Improving access to diagnosis constitutes a key step in the control of the Human immunodeficiency virus (HIV)/AIDS epidemic. Rapid testing is increasingly gaining interest as a powerful diagnostic tool to achieve this goal. The purpose of this study was to implement the rapid HIV test (RHT) in a clinical setting in order to evaluate its effectiveness in increasing HIV diagnosis and patient linkage to the healthcare system.
The RHT was offered to all patients attending a sexually-transmitted disease clinic in the City of Buenos Aires between March and December 2015. A total of 593 RHT were performed. The implementation of the RHT yielded an increase in frequency of diagnosis from 6.9% to 31.4% (p<0.001). The first steps of the care cascade showed high retention rates around 90%. RHT yielded an HIV prevalence of 6.3% (95% CI: 4.2–8.5) in this population. HIV prevalence tended to be higher in individuals with no previous HIV testing (p=0.09). Linkage to the healthcare system was associated with a higher probability of having been tested for HIV (p=0.008). The incorporation of the RHT resulted in a high retention of patients and an increase in both frequency of diagnosis and results reception when compared to the classic methodology.
El mejoramiento del acceso al diagnóstico constituye un punto clave en el control de la epidemia del virus de la inmunodeficiencia humana (Human immunodeficiency virus: HIV)/sida. El testeo rápido se ha convertido en una poderosa herramienta diagnóstica de interés para alcanzar este objetivo. El propósito de este estudio fue la implementación del test rápido del HIV (TRH) en un contexto clínico con el fin de evaluar su efectividad para incrementar la tasa de diagnóstico de HIV y la vinculación de los pacientes al sistema de salud. Se ofreció el TRH a todos los pacientes que acudieron a una clínica de enfermedades de transmisión sexual en la Ciudad Autónoma de Buenos Aires entre marzo y diciembre del 2015. Se realizaron un total de 593 TRH. La implementación del TRH resultó en un aumento en la frecuencia de diagnóstico del 6,9 al 31,4% (p<0,001). Los primeros pasos en la cascada de cuidados mostraron altas tasas de retención alrededor del 90%. La prevalencia del HIV según TRH en la población de estudio fue del 6,3% (IC 95%: 4,2-8,5), tendiendo a ser mayor en individuos sin testeo previo de HIV (p=0,09). La vinculación al sistema de salud se asoció a una mayor probabilidad de haber sido testeado previamente para HIV (p=0,008). La incorporación del TRH resultó en una mayor retención de los pacientes, así como en un aumento tanto en la frecuencia de diagnóstico como en la de retiro de resultados, en comparación con la metodología clásica.
Given the worldwide commitment to end the epidemic by 2030, UNAIDS has set a series of objectives for the year 2020: that 90% of all people living with HIV become aware of their infection, that 90% of those aware of their infection gain access to treatment and that 90% of those on treatment suppress their viral load one year after the infection17. This highlights the importance of improved access to diagnosis as the first step in the control of the HIV/AIDS epidemic. However, there still remains much to be done in this area, considering the high frequency of late diagnoses worldwide8,13. In this context, rapid HIV tests (RHT) are essential tools to achieve the 90–90–90 target due to their proved efficacy in facilitating access to diagnosis10.
In Argentina, HIV prevalence has been estimated to be 0.4% among young people and adults. The epidemic is mostly concentrated in men who have sex with men (MSM) and transgender women, with HIV prevalence rates of 12–15% and 34%, respectively. Moreover, it has been reported that approximately 37.5% of men and 30% of women receive a late HIV diagnosis2. Even when international studies clearly associated RHT introduction with increased HIV testing in several settings, only one research study on the implementation of the RHT has been performed in Argentina5. Given the need for local data on the implications of the incorporation of the RHT into the diagnostic algorithms, our goal was to test the effectiveness of a structural intervention: the incorporation of the RHT followed by immediate counseling, diagnosis confirmation and entry to care in a walk-in sexually-transmitted disease (STD) clinic located in a University Hospital (Programa de Enfermedades de Transmisión Sexual (PETS), Hospital de Clínicas “José de San Martin” Universidad de Buenos Aires).
Materials and methodsEnrollment of participantsA diagnostic algorithm was designed in line with those recommended by the national health ministry1, in particular the one-rapid-test screening algorithm. Between March and December 2015 the RHT was offered to all patients attending the STD clinic who met eligibility criteria. Eligible patients were men and women 18 years old or older who attended the clinic regardless of the reason for consultation. During the process of registration each patient was told that the test would be carried out from a finger prick blood sample and that the result would be informed to them directly by the physician during the consultation. Those who manifested interest were given an informed consent for them to read and sign, as well as a survey to complete in order to collect socio-demographical data and information related to sexual behavior, linkage to the healthcare system and previous HIV testing. Upon reading, completing and signing this documentation, they were enrolled in the study and individually led to a room which was especially conditioned for carrying out the test.
Rapid HIV test and delivery of resultsThe RHT was performed by collecting a blood sample through a finger prick, using the commercial kit Determine™ HIV-1/2 (Alere), an immunochromatographic test for the qualitative detection of antibodies against HIV-1/2 antigens. The test was carried out according to the manufacturer's instructions and the results were available within 15minutes These were interpreted by the technician and/or researcher in charge and delivered to the physician for him to read and to show to the patient. In the event of a non-reactive RHT, the physician gave the patient the necessary recommendations based on reported risk factors. In case of reactive RHT, on the other hand, the patient was informed about the need for further confirmatory studies and referred to the INBIRS diagnostic laboratory, which is located in the nearby School of Medicine (Facultad de Medicina, Universidad de Buenos Aires), approximately 100 meters away from the University Hospital.
HIV confirmatory studies and delivery of resultsA 15ml peripheral blood sample was taken from those individuals with reactive RHT who attended the INBIRS laboratory for confirmatory studies, which included ELISA (Genscreen Ultra HIV Ag-AbBio-rad), plasma viral load (Abbott Real Time HIV-1 RNA Version 3.0) and CD4+T cell count (flow citometry). The results were sent to the clinic and handed over to the patient by the same physician during a second visit. Patients with confirmed HIV diagnosis were referred to the Infectious Diseases Unit of the University Hospital (Departamento de Infectología, Hospital de Clínicas “José de San Martín”, Universidad de Buenos Aires) in order to be assisted. Patient follow-up was performed by regular reviewing of clinical records for a period of one year following the study period.
Statistical analysisFisher's exact test and Mann–Whitney U test were used to compare differences in categorical and numerical variables, respectively. All statistical analyses, including calculation of interquartile ranges (IQR) and 95% confidence intervals (95% CI) were carried out using IBM SPSS Statistics Base 22.0 (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.).
Ethical aspectAll the procedures were in line with the Declaration of Helsinki (Seoul 2008), the National Personal Information Protection Law (Law 25326) and followed the recommendations provided by national regulations for research. The protocol of the study, as well as the documentation used (namely, informed consent and survey) were evaluated and approved by the University Hospital Institutional Review Board (IRB) (Comité de Ética, Hospital de Clínicas “José de San Martín”, Universidad de Buenos Aires). Informed consent was obtained from all study participants.
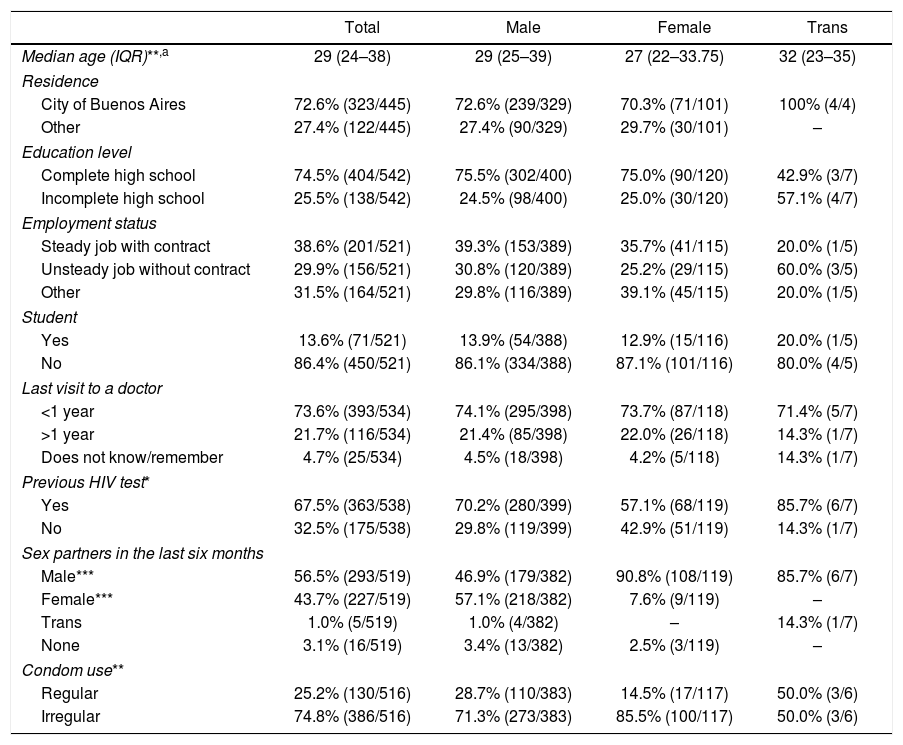
ResultsSocio-demographic characteristics of the study populationBetween March and December 2015, a total of 593 RHT were performed on 547 patients (39 patients were tested twice, six patients three times and one patient four times; there were no duplicate cases among reactive RHT). Unless otherwise noted, all analyses were performed based on the non-duplicate cases. Considering gender at birth, 76.8% (420/547) of participants were men and 23.2% (127/547) were women. Regarding self-identified gender, most individuals identified themselves as male (75.6%, 403/533), others as female (22.5%, 120/533) and a small minority as transgender (1.3%, 7/533). Table 1 shows all socio-demographic characteristics, stratified by self-identified gender. Briefly, the patients’ median age was 29 years (IQR: 24–38), with self-identified males being significantly older than females (males: 29 years old, IQR: 25–39; females: 27 years old, IQR: 22–33.75; Mann–Whitney test, p=0.001). Transgender individuals showed a higher median age (32 years old, IQR: 23–35). However, no statistically significant difference could be inferred either with males (p=0.84) or females (p=0.42). Most patients lived in Buenos Aires, the capital city (72.6%, 323/445), or in the Buenos Aires Province (26.3%, 117/445). Most individuals (74.5%, 404/542) reported having completed high school education level. Regarding work status, 38.6% (201/521) had a steady job, i.e. with a contract, 29.9% (156/521) had an unsteady job, i.e. without a contract, while the remaining individuals who reported not having a job were unemployed, retired or students.
Socio-demographic characteristics of the 547 participants included in the study between March and December 2015 in the STD clinic in Buenos Aires, Argentina
| Total | Male | Female | Trans | |
|---|---|---|---|---|
| Median age (IQR)**,a | 29 (24–38) | 29 (25–39) | 27 (22–33.75) | 32 (23–35) |
| Residence | ||||
| City of Buenos Aires | 72.6% (323/445) | 72.6% (239/329) | 70.3% (71/101) | 100% (4/4) |
| Other | 27.4% (122/445) | 27.4% (90/329) | 29.7% (30/101) | – |
| Education level | ||||
| Complete high school | 74.5% (404/542) | 75.5% (302/400) | 75.0% (90/120) | 42.9% (3/7) |
| Incomplete high school | 25.5% (138/542) | 24.5% (98/400) | 25.0% (30/120) | 57.1% (4/7) |
| Employment status | ||||
| Steady job with contract | 38.6% (201/521) | 39.3% (153/389) | 35.7% (41/115) | 20.0% (1/5) |
| Unsteady job without contract | 29.9% (156/521) | 30.8% (120/389) | 25.2% (29/115) | 60.0% (3/5) |
| Other | 31.5% (164/521) | 29.8% (116/389) | 39.1% (45/115) | 20.0% (1/5) |
| Student | ||||
| Yes | 13.6% (71/521) | 13.9% (54/388) | 12.9% (15/116) | 20.0% (1/5) |
| No | 86.4% (450/521) | 86.1% (334/388) | 87.1% (101/116) | 80.0% (4/5) |
| Last visit to a doctor | ||||
| <1 year | 73.6% (393/534) | 74.1% (295/398) | 73.7% (87/118) | 71.4% (5/7) |
| >1 year | 21.7% (116/534) | 21.4% (85/398) | 22.0% (26/118) | 14.3% (1/7) |
| Does not know/remember | 4.7% (25/534) | 4.5% (18/398) | 4.2% (5/118) | 14.3% (1/7) |
| Previous HIV test* | ||||
| Yes | 67.5% (363/538) | 70.2% (280/399) | 57.1% (68/119) | 85.7% (6/7) |
| No | 32.5% (175/538) | 29.8% (119/399) | 42.9% (51/119) | 14.3% (1/7) |
| Sex partners in the last six months | ||||
| Male*** | 56.5% (293/519) | 46.9% (179/382) | 90.8% (108/119) | 85.7% (6/7) |
| Female*** | 43.7% (227/519) | 57.1% (218/382) | 7.6% (9/119) | – |
| Trans | 1.0% (5/519) | 1.0% (4/382) | – | 14.3% (1/7) |
| None | 3.1% (16/519) | 3.4% (13/382) | 2.5% (3/119) | – |
| Condom use** | ||||
| Regular | 25.2% (130/516) | 28.7% (110/383) | 14.5% (17/117) | 50.0% (3/6) |
| Irregular | 74.8% (386/516) | 71.3% (273/383) | 85.5% (100/117) | 50.0% (3/6) |
Regarding linkage to the healthcare system, 73.6% (393/534) of patients reported receiving some kind of medical service during the previous year. Most patients (67.5%, 363/538) had been tested for HIV previously, and 94.5% (294/311) of them reported receiving their results. Probability of having been tested for HIV in the past was higher in patients who reported receiving any kind of medical service during the previous year as opposed to none (71.0% (277/390) vs. 58.6.% (82/140), p=0.008) and men with reported male partners as opposed to female partners (86.0% (141/164) vs. 60.7% (125/206), p<0.001). Inconsistent condom use was more frequent in patients with no previous HIV test when compared to those previously tested (82.9% (136/164) vs. 71.8% (249/347), p=0.006).
Sexual practices and condom useRegarding sexual practices, 51.3% (210/409) of men reported having sexual relationships only with women in the six months prior to the study, while 40.3% (165/409) reported having had male sexual partners. The remaining men had either both male and female (3.9%, 16/409), female and trans (0.7%, 3/409), only trans (0.5%, 2/409) or no sexual partners (3.2%, 13/409). In the case of women, the vast majority (95.2%, 120/126) reported having had only male sexual partners, while only 2.4% (3/126) claimed to have had no sexual partners during the previous six months. Only a handful of women reported both male and female (1.6%, 2/126) or only female (0.8%, 1/126) sexual partners.
Concerning condom use, only 25.2% (130/516) of participants reported consistent condom use during sexual relationships, while 9.7% (50/516) claimed never to use a condom and the rest reported inconsistent condom use (65.1%, 336/516). Consistent condom use was more frequent among men than women (29.3% (116/396) vs. 11.7% (14/120), p=0.0001). Patients without a previous HIV test also showed a higher frequency of inconsistent condom use (82.9% (136/164) vs. 71.8% (249/347), p=0.006) when compared with those previously tested.
HIV prevalence and associated risk factorsAccording to RHT, HIV prevalence was 6.3% (34/538, 95% CI: 4.2–8.5), with a significant higher prevalence in men than in women (7.5% (31/412) vs. 2.4% (3/126), p=0.04). Patients who had not been tested for HIV showed a tendency toward a higher HIV prevalence than those who had been tested once (9.1% (16/175) vs. 5.1% (18/354), p=0.09). When accounting for sexual partner type, a significant higher HIV prevalence was found among men who had exclusively male partners as opposed to exclusively female partners (15.0% (24/160) vs. 1.9% (4/207), p<0.001). No significant differences in HIV prevalence were observed between participants who reported consistent or inconsistent condom use (8.5% (11/129) vs. 5.8% (22/378), p=0.30).
Patient referral and first steps in the cascade of careAll patients with a reactive RHT (n=34) were referred to the laboratory for confirmatory studies; 94.1% (32/34) went to the laboratory, and 87.5% (28/32) came back in order to receive their results. The infection was confirmed in 93.8% (30/32) of patients, yielding two false reactive RHT results. Among HIV positive patients (n=30), the median CD4+T cell count was 441cells/μl (p25–p75, 288–608) and the median HIV viral load was 28764 RNA copies/ml (p25–p75, 4446–59177). The two cases classified as false positives were confirmed as HIV negative according to plasma viral load, fourth-generation ELISA and immunological parameters. Of the 30 confirmed HIV-positive individuals, we were able to do follow-up 13 of them, i.e. 43.3%. Among these, 69.2% (9/13) repeated the laboratory tests, namely CD4+T cell count and plasma HIV viral load, and 76.9% (10/13) initiated treatment. Among those who repeated the tests, 55.6% (5/9) managed to suppress their viral load during the course of the follow-up period. Regardless of the presence or absence of viral suppression, all patients, except for one patient, had a lower viral load by the time of the final examination, which took place 4–14 months after the base-line test.
Influence of rapid testing on frequency of HIV diagnosisTo evaluate the impact of the incorporation of the RHT on the frequency of patients who agreed to be tested for HIV, we compared the frequency of the previous year performed by the conventional methodology (referral to laboratory, peripheral blood collection, laboratory analyses (ELISA, Western Blot) and delivery of results within 15 days) with the frequency of patients tested by RHT in our study. In the year prior to the study, 6.9% (210/3060) of the patients who attended the clinic agreed to be tested, while this frequency rose to 31.4% (593/1887) during the study period (p<0.001)(this includes duplicate cases). We also observed a slight increment in the reception of confirmatory results from 76.2% (160/210) to 87.5% (28/32) (p=0.6746).
DiscussionThis study reveals for the first time in Argentina how the incorporation of the RHT in the diagnostic algorithm can cause a dramatic increase in the frequency of HIV diagnosis (almost fivefold) as observed in the comparison between the results of the present study and the period prior to the study, i.e. with and without the use of the RHT. This is consistent with previous studies performed in other countries comparing the uptake of HIV/AIDS services using RHT and conventional testing, where the implementation of RHT was associated with a threefold increase in the frequency of diagnosis14,18. Even when the use of RHT significantly increases the rate of HIV diagnosis, the frequency (≅30%) is still lower than that reported previously in other studies where acceptability of rapid tests ranged from 45% to 95%. However, uptake of RHT depended on the venue where testing was offered as well as the risk group to which clients belonged, for example, acceptability has been reported to be higher in pregnant women and lower among clients of sex workers (45%), black university students tested on campus (50%) and African and Caribbean clients in family practice (45%)3,12,15. Low RHT acceptability among some populations may be related to the fact that they did not expect to be offered a test or do not perceive themselves to be at risk for HIV. Due to the fact that at the time of the study it was not very common to find RHT offer in medical services; the poor acceptability can be related to the issues mentioned.
This rapid HIV testing approach not only effectively increased the number of individuals tested but also reached individuals at risk for HIV, as can be seen by the high HIV prevalence detected. This was found to be almost 6%, which is considerably higher than that of the general population (below 1%)2. The reason for this might lie in the fact that the individuals included in this study are already seeking medical attention for STD-related – real or suspected – problems, thus one could expect to find a higher-than-average prevalence of STDs in general, including HIV. We also found that HIV positive individuals reached in this study have a median CD4+T cell count below 500cells/μl, which suggests late HIV diagnosis. This result is of great importance considering the evidence that indicate that immediate antiretroviral therapy, with CD4+T cells count over 500cells/ml, can reduce disease progression, improving the likelihood of immunologic recovery while also increasing the percentage of individuals who achieve viral suppression7,16.
Our findings underscore the importance of increasing the rate of HIV diagnosis by improving patient linkage to the healthcare system. One in four patients had had no contact with any health service for over a year and HIV testing was significantly less frequent among them, which is consistent with previous studies4,6,11. This association between linkage to healthcare system and HIV testing highlights the importance of interventions to bring individuals to clinics/hospitals and identify opportunities for rapid HIV testing in these environments. The model tested in this study could be applied in other clinics with similar characteristics, as long as it does not involve additional time for the patient or moving to another place to be tested. From this finding, it would be pertinent to recommend including the RHT in STDs clinics. To achieve this, it would be imperative to have minimal requirements like funding, room, and trained personnel.
It has been previously reported that rapid testing reduces waiting times for the patient, favoring linkage and retention in care9. Accordingly, our approach to the first steps of the cascade of care with RHT shows encouraging outcomes, with high retention rates (around 90%). However, our study has certain limitations that need to be addressed. Our experimental design only allowed us to closely follow the first few steps of the care cascade – further inquiry is necessary to determine if the beneficial effect observed applies to the entire chain, from testing to treatment and viral suppression. Finally, a higher sample size might have enabled us to examine socio-demographics and conduct differences between other, less frequent populations, such as trans, which here were only found in very low numbers. New studies aimed at applying the implementation of HIV diagnosis via rapid testing would strongly benefit from these additions.
ConclusionsIn conclusion, results obtained in this study confirm those of previous interventions performed in other countries and highlight the importance of facilitating patient access to diagnosis and retention within the healthcare system. The incorporation of the RHT resulted in a high retention of patients and an increase in both the frequency of diagnosis and results withdrawal when compared to the conventional methodology. Other notable achievements of the study in this respect include the successful incorporation of RHT in a walk-in clinic, with high acceptance and efficacy. Last but not least, the testing methodology employed, based on the open offer of the RHT without disrupting the normal circuit of the clinic, is potentially applicable to various other clinical settings with similar features.
Conflict of interestThe authors declare that they have no conflicts of interest.
The authors would like to thank all the patients who agreed to participate in this study, as well as the staff of the clinic and the INBIRS laboratory.
This study was funded with a research project grant from the University of Buenos Aires: UBACYT 2014/2017 (project code: 20720130200010BA; principal investigator: Dr. María A. Pando). The University of Buenos Aires had no involvement in the study design, the collection, analysis and interpretation of data, the writing of the report or the decision to submit the article for publication.