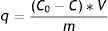The biotechnology sector is continually seeking sustainable and more economical bioprocesses. Fermentation media produced with cheap components or wastes reduce production costs. Moreover, if wastes are used, they contribute to avoid environmental pollution. In this work, microbial growth media based on molasses or acidified glycerol as carbon sources and fertilizer as nitrogen source were tested for the production of a whole-cell catalyst that could be used in Cr(VI)-containing wastewater treatments. Results showed that the highest biomass production yield was obtained with a medium containing acidified glycerol 5% v/v and fertilizer 0.6% v/v. The biomass produced using this medium was immobilized in calcium alginate beads and used as catalyst in the biotransformation of Cr(VI) into Cr(III). The catalyst could be efficiently used for 5 reduction cycles of 40mg/l Cr(VI) each. Cr(III) retention assays were performed to determine whether Cr(III) could be retained by the catalyst avoiding its solubilization in the supernatants. The retention capacity of the catalyst at 32°C and pH 3.0 was 3mg Cr(III)/g. Both an alternative and economical fermentation medium is here proposed for the optimization of Cr(VI)-containing wastewater treatment.
El sector industrial biotecnológico continuamente busca bioprocesos más económicos y sustentables. El uso de medios de cultivo producidos con componentes de bajo costo o con residuos reduce el presupuesto global del proceso y, particularmente si se utilizan residuos, se contribuye, además, a evitar la contaminación ambiental. En este trabajo se probaron medios de cultivo basados en melaza de caña o glicerol ácido como fuentes de carbono y energía, y fertilizante como fuente de nitrógeno, para la producción de un biocatalizador que podría ser usado para el tratamiento de aguas residuales que contienen Cr(VI). Los resultados mostraron que el mayor rendimiento de producción de biomasa se obtuvo con un medio que contenía 5% v/v de glicerol ácido y 0,6% v/v de fertilizante. Utilizando este medio se produjo la biomasa suficiente para la biotransformación de Cr(VI) a Cr(III), luego de ser inmovilizada en alginato de calcio. El proceso pudo ser aplicado eficientemente durante 5 ciclos de reducción de 40mg/l de Cr(VI) cada uno. Además, se realizaron ensayos de retención de Cr(III) para determinar si esta especie química podría ser removida de la solución por interacción con el biocatalizador. La capacidad de retención obtenida por el biocatalizador a 32°C y pH 3 fue de 3mg de Cr(III)/g. De esta manera, se propone un medio de cultivo alternativo y económico para la efectivización de un tratamiento de aguas residuales que contengan Cr(VI).
Alternative economic microbial growth media have been intensively developed in recent years3,19,29. The use of wastes or useless process by-products as substrates for microbial fermentation is an ordinary practice in bioprocesses to gain sustainability1,30,31. Molasses and acidified glycerol are good examples of process wastes with high potential use as medium components. Molasses is a sub-product obtained from the sugar production13,18, with high sugar and mineral contents suitable as carbon and energy sources for microbial growth. It is calculated that for every ton of processed sugar, 0.3tons of molasses are produced with limited uses up to now18. Acidified glycerol is a sub-product of the biodiesel production, obtained in an early purification step that consists in the addition of HCl to crude glycerol to remove oil and other impurities17. This sub-product has little use nowadays and generates a problem for the biodiesel industry since it requires a mandatory treatment prior discharge, taking into consideration that for every 10kg of biodiesel, 1kg of glycerol is produced14,19,26,27. This crude glycerol excess, therefore, increases the cost of the biodiesel production process19,25,27. The use of crude glycerol or acidified glycerol as carbon and energy sources in microbial fermentations is a possible solution for this problem. The substitution of the commonly used nitrogen sources for others that are more economical is also a matter of great importance. There are several publications illustrating the efforts done in this direction4,20,28,32.
Cr(VI)-containing wastewaters produced by industries8,12,24 are a great concern because of the highly toxic effects of hexavalent chromium6,21. Currently used Cr(VI)-containing wastewater treatments are often inefficient and costly so new alternative treatments are being investigated5,33. Biological Cr(VI) reduction to the less toxic and easier to remove Cr(III) is a treatment that is under research and could provide a cost/effective solution for the industry sector.
The aim of this work is to produce biomass of Pseudomonas veronii 2E, a Cr(VI)-reducing bacterium9, using economical growth media formulated with industrial by-products, immobilized in calcium alginate beads and applied as biocatalyst for the biotransformation of Cr(VI) into Cr(III). In addition, a Cr(III) retention assay was carried out to determine the possible fate of the generated Cr(III).
Materials and methodsMicroorganism and strain maintenancePseudomonas veronii 2E, is an autochthonous bacterium isolated from polluted environments belonging to sediments associated to the Reconquista River basin (Buenos Aires Metropolitan Area). This strain was identified by 500bp 16S r-RNA gene sequencing and is able to retain Cd(II), Zn(II) and Cu(II) and to biotransform Cr(VI) from aqueous systems, as described in previous studies2,9,22. P. veronii 2E was routinely maintained in plate count agar cultures. Growth was achieved at 32°C for 48h. Afterwards, the culture was stored at 4°C until use.
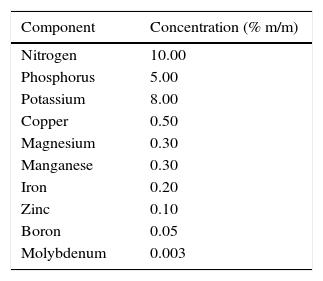
Growth mediaThe molasses-based media (MBM) were prepared using different proportions of heat sterilized (121°C, 15min) commercial molasses El Trebol® (0.1–2% m/v) and a previously filter-sterilized (0.45μm pore diameter) fertilizer solution (0–1.2% v/v; Afital® produced by Agro EMCODI S.A., located in Lanús, Buenos Aires, Argentina), whose composition is detailed in Table 1. The acidified glycerol was provided as a solution from a biodiesel plant located in Malvinas Argentinas, Buenos Aires, Argentina, which, after being sterilized, was mixed in different proportions (1–10% v/v) with the same fertilizer solution (GBM). All alternative media were neutralized at pH 7.0 with NaOH 6mol/l. Nutrient broth (NB, Merck) was prepared by dissolving 8g (5g beef peptone and 3g yeast extract) in 1l distilled water. Dextrose was added at 1g/l. NB was sterilized by heat at 121°C for 15min.
P. veronii 2E was inoculated in 10ml of NB in a 125ml Erlenmeyer flask. The culture was incubated at 32°C for 24h and at 120rpm. Then, 5ml of the culture was used as inoculum in 250ml Erlenmeyer flasks with 45ml of MBM or GBM containing different amounts of molasses or acidified glycerol respectively as carbon source (0.1, 0.5, 1, and 2% m/v molasses and 1, 2, 5, and 10% v/v acidified glycerol). The commercial fertilizer was added as nitrogen source at different concentrations (0, 0.6 and 1.2% v/v for MBM and 0.6% v/v for GBM). Incubation was carried out at 32°C until the cultures reached stationary phase (24h for cultures in NB and in MBM and 72h for cultures in GBM). Microbial growth was determined by measuring the OD600nm after 24h (NB or MBM) or by biomass dry weight after 72h (GBM). Biomass dry weight was obtained at 72h of growth in GBM when the cultures were centrifuged at 6300×g for 15min. The pellets were then dried at 60°C until constant weight. To compare the microbial growth level obtained with the different media, the biomass dry weight was determined only for the maximum biomass obtained in MBM and for NB after the 24h-cultures.
Cell immobilization and Cr(VI) biotransformationCells grown at 32°C for 72h in two 100ml-cultures in 500ml Erlenmeyer flasks with 5% v/v acidified glycerol and 0.6% v/v fertilizer were harvested after centrifugation at 6300×g for 15min. The obtained cell pellets were kept at −20°C until use. After storage, the frozen cell pellets were thawed, mixed and suspended in 40ml of distilled water. The final biomass concentration obtained was 56g/l. This suspension was mixed with 40ml of 1% m/v sodium alginate. The mixture was dripped over a 0.05mol/l CaCl2 solution at 4°C. The beads formed −40g of air-dried mass – that were kept in the solution at 4°C for 15min. Afterwards, the beads were washed with distilled water twice and suspended in 40ml of a solution containing 40mg/l Cr(VI) and 1% v/v acidified glycerol, previously neutralized with NaOH 6mol/l, as an electron donor for the reaction of Cr(VI) reduction. Incubation was performed at 32°C and pH 7.0 under constant agitation at 120rpm. Samples were taken periodically and Cr(VI) and total chromium concentrations were determined in the supernatants. After total Cr(VI) reduction, the beads were suspended in a new Cr(VI) solution starting a new reduction cycle. This procedure was repeated for five cycles. Abiotic control consisting of cell-free calcium alginate beads were incubated under the same conditions.
Cr(III) retention by immobilized cell alginate beadsTo test Cr(III) retention, 10g of the immobilized cells alginate beads were suspended in 10ml of a solution containing different amounts of Cr(III) (250, 500, 1000, 2000, 5000, 7000 and 10 000mg/l), prepared with Cr(NO3)3. The suspension was incubated at 32°C for 24h and pH 3.0 under constant agitation at 120rpm. Total chromium concentration was determined in the supernatants. As control, 10g of cell-free alginate beads were used in 10ml of a solution with 500mg/l Cr(III) under the same incubation conditions. The capacity of retention was calculated as:
where q is the specific retention capacity (mg Cr(III)/g alginate beads); C0 is the initial Cr(III) concentration (mg/l); C is the Cr(III) equilibrium concentration (mg/l); V is the volume (l) and m is the total mass of alginate beads (g).Cr(VI) analytical determinationFor Cr(VI) quantification in the supernatant samples the 1,5-diphenylcarbazide (DPC) method was used23. Briefly, 1ml sample was mixed with 1ml H2SO4 0.5mol/l and 0.2ml of reactive solution composed by 0.25% m/v DPC and 4% m/v phthalic acid in ethanol 96% v/v. The mixture was then left for 10min for color development. The absorbance of the solution was measured at 540nm. Distilled water was used as blank.
Total chromium determinationFor total chromium determination, the pH of supernatant samples was raised to 12.0 with NaOH 6mol/l. Then, H2O2 was added to a 1.4mol/l final concentration and the mixture was incubated at 60°C for 40min29 to ensure the complete oxidation of all chromium-species. The Cr(VI) obtained was determined as previously described23. The detection limit of this analytical technique was 3mg chromium/l.
Statistical analysisAll the determinations were done in duplicate and experiments were repeated at least once. Data plotted in figures correspond to mean values and error bars correspond to the standard deviation. Results were analyzed using MINITAB 17 (PA, USA). To determine if the mean values were significantly different, one-way analysis of variance (ANOVA) and the Tukey's test were used. Values which presented a p<0.05 were considered significantly different.
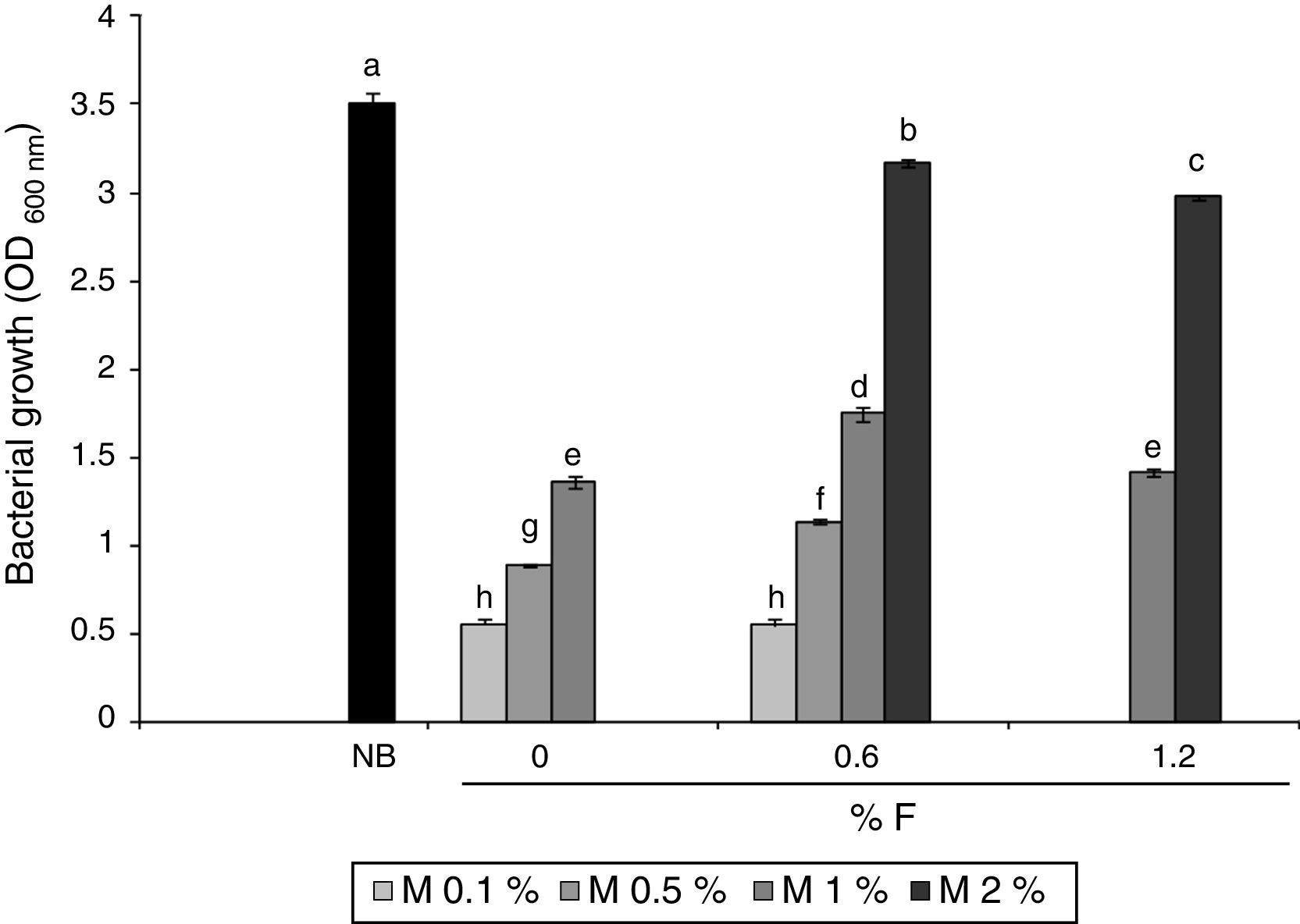
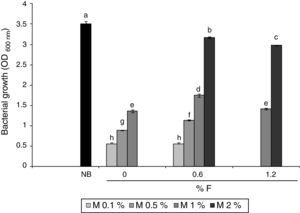
Results and discussionMicrobial growth in MBM and GBMFigure 1 shows microbial growth levels with different MBM composition. Maximum growth was achieved with NB. However, similar cell densities were obtained with 2% m/v molasses and 0.6% v/v fertilizer-MBM and NB. A slight decrease in the biomass obtained was observed at fertilizer concentrations higher than 0.6% v/v, both with 1 and 2% m/v molasses.
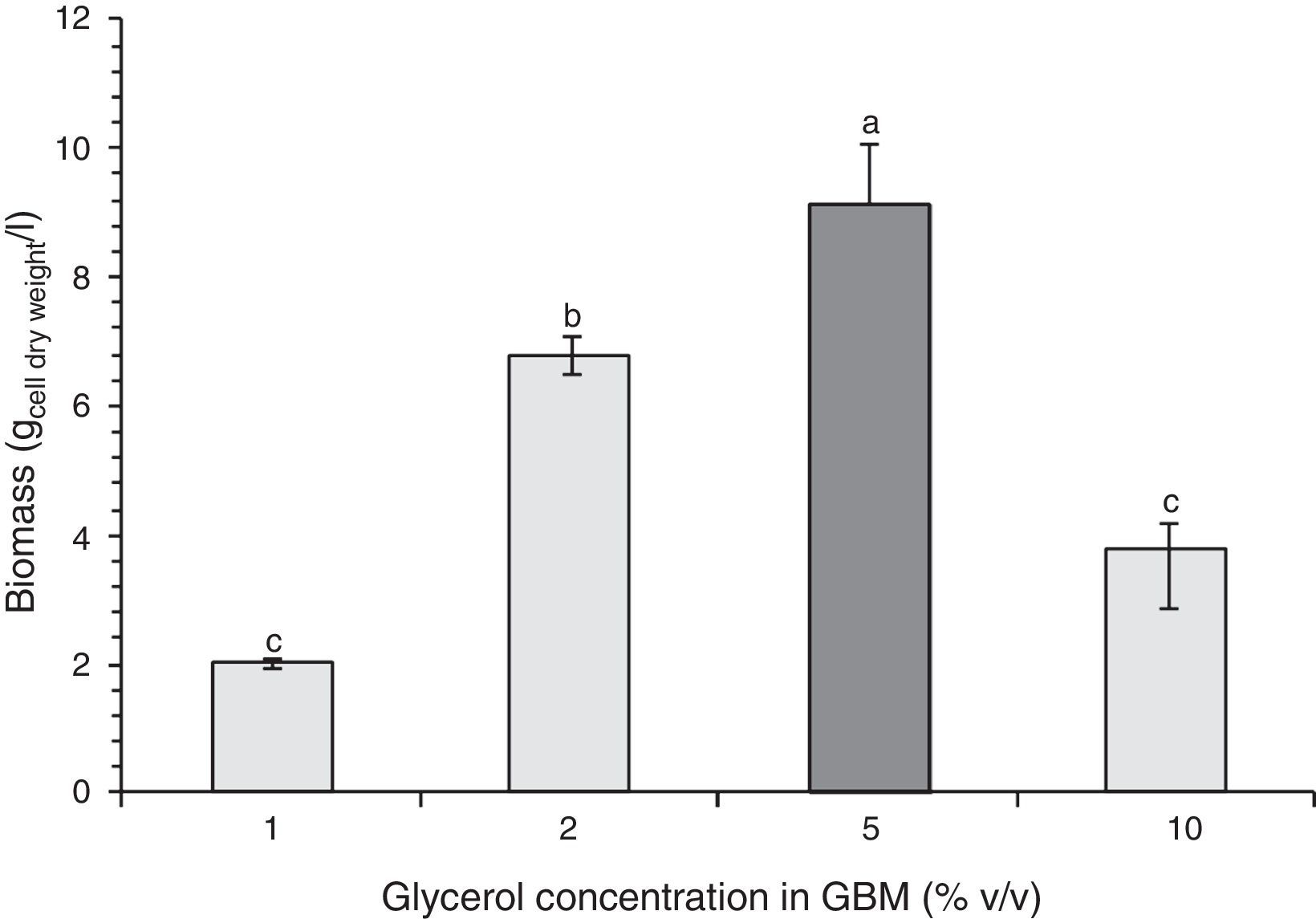
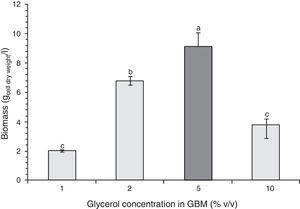
Figure 2 shows microbial growth in GBM containing 0.6% v/v fertilizer and different amounts of glycerol. In this case, maximum growth level was achieved with 5% v/v glycerol.
The maximum dry biomass obtained in the entire cultures at the corresponding stationary phases with 2% m/v molasses and 0.6% v/v fertilizer-MBM, NB and 5% v/v glycerol and 0.6% v/v fertilizer-GBM were 4.8, 4.9 and 9.5g dry biomass/l respectively. While the growth levels in MBM and in NB were similar, the growth level in GBM was 94% higher, the latter being the optimal composition to achieve the maximum bacterial biomass.
Microbial growth using 2% m/v molasses and 0.6% v/v fertilizer-MBM was similar to the microbial growth produced with NB. The addition of fertilizer slightly increased biomass production. Stationary phase was reached faster in MBM and in NB than in GB, which was probably due to the fact that the bacterium was already adapted to the carbon source in MBM and in NB since the pre-culture contained the same carbon source. Although the maximum biomass obtained in the entire culture was higher with 5% v/v glycerol and 0.6% v/v fertilizer-GBM than with NB and 2% m/v molasses and 0.6% fertilizer-MBM, the productivity was the lowest: 6.70mg/h±0.15mg/h. The productivity with MBM and NB showed no statistically significant difference: 9.38mg/h±0.88mg/h and 10.31mg/h±0.15mg/h respectively. Since the carbon content of the alternative media was unknown, it was not possible to determine their biomass production efficiency. However, it was demonstrated that the use of alternative carbon sources was feasible and more economically viable than the use of the standard NB. The cost of crude glycerol is estimated in U$D 0.10/kg25, the cost of molasses is U$D 0.13–0.16/kg34 and the cost of the fertilizer is U$D 0.4/l (Afital®). Moreover, the cost of beef peptone is U$D 8–15/kg (Yantai Huahai Biochemical Product Co.) and the cost of yeast extract is U$D 5.8–6.5/kg (Hangzhou Ruijiang Chemical Co.). These latter amounts individually surpass the cost of all the ingredients of the alternative media tested in this work.
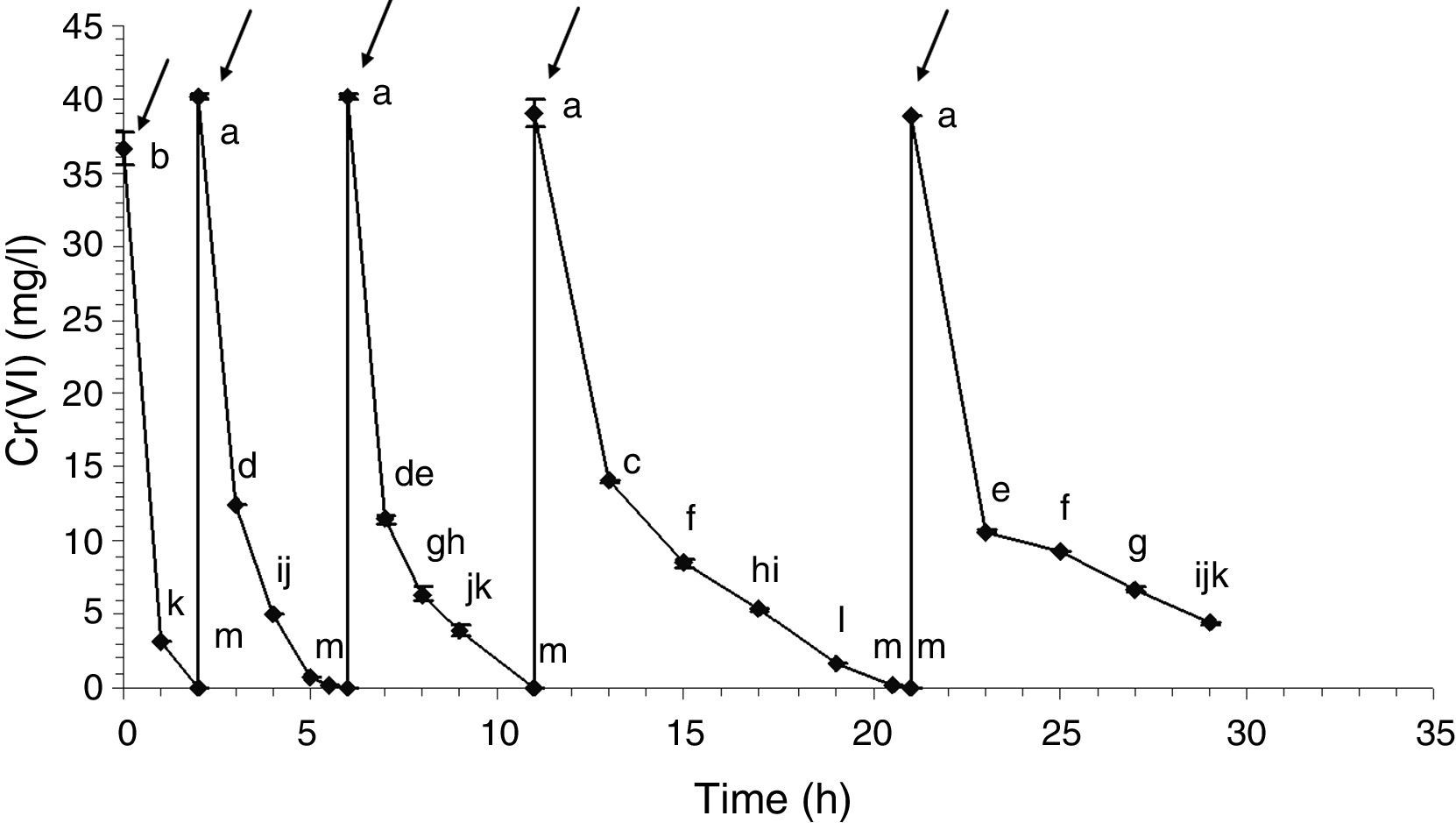
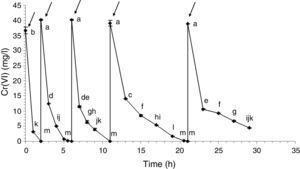
Cell immobilization and Cr(VI) biotransformationHarvested cells from 5% v/v glycerol and 0.6% v/v fertilizer-GBM 72h-cultures were immobilized in calcium alginate beads to simulate a wastewater biotreatment for Cr(VI) removal by its biotransformation into Cr(III). Figure 3 shows the Cr(VI) abatement from an artificial wastewater for each biotransformation cycle. During the first four cycles, 100% of Cr(VI) was reduced in less than 10h: 36.65mg/l in the first cycle; 40.17mg/l in the second and third cycles and 39.06mg/l in the fourth cycle. In the fifth cycle, 88.7% Cr(VI) was reduced in 8h (38.94–4.4mg/l). In fact, a period of 12h was necessary during this fifth cycle to achieve the decrease of Cr(VI) levels in agreement with the international limits for wastewater discharges. In addition, Cr(III) was undetected in all of the supernatants tested: total chromium concentrations were similar to the detected Cr(VI) concentrations. The abiotic control showed 27% of Cr(VI) removal in 8h. However, the totality of the Cr(VI) was recovered when the beads were washed with distilled water, indicating that abiotic reduction was negligible under these conditions.
Cr(VI) biotransformation cycles by entrapped cells in calcium alginate beads. Arrows indicate Cr(VI) solution renewal. Statistically, the Cr(VI) concentration values that were significantly different by the statistical analysis can be identified because they do not share the letters inserted in the right side of each point (a, b, c, d, e, f, g, h, i, j, k, l or m).
During this Cr(VI) biotransformation assay, the chromate reductase activity decayed with every cycle. This was observed by several authors: Ge et al. (2013)11 observed this activity loss when using free cells of Pseudochrobactrum sp. and Proteus sp. during 3 cycles of 20mg/l Cr(VI) reduction. Konovalova et al. (2003)16 used immobilized bacterial cells in agar to reduce Cr(VI) (20mg/l) during 6 cycles. Activity loss was attributed to the accumulation of toxic metabolites inside the immobilization matrix. Zhu et al. (2006)35 achieved the reduction of 7mmol/l Cr(VI) during 8 consecutive batches using free bacterial cells. The activity loss was produced because of the depletion of the electron donor. In this work no Cr(III) was found in the supernatant nor in any insoluble phase; therefore, it could be inferred that Cr(III) could be accumulated inside the beads or inside the cells, being this latter case the probable reason of the detected activity loss. Since the fate of the Cr(III) produced as a result of the reduction of Cr(VI) remained unknown, Cr(III) retention assays were carried out to determine if there would be an interaction with the immobilized cells or the matrix (calcium alginate beads). If that were the case, the obtained results would indicate that the totality of the Cr(III) produced during the five Cr(VI) reduction cycles would be retained in the 40g of beads used. Part of the Cr(III) could be absorbed by the bacterial surface or bioaccumulated and the remaining Cr(III) retained in the alginate structure.
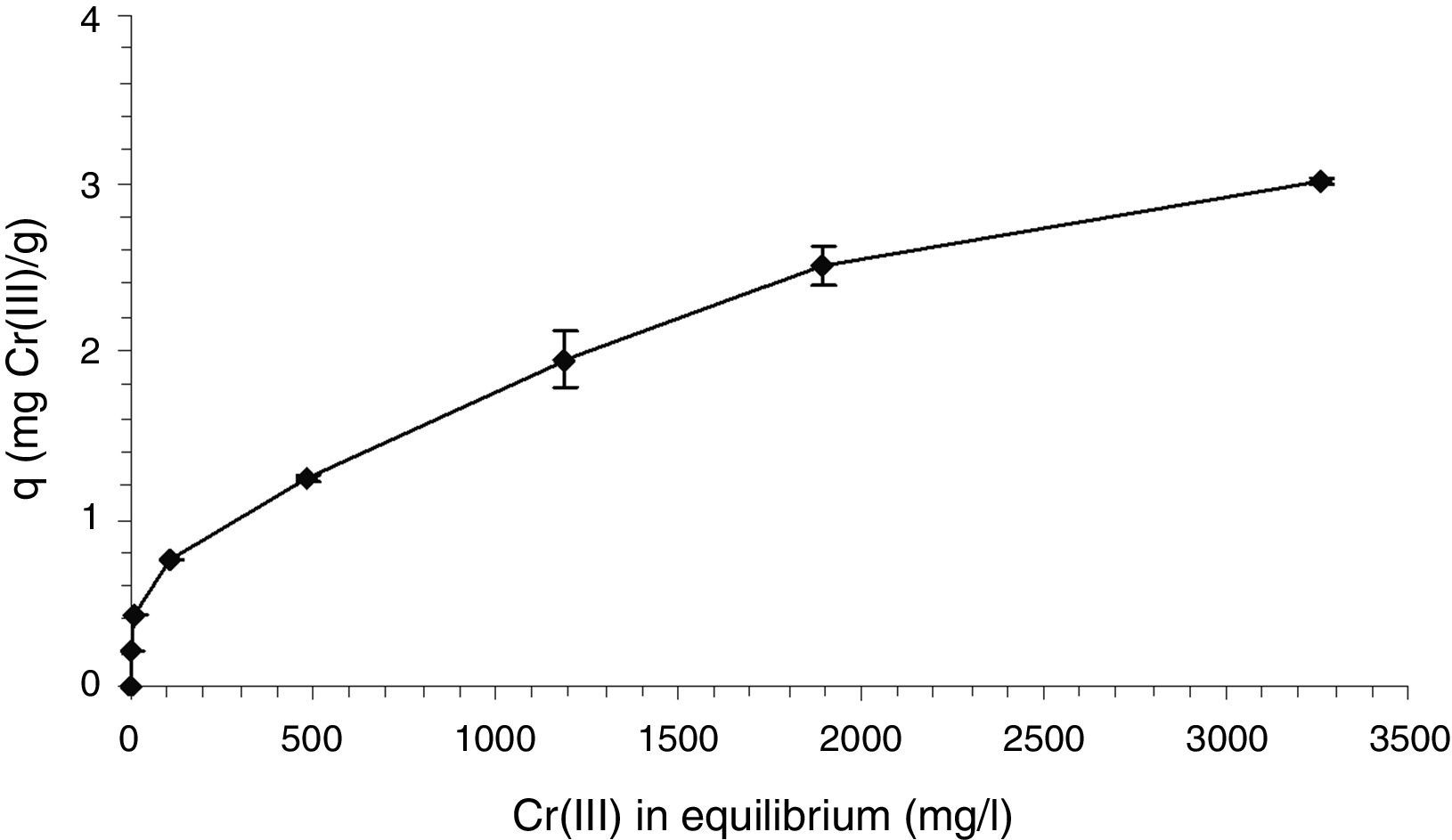
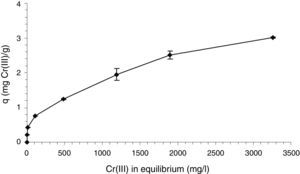
The Cr(III) retention capacity of the alginate beads with immobilized cells is shown as an isotherm in Figure 4. The maximum retention capacity (q) obtained in the assayed experimental conditions was about 3mg Cr(III)/g beads without showing saturation. With an initial Cr(III) concentration of 500mg/l, the percentage of Cr(III) retention of the alginate beads with immobilized cells was 98.3%, while the calcium alginate beads without bacteria presented a Cr(III) removal of 87.3%. There is a clear interaction between the matrix and Cr(III), directed by the carboxylate groups present in its chemical structure accompanied by the displacement of Ca2+, as reported in the literature7. This interaction is enhanced by the entrapped bacterial cells, which could contribute with a combination of biosorption and bioaccumulation phenomena. Several works reported higher Cr(III) retention capacities using different materials10,15, which resulted strictly dependent on pH. The acidic conditions used for Cr(III) retention experiments in this work were necessary to ensure Cr(III) availability and to avoid any complexation and precipitation interfering phenomena. Working at neutral pH as in the assayed biotreatment, an enhanced Cr(III) retention was expected within the matrix by the appearance of insoluble Cr(III) species extracted from the analysis of the corresponding speciation diagram.
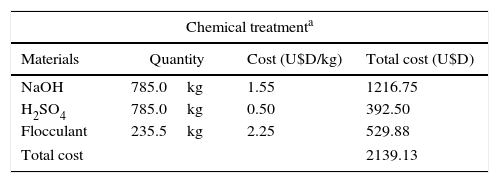
When the Cr(VI) concentration in a wastewater is below 1mg/l but higher than 0.1mg/l, a biological treatment results more environmentally friendly than the chemical treatment usually applied with sodium metabisulfite, sulfuric acid, sodium hydroxide and flocculants. Regarding the economic perspective, this proposed process developed with P. veronii 2E immobilized in calcium alginate beads would allow the treatment of 5m3 wastewater/h with a hydraulic retention time of 30min in a column of 2.5m3. For the preparation of the column, 6m3 of the bacterial suspension with 55 g biomass/l (prepared from an initial culture of 36 m3), 60 kg sodium alginate and 88.8 kg CaCl2 would be needed. Thus, the GBM should be prepared with: 360 l acidified glycerol, 216 l fertilizer and 7.8kg NaOH. For the treatment of 785m3 of a Cr(VI) loaded wastewater (the maximum volume than can be treated before renewing the biocatalyst), 7950l acidified glycerol (as electron donor) and 113kg NaOH would be required. The total cost of this biotreatment would be lower in comparison with the chemical treatment currently applied in electroplating industries in Argentina. Table 2 details the cost of only the NaOH, H2SO4 and flocculant needed for the chemical treatment of 785m3 of wastewater (Cr(VI)<1mg/l) and the cost of the materials needed for the biological treatment of the same wastewater, supporting the convenience of the implementation of the biological treatment proposed in this work.
Comparison of the cost of materials for chemical and biological treatments
| Chemical treatmenta | |||
|---|---|---|---|
| Materials | Quantity | Cost (U$D/kg) | Total cost (U$D) |
| NaOH | 785.0kg | 1.55 | 1216.75 |
| H2SO4 | 785.0kg | 0.50 | 392.50 |
| Flocculant | 235.5kg | 2.25 | 529.88 |
| Total cost | 2139.13 | ||
| Biological treatmenta | |||
|---|---|---|---|
| Materials | Quantity | Cost (U$D) | Total cost (U$D) |
| Acidified glycerol | 360.0l | 0.10/l | 36.00 |
| Acidified glycerol (electron donor) | 7950.0l | 0.10/l | 795.00 |
| Fertilizer | 216.0l | 0.40/l | 86.40 |
| NaOH | 120.8kg | 1.55/kg | 187.24 |
| Sodium alginate | 60.0kg | 11.00/kg | 660.00 |
| CaCl2 | 88.8kg | 0.2/kg | 17.76 |
| Total cost | 1782.40 | ||
The use of alternative economic growth media allows the sustainable production of microbial biomass. In this work, media with low cost components as commercial fertilizer as nitrogen source and molasses or acidified glycerol as carbon sources were developed. The best alternative medium composition was used to generate biomass, efficiently applied as a catalyst for the reduction of Cr(VI) to Cr(III). Thus produced, this catalyst could be useful for Cr(VI)-containing wastewater treatments. Therefore, an economic biomass production for the development of a whole cell catalyst is here proposed.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis work was supported by the del Consejo Nacional de Investigaciones Cientificas y Tecnologicas, Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), PICTO N°00086 and Universidad Nacional de General Sarmiento de Argentina.
Conflict of interestThe authors declare that there are no conflicts of interest.
This work was funded by the National University of General Sarmiento, National Council of Scientific and Technical Research (CONICET) and the National Agency for Scientific and Technological Promotion (ANPCyT), PICTO No. 00086. The authors thank Ms. Leticia Rossi for reviewing the English language.