxicillin/clavulanic acid (1g/12h) and then with ceftriaxone (1g/12h) failed and the infection evolved to sepsis. Finally, treatment with vancomycin (1g/12h) plus piperacillin/tazobactam (4.5g/8h) was effective. Lemierre's syndrome was ruled out. To the best of our knowledge, this is the first case of bacteremia by A. haemolyticum reported in Argentina.
Se describe el caso de un paciente varón inmunocompetente de veinte años de edad que se presentó en la sala de emergencias con faringitis y fiebre. Se extrajeron muestras para realizar hemocultivos y se recuperó Arcanobacterium haemolyticum (biotipo rugoso). Se investigó la presencia del gen de la arcanolisina por un método molecular, y se amplificó y secuenció la región upstream de dicho gen para determinar su correlación con los biotipos lisos o rugosos. Aunque el aislamiento fue sensible a la penicilina, la vancomicina y la gentamicina, los tratamientos empíricos primero con amoxicilina/ácido clavulánico (1g/12h) y luego con ceftriaxona (1g/12h) no fueron efectivos, y la infección evolucionó a sepsis. Finalmente, el tratamiento con vancomicina (1g/12h) más piperacilina/tazobactam (4,5g/8h) fue efectivo. Se descartó la presencia del síndrome de Lemierre. Según nuestro conocimiento, este es el primer caso de bacteriemia por A. haemolyticum reportado en Argentina.
Arcanobacterium haemolyticum (formerly Corynebacterium haemolyticum) is an aerobic or facultative anaerobic, non-motile and non-spore-forming, slightly curved gram-positive rod with pointed ends. It does not hydrolyze esculin, urea or gelatin12. It is the causative agent of skin infections and sore throat such as exudative pharyngitis and tonsillitis. Although A. haemolyticum is associated with pharyngitis in young adults, the incidence is low (0.5–2.5%). Moreover, it is not easily detected in the microbiological laboratory because of its slow growth and many characteristics resembling β-hemolytic streptococci such as being catalase negative, β-hemolysis, susceptibility to bacitracin (0.04U) and cross-agglutination with antisera for Lancefield's groups A, B, C, D, F or G1. Moreover, this species produces α-mannosidase and yields a positive result in the CAMP test using Streptococcus agalactiae ATCC 13813 and a reverse CAMP test using Staphylococcus aureus ATCC 259235.
No risk factors for infection have been identified yet, although two distinct subsets are recognized: healthy young adults presenting with upper respiratory tract infections and older, often immunocompromised, patients presenting with skin and soft tissue infections14.
Here, we report a case of bacteremia caused by A. haemolyticum in a patient who presented to the emergency room with pharyngitis and fever. Moreover, the presence of Lemierre's syndrome was suspected and investigated.
To the best of our knowledge, this is the first case of bacteremia by A. haemolyticum reported in Argentina.
Case report: a twenty-year old immunocompetent male patient, undergoing a strong emotional shock, consulted at the emergency room for pharyngitis, cutaneous rush and fever for over a week. He had been under antibiotic treatment with amoxicillin/clavulanic acid, 1g by mouth twice daily for three days. Blood tests had been performed two days before and revealed leukocytosis: 12 300 white blood cells/mm3 (84.8% neutrophils) (Fig. S1, panels a and b), thrombocytopenia: 63 000 platelets/mm3 (Fig. S1, panel c), increased erythrocyte sedimentation rate: 44mm and mild hepatic inflammation (GOT: 59.0U/l, GPT: 75.0U/l, total bilirubin: 2.03mg/dl, direct bilirubin: 0.29mg/dl, ALP: 378U/l). Laboratory tests were checked with a new sample and blood cultures were carried out. Chest X-ray showed no abnormalities. Antibiotic treatment was changed to ceftriaxone, 1g given intravenously every 12h. Dipyrone was applied in the buttock. Thrombocytopenia persisted until day five (Fig. S1, panel c) and anemia developed from day five until day thirteen (Fig. S1, panel d). Cardiac Doppler and abdominal ultrasounds showed absence of abnormalities. Infectious mononucleosis, streptococcal pharyngitis, hematological alterations, syphilis, HIV, cytomegalovirus and leptospirosis were ruled out. Laboratory reported the presence of diphtheroid gram-positive bacilli in blood cultures (both aerobic and anaerobic bottles), which did not grow in chocolate agar under candle after 48h and were considered presumptively anaerobic. The bottles were sent to a laboratory specialized in anaerobic bacteriology. Additionally, chest and abdominal computed tomography scans were performed (Fig. S3, panels a and b). Defined and irregular nodular opacities of bilateral distribution that did not exceed 2.5cm in diameter were detected, some of which were cavitated, predominantly subpleurally located (Fig. S3, panel a). In addition, a condensation was observed with an air bronchogram at the left lung base associated with a small amount of pleural effusion and bibasal fibroretractable changes. No focal lesions of the parenchyma of both lungs of primary or secondary appearance were detected. There was no evidence of pulmonary hilar or mediastinal lymph nodes. These findings matched those detected in a new chest X-ray that showed left-sided infiltrate and two images of small cavitated subpleural abscesses in the right lung. Furthermore, an abscessed collection of 10×6cm was observed in the left gluteus medius muscle that displaced and compressed surrounding structures (Fig. S3, panel b). In addition, a soft tissue ultrasound (hip and left gluteus) was performed. A collection that agreed with the tomographic findings was found. Despite six days under treatment with ceftriaxone, fever persisted. Then, treatment with the combination vancomycin (1g every 12h) plus piperacillin/tazobactam (4.5g every 8h), both administered intravenously, was started. The patient was admitted to the intensive care unit because of hypotension and sepsis. He evolved without fever. Drainage of the gluteal abscess was performed by puncture under local anesthesia and a collection of purulent fluid was obtained and sent to the laboratory for culture. Gram stained smears of the material showed plentiful polymorphonuclear leukocytes but no bacteria, and cultures were negative. Subsequently, it was necessary to perform surgical drainage of the abscess. On day 19 of hospitalization the antibiotic scheme was completed (13 days) and the patient was discharged. The patient returned a few days later to undergo a color Doppler ultrasound of the neck arteries on both sides with the intention of ruling out Lemierre's syndrome. The study showed normal myointimal thickening without evidence of thrombosis in both internal jugular veins. On that occasion, a d-dimer test was also performed: <100ng/ml.
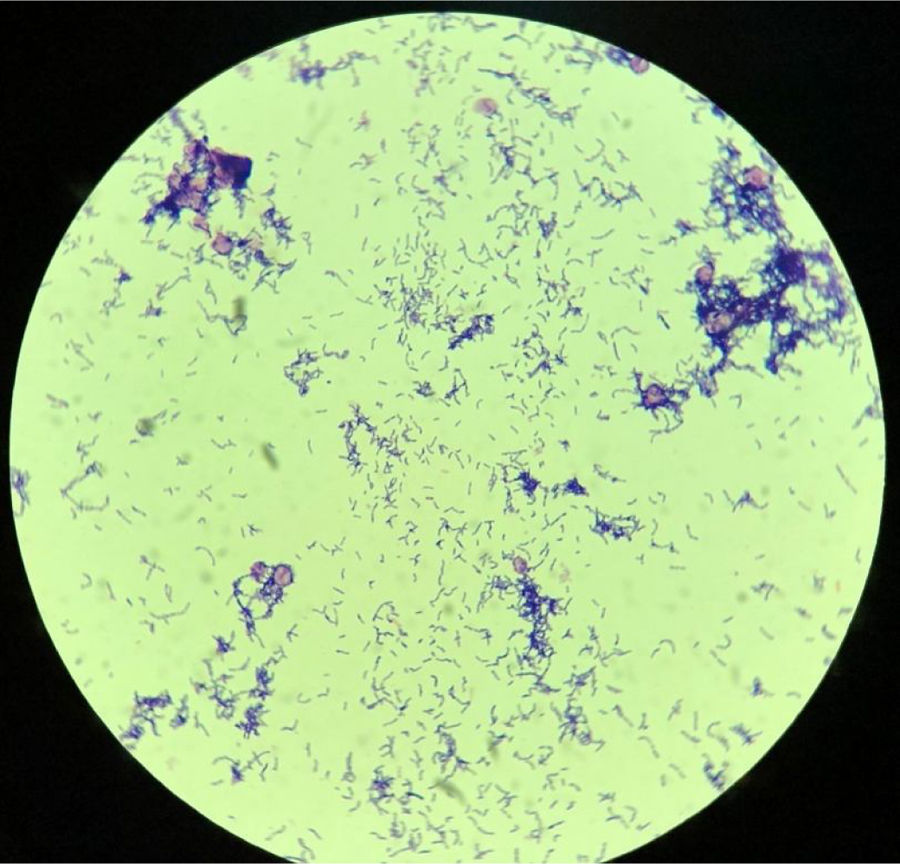
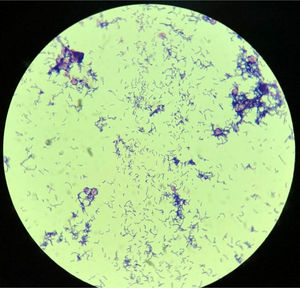
Once at the laboratory specialized in anaerobic bacteriology, the two positive blood culture bottles (Adult blood culture bottles, Laboratorios Britania S.A., Argentina) were subcultured into 5% leaked blood agar supplemented with vitamin K, hemin, tween 80, sodium bicarbonate, pyruvate and yeast extract and incubated at 37°C for 2 days under anaerobic conditions. Identification of the recovered microorganism was initially performed based on its microscopic appearance (diphtheroid gram-positive bacillus) (Fig. 1), its ability to grow under aerobic and anaerobic atmospheric conditions, a negative catalase test, partial β-hemolysis on sheep blood agar, total hemolysis on human blood agar and a positive reverse CAMP test (narrowing of the hemolytic zone produced by staphylococcal β-lysine) (Fig. S2). Additional tests performed: β-galactosidase (+), nitrate reduction (−), urease (−), heat-stable nuclease (DNasa) (+). The identification of the isolate was confirmed using Matrix Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS) technology (Vitek MS, bioMérieux, Marcy l’Etoile, France) as A. haemolyticum (99.9%). On day 14 of hospitalization, A. haemolyticum (strain 5612) was detected in blood cultures (both aerobic and anaerobic bottles).
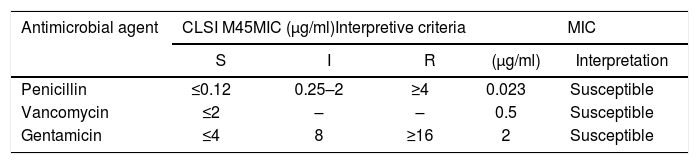
The isolate was tested for its susceptibility to penicillin, gentamicin and vancomycin by the Epsilometer test (E-test; AB Biodisc, Solna, Sweden) following the original manufacturer's recommended guidelines (AB Biodisk. 1996. E-test technical guide 1B: susceptibility testing of anaerobes). The breakpoints recommended by the Clinical and Laboratory Standards Institute (CLSI) were used (Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria, 3rd ed. Approved standard M45. CLSI, Wayne, PA; 2015). The strain was susceptible to penicillin, vancomycin and gentamicin (Table 1).
Minimal inhibitory concentration (MIC) of A. haemolyticum 5612.
| Antimicrobial agent | CLSI M45MIC (μg/ml)Interpretive criteria | MIC | |||
|---|---|---|---|---|---|
| S | I | R | (μg/ml) | Interpretation | |
| Penicillin | ≤0.12 | 0.25–2 | ≥4 | 0.023 | Susceptible |
| Vancomycin | ≤2 | – | – | 0.5 | Susceptible |
| Gentamicin | ≤4 | 8 | ≥16 | 2 | Susceptible |
S: susceptible, I: intermediate, R: resistant.
Afterwards, the isolate was sent to a laboratory specialized in molecular biology to investigate A. haemolyticum arcanolysin (aln/ALN), a member of the cholesterol-dependent cytolysin (CDC) family. The aln gene was amplified by polymerase chain reaction (PCR) employing oligonucleotide primers designed in-house called aln F (forward) and aln R (reverse) with the following sequences: 5′-GTCAAGTTATGCCGGGAATG-3′ and 5′-CGATGTTCTTGAACCAAGG-3′, respectively, yielding a 1984bp amplicon. According to Ruther et al.13, the upstream region of aln was also amplified using primers DM1078 (forward) and DM1080 (reverse) yielding an 830bp amplicon. The final PCR product was sequenced in order to compare with rough or smooth previously reported A. haemolyticum strains. In silico restriction pattern was obtained using NEB cutter V 2.0 free software.
Above, we reported a case of bacteremia by A. haemolyticum with pharyngitis as its starting point. Bacteremias caused by this microorganism are rare and only a few cases have been reported6. Pharyngitis by A. haemolyticum affects mostly children and young adults between 10 and 30 years of age12. As it is the case, it is considered an opportunistic pathogen. Occasionally, it can lead to episodes of sepsis. Moreover, it liberates extracellular enzymes such as phospholipase D, neuraminidase, hemolysin and DNases which are responsible for its pathogenesis10.
A. haemolyticum has two biotypes: smooth and rough. Smooth biotypes show smooth colony edges, moderate to strong β-hemolysis and predominately cause wound infections. Rough biotypes possess rough and irregular colony edges, have weak to no β-hemolysis and are isolated mainly from the respiratory tract. Moreover, there are biochemical differences between strains: rough biotypes are sucrose and trehalose-negative while smooth ones are β-glucuronidase-negative. Although the reason of these two variants is unknown, there seems to be a strong correlation with a polymorphic region upstream of the arcanolysin gene (aln)13. This enzyme is a recently discovered hemolysin from A. haemolyticum and, as member of the CDC family, is produced and secreted as a monomer that, upon contact with a eukaryotic membrane, oligomerize to form a large β-barrel pore resulting in the lysis of the cell.
A. haemolyticum 5612 displayed a rough colony morphology. PCR amplification of the aln open-reading frame and aln upstream region from strain 5612 rendered the 1984bp and 830bp expected amplicons, respectively. Sequence analysis of the 830bp upstream region of aln revealed that the strain 5612 recovered in this work was 100% identical to the rough isolate B0961-98 (GenBank accession number: KP668885) isolated from a Finnish patient with pharyngitis. According to Ruther et al.13, the polymorphisms in the intergenic region of aln allow to obtain different restriction enzyme patterns. Thus, the intergenic region from rough isolates can be cleaved by XcmI but not ClaI (X restriction pattern)13. The in silico sequence analysis also allowed to identify the X pattern in A.haemolyticum 5612.
Even though the isolate was susceptible to penicillin (Table 1), treatments with amoxicillin/clavulanic acid or ceftriaxone were not effective. Fairly sure, the intramuscular dipyrone injection applied in the buttock at entrance in the course of thrombocytopenia caused hematoma and, most likely, the gluteal abscess would have resulted from intravascular seeding of the hematoma during bacteremia. The possibility of treatment failures when A. haemolyticum is treated with β-lactams9 should be taken into account. Nyman et al. concluded that A. haemolyticum is often penicillin-tolerant, suggesting that phenoxymethylpenicillin administration would be ineffective in eradicating it from the pharynx11. In this particular case, treatment failure with amoxicillin/clavulanic acid before entrance warned about the possibility of treatment failure with any β-lactam. Moreover, there is a report of a case of bacteremia secondary to a sacral eschar infection in which treatment with amoxicillin/clavulanic acid was not effective despite in vitro susceptibility, and the patient had fatal sepsis6. In another report, empiric treatment with ceftriaxone/metronidazole also failed7. In infective endocarditis, A. haemolyticum has also demonstrated both penicillin and ampicillin tolerance, resulting in no clinical improvement on empirical therapy with ampicillin/gentamicin2. Regardless, the combination vancomycin plus piperacilin/tazobactam was chosen and our patient improved.
With regard to the hematological parameter, the hematocrit evidenced a hemolytic process possibly caused by arcanolysin (Fig. S1, panel d). As expected, leukocytes were elevated and neutrophils showed a straightforward trend in time compatible with a monomicrobial infection (Fig. S1, panels a and b). Platelets started very low and reached very high values (Fig. S1, panel c). This could be the consequence of a break of its production caused by the septic process from the beginning followed by an acute phase reactant behavior.
Furthermore, Lemierre's syndrome refers to septic thrombophlebitis of the internal jugular vein and disseminated metastatic infection that most often develops as a complication of a bacterial sore throat infection in the young. Lemierre's syndrome is most often caused by Fusobacterium necrophorum (human necrobacillosis). Only a few cases of Lemierre's syndrome caused by A. haemolyticum have been reported in the medical literature4,7,15. Therefore, the occurrence of Lemierre's syndrome in this patient was worth investigating since he met three out of four criteria to suspect it: primary oropharingeal infection, septicemia, metastatic abscess but not septic or embolic phlebitis of jugular vein. For this purpose, it was necessary to evaluate the presence of any trail of thrombophlebitis of the internal jugular veins by Doppler ultrasound and d-dimmer test. As the results were negative, Lemierre's syndrome was ruled out. Notwithstanding, the diagnosis of Lemierre's syndrome has been achieved elsewhere without the presence of jugular vein thrombophlebitis8.
Finally, infections by A. haemolyticum must be considered in patients who underwent treatments with trimethoprim/sulfamethoxazole because of its natural resistance and selection3.
Infections by A. haemolyticum represent a challenge for microbiologists and physicians. Gram stains should always be performed to organisms obtained from throat samples to differentiate them from Streptococcus pyogenes. Physician must be aware of the possibility of treatment failures using β-lactams.
FundingsThis research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflict of interestThe authors declare that they have no conflicts of interest.
We thank Dr. Horacio Lopardo for his conscious contributions while revising this manuscript.