Leptospirosis is considered an endemic disease in Buenos Aires province, Argentina, with human cases reported annually from rural and urban areas. The aim of the study was to describe the variables that influence the delay in the serological confirmation of leptospirosis in human cases (period 2006–2014) from Buenos Aires province. Sixty-four percent (64%) of cases could be confirmed by microscopic agglutination test (MAT) with the first sample. The time of confirmation of the human leptospirosis cases was on average 21 days from the onset of the first clinical signs, and varied depending on the distance of the different sanitary regions. The geographical distribution of the confirmed leptospirosis human cases, in addition to the high number of suspected cases and probable cases (which could not be confirmed by MAT), demonstrate that leptospirosis is endemic and underreported in Buenos Aires province, and that distance and lack of resources could be determinant factors of this situation.
La leptospirosis se considera una enfermedad endémica en la provincia de Buenos Aires, con casos humanos registrados anualmente en áreas rurales y urbanas. El objetivo de este estudio fue describir las variables que influyen en el retraso de la confirmación serológica de la leptospirosis en humanos, a partir del análisis de los casos ocurridos en el período 2006-2014 en la provincia de Buenos Aires. El 64% de los casos fue confirmado por microaglutinación (MAT) con la primera muestra clínica. El tiempo de confirmación promedio fue de 21 días desde el inicio de los primeros signos clínicos; este valor se vio afectado por la distancia entre los centros de salud y los laboratorios de referencia en las distintas regiones sanitarias. La distribución geográfica de los casos confirmados de leptospirosis en humanos, además del alto número de casos sospechosos y probables (que no pudieron ser confirmados por MAT), demuestra que esta afección es endémica y se encuentra subregistrada en la provincia de Buenos Aires, y que la distancia y la falta de recursos serían factores determinantes de estas características.
Leptospirosis is a widely recognized zoonosis (caused by Leptospira spp.) with a worldwide distribution. The incidence of the disease in the human and animal population is highly variable, depending on the type of ecosystems (urban and rural), the variations in environmental conditions, and the presence of the host7. Although rodents and canines are considered the main sources of infection for humans, other hosts such as cattle and swine have been associated with human cases of leptospirosis. The infected animal excretes the microorganism in the urine, intermittently and for a variable period of time, (depending on the animal species and serovar); the survival of leptospires in the environment will depend on the environmental conditions (temperature and humidity) and the pH of the soil, requiring a neutral to slightly alkaline pH. Infections in humans occur through direct contact with the urine of infected animals or indirectly by contact with contaminated water. In Argentina, leptospirosis is an important public health concern due to the occurrence of human cases and outbreaks of disease9. Buenos Aires and Santa Fe provinces have recorded the highest number of leptospirosis cases in Argentina, due in part to the fact that these districts have diagnostic laboratories that allow to identify the cases. Outbreaks in both humans and animals are usually related to floods, periods of heavy rainfall and high humidity7,8.
To perform the microscopic agglutination test (MAT), the International Leptospirosis Society of the World Health Organization (WHO-ILS) suggests the use of 19 strains in the antigen panel. However, based on previous studies, in order to expedite the diagnosis, reduce costs and facilitate the management and maintenance of the panel used in the routine MAT, most of the reference laboratories in Argentina use 10–12 of the 19 suggested strains4. In a study carried out by Jacob et al.4 with samples from all over the country, it was observed that the serogroups presumably infecting humans were: Icterohaemorrhagiae (31%), Pomona (15%), Ballum (14%), Canicola (10%), Sejroe (4%) and Tarassovi (2%)4. However, in Buenos Aires province, according to previous serological studies, Canicola, Sejroe and Ballum serogroups were the most reactive serogroups in human cases7.
Leptospirosis is considered an endemic disease in Buenos Aires province, with human cases recorded annually from different cities in both rural and urban areas. The highest notification rate of human cases (single cases and multiple cases in outbreaks) is recorded mainly in the period of March-July of each year. Confirmed cases of leptospirosis predominantly correspond to male adults. In relation to the probable source of infection in the region, human cases are associated with contact with water sources, rodents and domestic animals, especially dogs5,7,8.
Buenos Aires province is currently divided into 12 Health Regions (HR), and is composed of 135 cities (Fig. 1). The Department of Rural Zoonoses (which is located Azul city) receives human clinical samples from seven HR (I, II, III, IV, VIII, IX and X); although occasionally some samples are referred from the other regions (V, VI, VII, XI and XII), with headquarters in San Martin, Lomas de Zamora, Moreno, Ensenada and La Matanza respectively. MAT is used in the diagnosis of the disease and for epidemiological studies (serological prevalence); this technique is considered the “gold standard” in the serological diagnosis of leptospirosis in human and animals1,2. Although it is a highly specific test, its sensitivity depends on the days of evolution of the disease, being lower in the acute stage (first week). The dilution of the serum is kept in contact with an equal volume of a leptospiral suspension. MAT titers may be affected by the quality of the antigen suspensions and the strains used, and the significance of a titer in a single sample depends on residual titers due to past infections3. The aim of this study was to describe the variables that influence the delay in the serological confirmation of leptospirosis in human cases (period 2006–2014) in Buenos Aires province.
Geographical distribution of the health regions in Buenos Aires province: HR I (Bahia Blanca), HR II (Pehuajó), HR III (Junín), HR IV (Pergamino), HR V (San Martin), HR VI (Lomas de Zamora), HR VII (Moreno), HR VIII (General Pueyrredón), HR IX (Azul), HR X (Chivilcoy), HR XI (Ensenada) and HR XII (La Matanza). The location of the provincial reference laboratory is indicated with a black star.
Samples and epidemiological information from suspected cases of leptospirosis from Buenos Aires province are referred to the Leptospirosis Laboratory of the Department of Rural Zoonoses. A retrospective analytical study was conducted using epidemiological and serological data from confirmed human cases of leptospirosis in Buenos Aires province during the period 2006–2014. All confirmed human cases of leptospirosis were included in the notification form, and all the variables analyzed were recorded; confirmed human cases that did not have epidemiological data (date of onset of the disease, time of clinical sample collection for the diagnosis of the disease, date of admission to the reference laboratory and date of confirmation of the case) were excluded.
MAT was performed using a panel of live strains of leptospires (Canicola, Hardjo, Hebdomadis, Pomona, Copenhageni, Pyrogenes and Wolffi serovars of Leptospira interrogans, Castellonis and Tarassovi serovars of Leptospira borgpetersenii, and Grippotyphosa serovars of Leptospira kirschneri) maintained on Ellinghausen–McCullough–Johnson–Harris medium (EMJH medium: Difco Laboratories, Detroit, Michigan, USA). For MAT, an initial dilution of sera of 1:25 was started, and the positive ones were diluted until a negative result was obtained. Any sample with an absence of 50% of free leptospires or agglutination thereof with respect to the control was considered to be positive. In the confirmation of human cases of the disease, the cross-reaction to different serogroups, the titers reached, the onset date of the clinical manifestations and the kinetics of antibodies from paired samples were taken into account. A confirmed case was defined as a titer of ≥1:200 for three or more serogroup, or when the serological conversion was observed in paired samples.
With the epidemiological and serological diagnostic data obtained from 90/150 confirmed human cases of leptospirosis (period 2006–2014) by the Department of Rural Zoonoses, the following was analyzed:
- •
Days between:
- ∘
The onset of illness (clinical manifestation) and date of serum sample collection for diagnosis (time of clinical suspicion).
- ∘
Collection of the clinical sample and arrival at the reference laboratory.
- ∘
The onset of the disease and serological confirmation of the case.
- ∘
- •
Detection of antibodies by MAT, number of reactive serogroups and titers reached.
Analysis of the main components and the Kruskal–Wallis test was performed with the cases that required a sample and two samples separately using the INFOSTAT statistical package.
In the 2006–2014 periods, the provincial reference laboratory received a diagnostic request for 2162 human suspected cases of leptospirosis from eleven health regions (HR IV, HR VIII and HR IX demanded 66.2% of the clinical samples for leptospirosis diagnosis). In 6.9% of the human cases (150/2162) it was possible to obtain serological confirmation by MAT; however, only in 90 of 150 confirmed human cases it was possible to obtain the information required for the analysis. With regard to the geographical distribution, the confirmed human cases were distributed in 22 cities of the province of Buenos Aires, belonging to the following health regions: HR IV (31/90), HR VIII (26/90), HR II (9/90), HR III (9/90), HR IX (8/90), HR I (5/90), HR X (1/90) and HR XI (1/90).
Sixty-four percent of human cases could be confirmed by MAT with the first clinical sample, while the remaining 36% required a second sample for detection of serological conversion. Seventeen percent of the human cases (15/90) also required the serological diagnosis of other diseases with clinical and compatible epidemiology such as Hantavirus and the Argentine hemorrhagic fever disease.
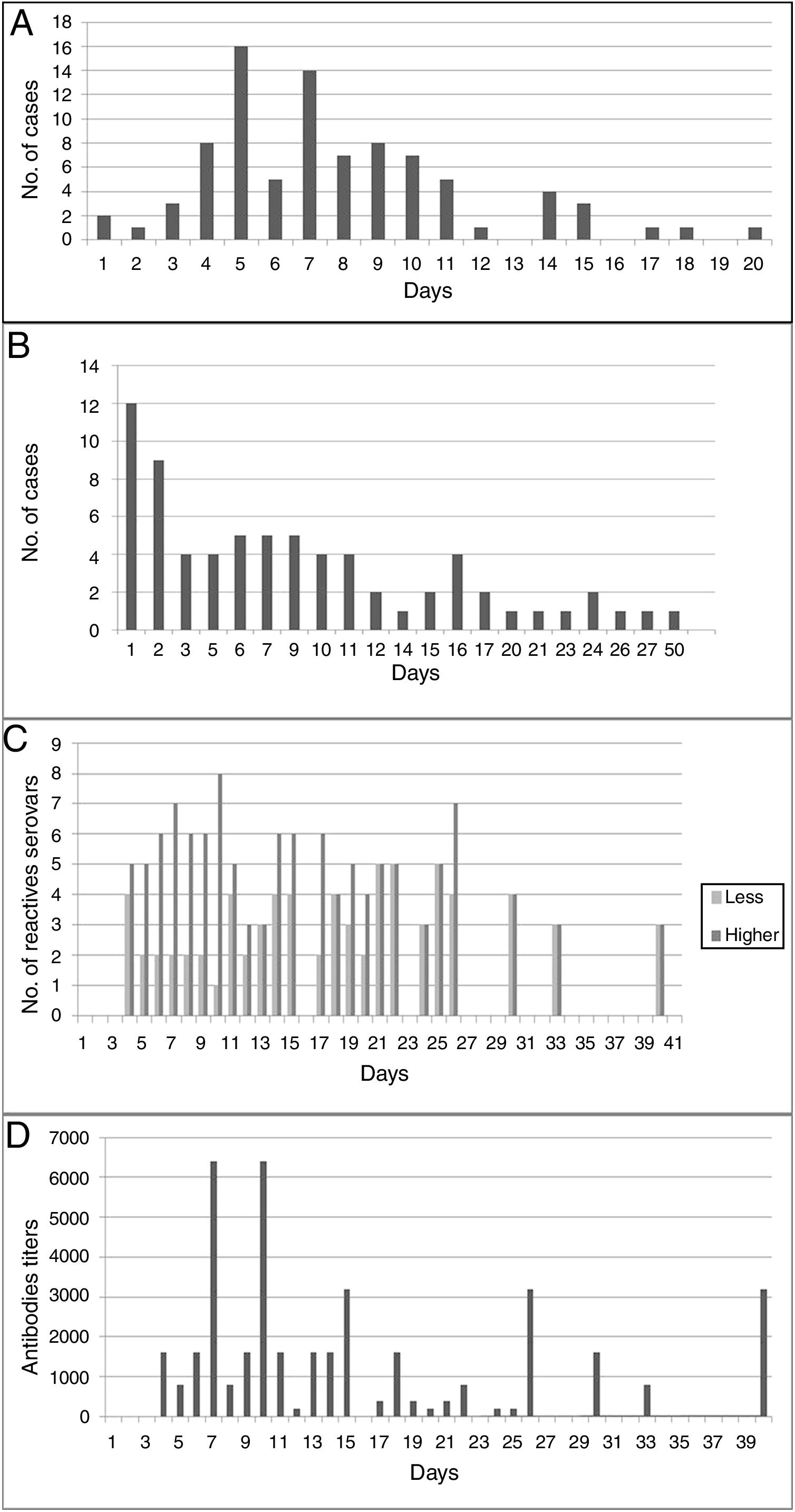
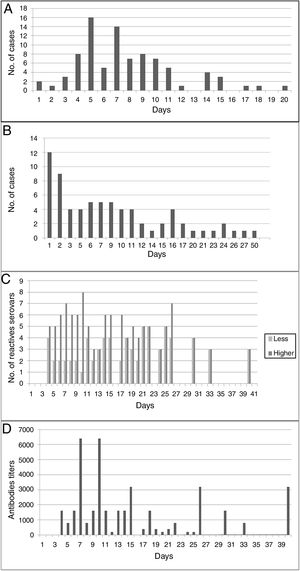
An average of 7.75 days (median: 8 days) elapsed between the onset of clinical manifestations and the sample collection time for the corresponding diagnosis (suspicion of disease); however, in 6.9% of human cases it was delayed between 15 and 20 days (Fig. 2A). Most of the samples (68.6%) reached the reference laboratory in about 10 days, 22.9% in about 20 days, and 8.6% in 21–27 days; with an average of 13.5 days and a median of 13 days (Fig. 2B).
(A) Days elapsed between the onset of clinical manifestations and the collection of the first sample for the serological diagnosis of the disease. (B) Days between the collection of the first clinical sample for serological diagnosis and admission at the specific diagnostic laboratory. (C) Cross-reaction between serogroups used as antigens in MAT, according to the days of the course of disease. (D) Antibody titers for serogroups used as antigens in MAT, according to the days of the course of disease.
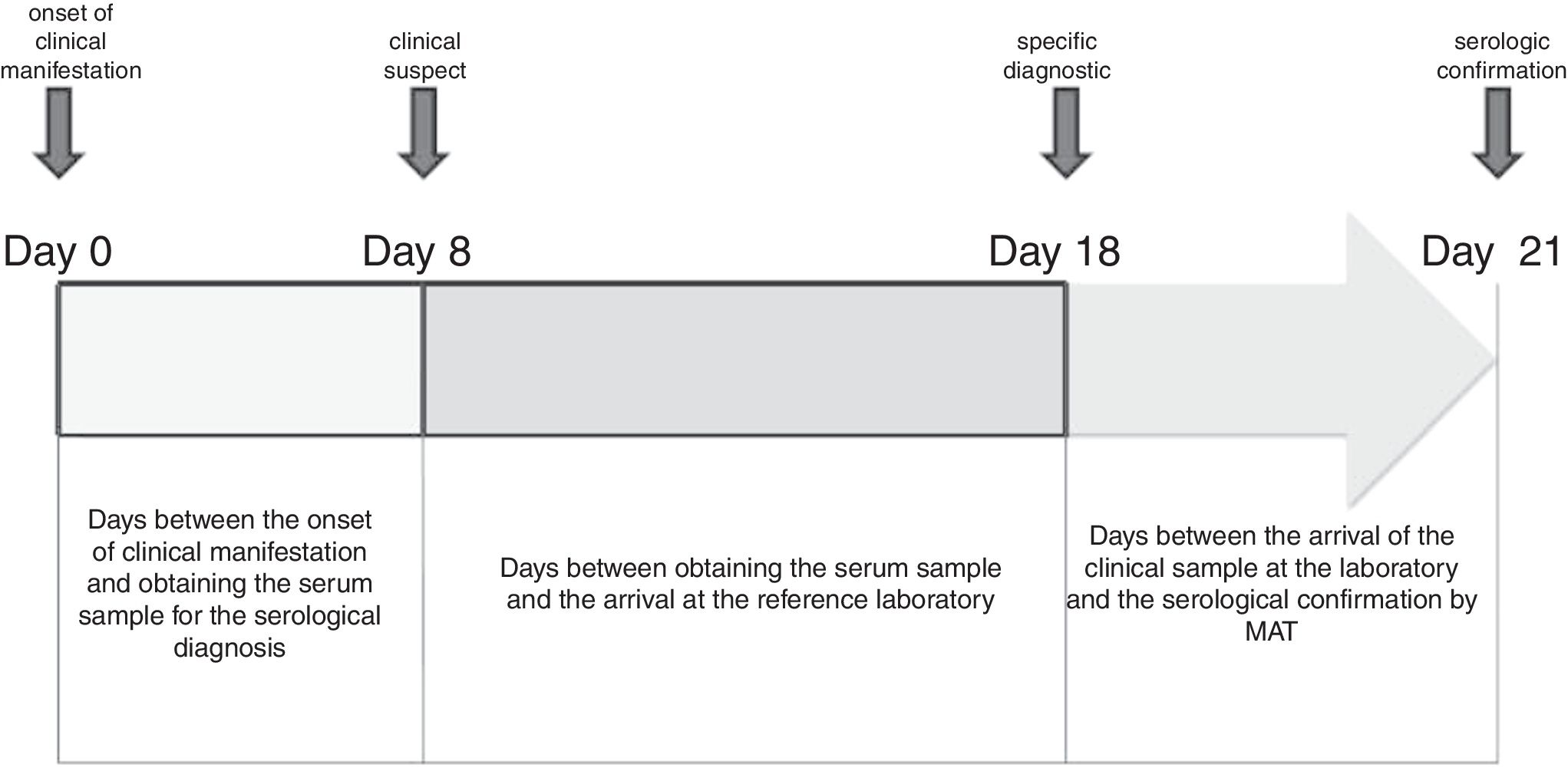
Human cases were confirmed by MAT on an average of 21 days (range=6–65) from the onset of the first clinical signs (usually fever). With MAT, antibodies were detected on the fourth day after the onset of clinical manifestations (Fig. 2D). The cross-reaction between serogroups was evidenced from day 4 to day 40 of the course of the disease; on average, positive sera reacted with four serogroups (Fig. 2C). Ballum–Canicola was the most observed pattern, with titers ranging from 1:50 to 1:6400. The reactive serogroups used in MAT in positive human serum samples were Tarassovi, Canicola and Ballum.
By analyzing the main components, we could explain the behavior of the variables both for the first samples (99.7%) and for the second ones (94.6%).
In the first clinical samples a high correlation was observed between the variable “days between the onset of clinical signs and clinical sample collection” and the variable “days of detection of antibodies”. The time of confirmation of the human cases of leptospirosis was affected depending on the distance of the sanitary regions (from 5km to 450km) with the specific diagnostic laboratory (p=0.0009).
The geographical distribution of confirmed human cases of leptospirosis, in addition to the high number of suspected cases and probable cases (which could not be confirmed by MAT), demonstrate that leptospirosis is endemic and underreported in the province of Buenos Aires. Clinical manifestations of human leptospirosis may be fever, jaundice, renal, hemorrhagic, meningeal and respiratory symptoms, and may cause death. The inclusion of Hantavirus and the Argentine hemorrhagic fever disease is common in the differential diagnostic (due to the clinical and epidemiological characteristics) in the region.
Based on the number of suspected human cases of leptospirosis reported in the region, the confirmation rate by serology is very low; partly because the laboratory does not have a second clinical sample, only first samples with less than a week of evolution. Although the presence of antibodies was observed in sera from patients with less than seven days of disease evolution, and taking into account that the titers can persist for a long time, it would be necessary to rule out whether these antibodies corresponded to a past exposure, thus interfering with the current diagnosis. Another important aspect to avoid false negative results in MAT is the inclusion in the panel of all antigen serogroups circulating in the region.
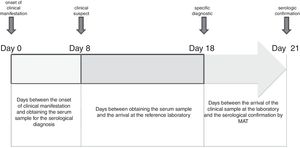
Serological confirmation of human cases was done on an average of 21 days after the onset of the illness (Fig. 3). The arrival time of the clinical sample to the reference laboratory depended on the geographical location of the HR, the city or the health care center where the patient is hospitalized. In the case of HR I (located in the city of Bahía Blanca) and HR IX (located in the city of Azul), this variable did not influence the confirmation time. In addition to the distance (kilometers), in the health system of the Buenos Aires province there is no mechanism for collection and distribution of clinical samples from the health centers to the reference laboratory, being observed that 34% of the cases are delayed more than 10 days.
Another important aspect of the serological diagnosis was the cross-reactivity phenomenon (between two to eight serogroups), which was observed from day 4 of the disease until day 40, with antibody titers ranging from 1:50 to 1:6400. It was striking to observe (in seven patients) the presence of cross-reactivity with titer ≥1:800 from the fourth and fifth day; however, a possible error in the anamnesis and data record should be ruled out. The number of serogroups that cross-reacted decreased as the disease progressed. In the first 30 days, in 66% of sera a cross-reaction was observed for five serogroup on average. Of the 9 serogroups used as antigens in MAT, Ballum–Canicola was the most frequently observed pattern. Because the cross-reaction among serogroups can persist for more than 40 days, if it is desired to associate the antigenic response to the possible infectious serogroup, it will be necessary to obtain a serum sample after a few months, when the immune response becomes even more specific. However, canines and rodents in the region are considered potential risk factors for humans; this sometimes makes it possible to suspect the serogroups Canicola and Icterohaemorrhagiae as possible infectious agents.
MAT is an important tool in the serological diagnosis of human leptospirosis in Buenos Aires province, allowing to confirm human cases in practically three weeks, and sometimes (64% of cases) with a single sample obtained seven or more days after the onset of the clinical manifestations. However, the serological techniques should be complemented with the polymerase chain reaction (PCR) and bacteriological tests (blood sample obtained in the first week of disease evolution) in the region. However, molecular testing is not available in restricted resource areas. By means of PCR, suspicious cases of the disease could be confirmed in the samples of patients within few days from the onset of clinical manifestation and when it is sometimes not possible to have a second clinical sample, mainly in fatal cases. We have observed that PCR has lower sensitivity than MAT in the acute period of the disease (1–7 days) when serum samples are used; however, this could be improved by using whole blood6. In a recent study, PCR was positive in serum samples from patients with a late course of the disease (more than 14 days), not agreeing with the accepted pathogenesis for leptospirosis6. In this work, real-time PCR reactions were used to detect Leptospira DNA, using LipL32-270F and LipL32-692R primers that amplify a 423-bp fragment, and Lepto F and Lepto R primers that generate an 87-bp amplicon from the DNA of Leptospira spp.6 In some serum samples (7/14) we have observed positivity using primers that amplify fragments with less than 100bp in absence of amplification with primers that generate longer DNA fragments (423bp), which could be attributed to the high levels of degradation that this type of samples usually present when stored and transported from health centers, in many cases with deficient refrigeration. For this reason, for the performance of a correct molecular diagnosis, it is important to use primers that amplify fragments lower than 300-bp long to avoid false negative results due to the high DNA degradation of the sample. PCR is an important diagnostic tool and complementary to MAT. It is necessary to study more samples, improving the procedures used in our region and taking into account the difficulties of shipping and storage of the clinical materials.
Furthermore, having a whole blood sample during the first week of the clinical course of the disease (prior to antibiotic therapy) would allow to attempt isolation and also to improve the sensitivity of the molecular diagnosis. Bacteriology is rarely used as a diagnostic method of human leptospirosis in the region, due to the need of a prolonged culture; however, this technique plays an important role in the epidemiology of the disease, providing information about the serotypes that are circulating in the region and their possible association with clinical manifestations in humans.
The distance from the health centers to the reference laboratory was the most important variable that affected the time of confirmation of human leptospirosis cases, and the clinical samples took several days to reach it. The health system of Buenos Aires province should develop a mechanism for the collection and adequate distribution of clinical samples.
Conflict of interestThere are no conflicts of interest to declare.