Lactic acid bacteria are known for their biotechnological potential. In various regions of Ecuador numerous indigenous biological resources are largely undocumented. In this study, we evaluated the potential probiotic characteristics and antagonistic in vitro properties of some lactic acid bacteria from native niches of the subtropical rain forests of Ecuador. These isolates were identified according to their morphological properties, standard API50CH fermentation profile and RAPD-DNA polymorphism pattern. The selected isolates were further evaluated for their probiotic potential. The isolates grew at 15°C and 45°C, survived at a pH ranging from 2.5 to 4.5 in the presence of 0.3% bile (>90%) and grew under sodium chloride conditions. All selected isolates were sensitive to ampicillin, amoxicillin and cefuroxime and some showed resistance to gentamicin, kanamycin and tetracycline. Moreover, the agar well diffusion assay showed that the supernatant of each strain at pH 3.0 and pH 4.0, but not at pH 7.0 exhibited increased antimicrobial activity (inhibition zone >15mm) against two foodborne pathogens, Escherichia coli and Salmonella spp. To our knowledge, this is the first report describing the antagonistic activity against two foodborne pathogens and the probiotic in vitro potential of lactic acid bacteria isolated from native biota of Ecuador.
Las bacterias ácido lácticas (BAL) son conocidas por su potencial biotecnológico. En diversas regiones del Ecuador existen recursos biológicos nativos, que en su mayoría no han sido documentados. En este estudio se evaluaron in vitro las posibles características probióticas y antagónicas de algunas BAL aisladas de nichos nativos de la selva subtropical. Estas cepas se clasificaron en función de sus propiedades morfológicas, el perfil estándar de fermentación API50CH y los patrones de polimorfismo RAPD-ADN. Diez microorganismos fueron seleccionados y se probó su potencial probiótico. Todas las bacterias crecieron a 15°C y 45°C, sobrevivieron en el rango de pH ácido de 2,5 a 4,5 en presencia de 0,3% de bilis (> 90%), mostraron tolerancia a los tratamientos en cloruro de sodio y diferentes perfiles de sensibilidad a antibióticos. Todas las cepas fueron sensibles a ampicilina, amoxicilina y cefuroxima, y algunas fueron resistentes a gentamicina, kanamicina y tetraciclina. Por otra parte, el ensayo de pruebas de difusión en agar mostró que el sobrenadande de cada cepa cultivada a pH 3,0 y pH 4,0, pero no a pH 7,0, presentó elevada actividad antimicrobiana (zona de inhibición>15mm) frente a 2 agentes patógenos alimentarios, Escherichia coli y Salmonella spp. Este estudio describe por primera vez la actividad antagonista frente a patógenos de origen alimentario y el potencial probiótico in vitro de BAL aisladas de nichos ecológicos nativos del Ecuador.
Lactic acid bacteria (LAB) widespread in nature are among the most valuable microorganisms known for the production of thousands of fermented foods or for their favorable probiotic use. LABs are non-pathogenic bacteria, technologically suitable for industrial processes and their capacity to produce antimicrobial compounds makes them beneficial for health2,24,25. Obtaining genetically stable strains to be used in probiotic products has been a concern for researchers in the field. Despite their human origin, recent studies have described novel sources for isolating LAB with potential probiotic benefits, such as wild-type fruits and fermented vegetables4,9,29,32. However, numerous lactobacilli were found to be abundant in the pollen, suggesting their role in suppressing the growth of molds and other spoilage organisms18,31.
The insufficient viability and survival of the bacteria in commercial food products remains a problem to be investigated, since the probiotic characteristics are known to be species-specific5. Thus, the screening and the selection of novel probiotic strains with higher viability can be achieved.
In compliance with the new territorial redistribution of Ecuador (2008), undeveloped natural areas were included in the governmental policy as important resources to be exploited as reservoirs of unknown microorganisms that could become potential areas for biotechnological research, food sovereignty and security. Due to the importance of probiotics and the lack of information regarding the presence of LAB in the native micro flora, the exploitation of natural resources for the identification of the new potentially valuable probiotic strains becomes a priority. Therefore, the aim of this study is to identify new probiotic candidates in a collection of LABs isolated from native un-exploited biota as well as to investigate their potential antimicrobial activity in vitro.
Materials and methodsSamplingSamples consisted of subtropical rain forest fruits (wild Citrus sinensis, immature and mature berries of Rubus sp., Psidium guajava, Fragaria vesca, Bactris gasipaes) and flower inflorescences (Heliconia sp., Fucsia sp., Bromelia sp.) collected aseptically from a subtropical humid mesothermal region of Santo Domingo de Los Tsachilas Province, 43km away from Quito, the capital city. Samples were packaged in clean bags, then stored at 4°C for further analysis.
Screening, isolation and phenotypic characterizationApproximately ten grams of each sample were transferred into Erlenmeyer flasks (500ml) containing sterile water (100ml) and incubated statically for up to 5 days at room temperature. MRS11 agar plates were used for the inoculation and the samples were incubated under anaerobic conditions at 37°C for 72h and isolated individual colonies were randomly selected and purified by replating in the same medium. The purified colonies (>100 colonies/each sample) were Gram stained, tested for mobility, indole-, catalase-production, spore formation and gas production from glucose. Cell morphology and colony characteristics on MRS agar were examined and based on these results the colonies were preliminary classified as: (1) presumptive lactococci, gram-positive, having coccal morphology, catalase-negative and non-motile, positive for gas production from glucose, and (2) gram-positive, with morphological aspect of rods, catalase-negative, non-motile, positive or negative for gas production from glucose, presumptive lactobacilli, stored at (−) 80°C in 20% glycerol. Moreover, ten isolates were selected to evaluate their probiotic properties. Lactobacillus fermentum CNCM 1-2998 (API50CH, 80% identity) recovered from an available commercial probiotic, Lacteol Fort (Lactobacillus LB, Axcan Pharma, France) was used as reference8.
Metabolic API50CH and RAPD electrophoretic band profilesThe API50CH strips (Biomerieux, Marcy l’Etoile France, cat # 50300) were used according to the manufacturer's instructions. Briefly, the isolates were cultured overnight in MRS-agar and a 2 McFarland turbidity inoculum suspension was used to fill de ampoules of API strips. The change in color of each well was evaluated after 24 and 48h incubation at 37°C and the results were generated using the Biomerieux ApiwebV.5.1 web system. Distinctive RAPD fingerprints were generated by the amplification of genomic DNA (PureLink™ Genomic DNA minikit, #K1820-00, Invitrogen) of the LAB isolates with arbitrary 10-mer oligonucleotide primers (Roth, Karlsruhe, Germany). Two random oligo sets (013A, kit HP22.1 and 08B, HP23.1 respectively) were tested33. The reactions were performed in a Termocycler device (MultiGene, Labnet International Inc.) with a Taq Platinum DNA Kit (Invitrogen) in a total volume of 50μl consisting of 1X Taq Polymerase buffer, 10mM MgCl2, a 200mM concentration of each dNTPs, 1mM concentration of each primer, 50ng of bacterial DNA, and 1U of Taq Platinum DNA polymerase. The amplification profile was as follows: 1 cycle of 10min at 94°C; 40 cycles of 45s at 94°C, 45s at 36°C, and 2min at 72°C; and 1 cycle of 5min at 74°C. RAPD amplification products were electrophoresed in 1.5% agarose gel in TBE (Tris–Borate–EDTA) buffer at 100V for 1.5h, using a 100 base-pair ladder as a fragment size marker (Invitrogen) and visualized by SYBR green staining. Each sample was repeated twice in a separate amplification reaction. The polymorphic bands were analyzed using SPSS software to calculate genetic diversity among the isolates and the dendrogram was plotted using UPGMA method19.
Survival under acidic and bile conditionsSurvival was determined by using 7log CFU/ml of the overnight culture of each of the selected LABs by the plate-agar method16. Briefly, after incubation, the bacterial cells were centrifuged at 5000×g for 5min at 4°C, the biomass was rinsed twice with sterile 1X PBS (Phosphate-Buffered Saline, pH 7.2) solution and resuspended in PBS with a pH of 2.5, 3.0, 3.5, 4.0 and 4.5 and incubated from 1 to 3h at 37°C. After each hour of incubation, 100μl of the cell culture were plated on MRS agar and incubated for 24h and the viable bacteria were counted. In the case of bile, the cells were incubated in MRS containing 0.3% bile at 37°C for 4h and the growth was monitored at OD600 an the percentage of resistance was determined as: (increment of OD600 of each isolate in MRS broth with bile/increment of OD600 in MRS broth without bile)×100 and relative to reference strain23. Strains showing more than 50% percentage resistance were considered as bile-resistant. Moreover, we determined the survival in bile 0.3% by plating 100μl bacterial cells on MRS agar. Not modified MRS was used as control and the experiment was run in triplicates starting from different batches of culture.
Optimum temperature and growth tolerance in the presence of sodium chlorideOvernight cultures (7log CFU/ml) of each isolate were inoculated in tubes containing MRS and incubated at 15°C and 45°C for 24h and the absorbance at 600 nm (Nova60, Millipore, Merck) was measured. To evaluate the tolerance in the presence of sodium chloride, the overnight culture of LAB was inoculated in MRS containing 2%, 4%, and 6% sodium chloride for 24h after incubation at 15°C and 45°C. Cell growth was monitored for each treatment and the effect of sodium chloride on cell survival was determined using the plate-agar method16. Not modified MRS was used as control and the experiment was run in triplicate starting from individual batches of bacterial culture.
Antibiotic susceptibilitySusceptibility to several antibiotics was determined using commercial disks of Ampicillin (10μg), Gentamicin (10μg), Kanamycin (30μg), Amoxicillin/Clavulanic Acid (20/10μg), Tetracycline (30μg), Cefuroxime (30μg) at the concentrations recommended by the Scientific Committee on Animal Nutrition (disks provided by Merck) by the disk diffusion assay. Freshly grown bacterial colonies (7log CFU/ml) were streaked on MRS agar plates to form a growth lawn and the antibiotic disks were placed on the streaked plates at appropriate distances and incubated for 48h at 37°C. After incubation the clear zone formed by each antibiotic was measured at different intervals of incubation time (18–24–36–48h). The inhibitory effect was expressed in millimeters of the inhibition zone diameter20. The experiment was run in triplicates starting with different batches of bacteria culture and the disks were verified by Escherichia coli ATCC 25922, a reference strain for quality control. Using a similar approach, the minimum inhibitory concentration (MIC) distribution within lactobacilli group for ampicillin, gentamicin and tetracycline were measured using the E-test (Biomerieux, E-testR) assay following the manufacturer's instructions. The culture conditions were identical to those in the disk diffusion assay. The microbiological breakpoints reported by the FEEDAP document15 were used to categorize lactobacilli as susceptible or resistant. The strains showing a MIC higher than the EFSA breakpoint15 were considered resistant.
Antimicrobial activity assayAntimicrobial activity was performed using the agar well diffusion method under anaerobic conditions16. Both foodborne pathogens E. coli O157 and Salmonella Typhimurium were previously isolated from fresh cheese and meat purchased from the local market using conventional techniques21,22. The LAB isolates were grown in MRS broth at 37°C for 16h and the supernatants were collected by centrifugation at 13000×g for 20min were adjusted to pH 3.0, 4.0 and 7.0 and sterilized by using 0.22μm filter. The indicator strains (100μl) grown in broth medium (7log CFU/ml) were mixed with 1.5ml of soft MRS agar (0.75%) and were overlaid on the nutrient agar plates and incubated at 37°C for 2h. The supernatant (100μl) was spotted onto the wells (7mm) on overlaid agar, incubated at 37°C and subsequently examined for inhibition zones at different intervals of time (18–24–36–48h). The experiments were run in triplicate, the mean values of the inhibition zones were estimated and we considered that the isolates showed higher inhibitory activity when the diameter of the inhibition halo was >15mm and lower inhibitory activity when the diameter was lower than 7mm.
Statistical analysisThe means were calculated from repeated measurements performed three times. The statistical analysis was carried out by one-way analysis of variance (ANOVA), and the Tukey's post hoc test was used to determine significant differences between the means. The statistical significance considered was p<0.05 (SPSS version 10.0.6, USA).
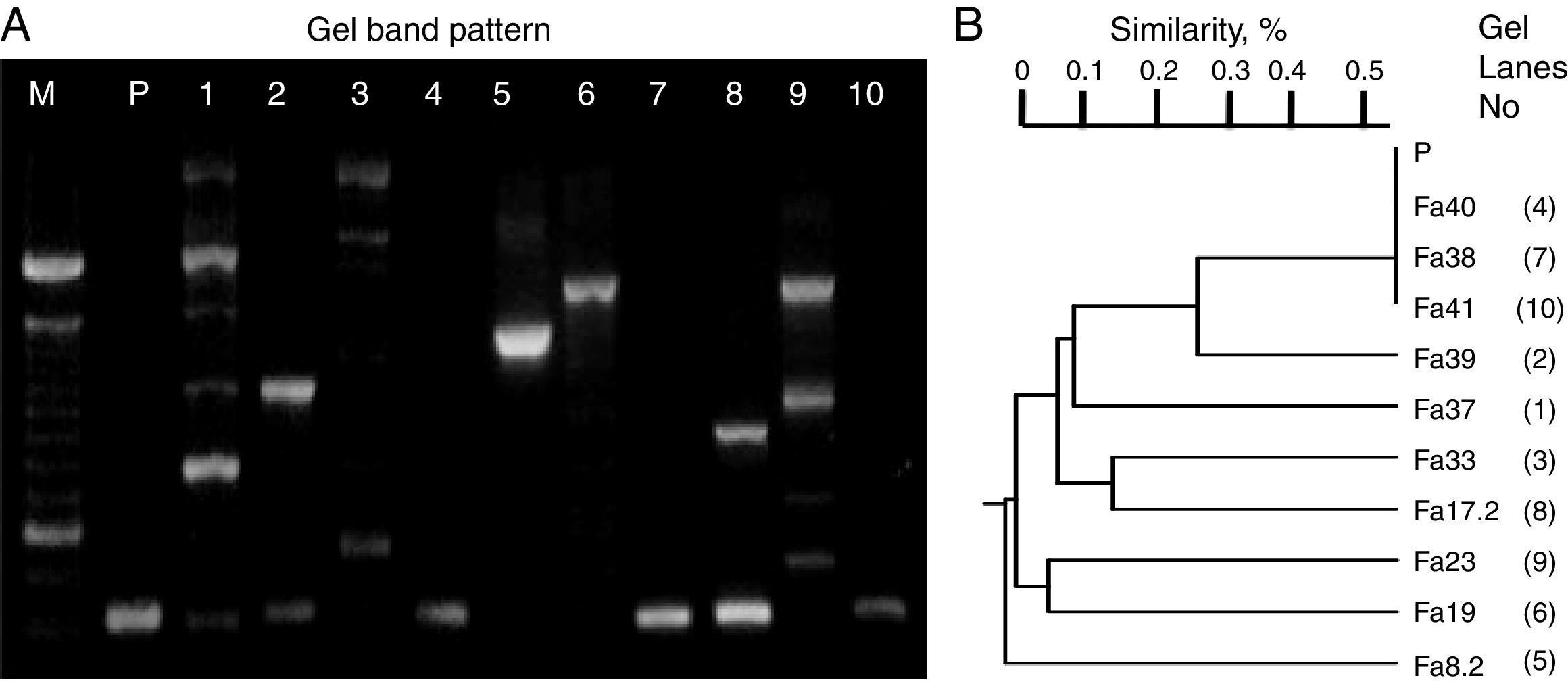
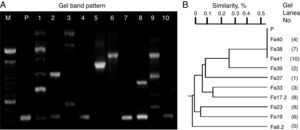
ResultsScreening identification of individual LAB isolatesIn this study, the preliminary phenotypic analysis suggested the relatedness of the bacterial isolates from wild-type fruits and mature inflorescences of several tropical flowers (>100 colonies/sample) with LAB, which were affiliated to Lactococcus (54%) and Lactobacilli (46%) groups. Moreover, carbohydrate and polymorphic profiles were conducted on ten randomly selected isolates related to each type of biological material (sample of origin). Thus, the isolates assigned UTNFa38, UTNFa40 and UTNFa41 were identified as Lactococcus lactis subsp. lactis, with 90–99% identity, the isolate UTNFa37, as Lactobacillus collinoides (99%), UTNFa39, as Lactobacillus brevis 3 with 98% identity, while UTNFa19, UTNFa23 and were identified as Lactobacillus paracasei subsp. paracasei 1 with 99.7% and 98.2% identity, respectively. The isolates UTNFa33 and UTNFa17.2 were identified as L. paracasei subsp. paracasei 3 with 99.6% and 97.9% identity and UTNFa 8.2 was identified as Lactobacillus pentosus with 98.3% identity. RAPD electrophoretic bands profiles (Fig. 1A) showed a clearly distinct and typical patter of the isolates, which were clustered according to their corresponding molecular weight. Both metabolic and DNA profiles showed the formation of five distinct groups (Fig. 1B). Although the carbohydrate profile of the control strain was distinct, the polymorphic band profile clustered with the Lactococcus group.
RAPD-DNA electroforesis band pattern (A) and clustering (B) according with the UPGMA analysis based on Jaccard similarity index. M – molecular marker (100bp, Invitrogen), P – L. fermentum CNCM 1-2998, 1-Fa37, 2-Fa39, 3-Fa33, 4-Fa40, 5-Fa8.2, 6-Fa19, 7-Fa38, 8-Fa17.2, 9-Fa23, 10-Fa41.
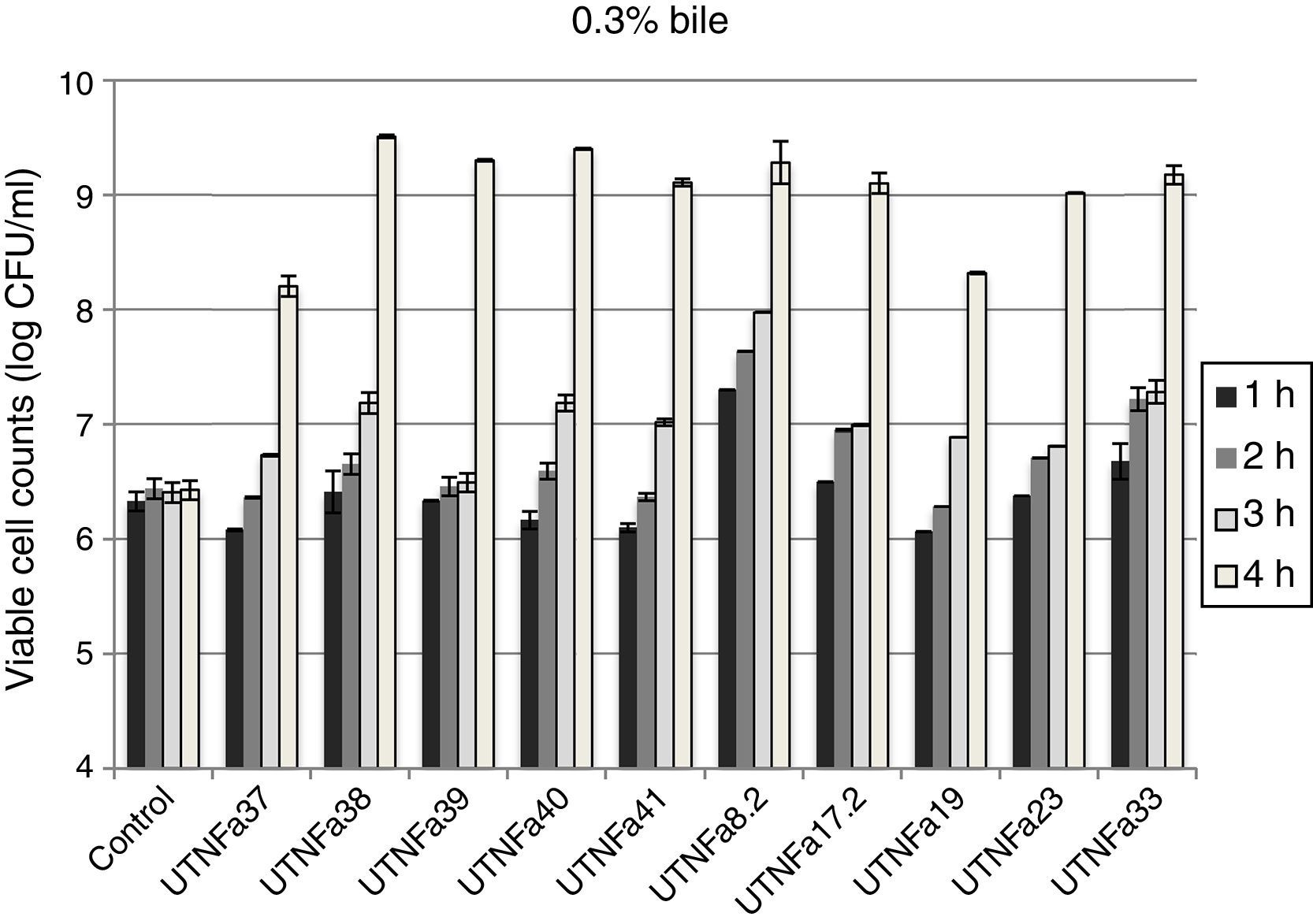
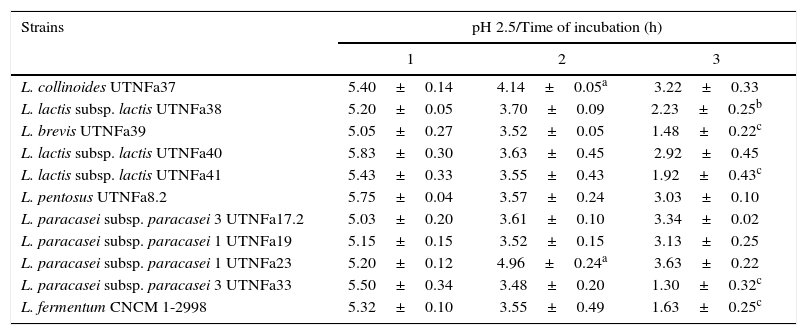
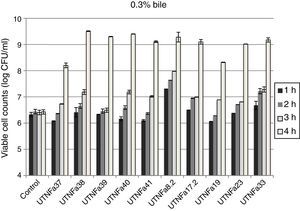
All LAB isolates were highly tolerant to acidic conditions during 3h of incubation. The selected LAB could withstand exposure to pH 3.0, 3.5, 4.0 and 4.5 conditions superior to pH 2.5. However, at pH 2.5, no significant difference in the viable cells was recorded after 1h of incubation, while after 3h of incubation, seven strains remained highly acid tolerant, although a decrease in the cell number was recorded (Table 1). The most acid-tolerant strains were UTNFa37, UTNFa8.2, UTNFa19, UTNFa23 and UTNFa17.2, while a significant loss in the viable cells was observed for isolates UTNFa39, UTNFa41, UTNFa33 as well as the reference strains (p<0.05) (Table 1). At the other pH tested, no significant decrease in viability was recorded and the mean values varied among the groups from 6.57 (±0.01) and 6.70 (±0.12) log CFU/ml at pH 3.0 and pH 3.5, respectively and 6.88 (±0.72) and 6.95 (±0. 25) log CFU/ml at pH 4.0, and pH 4.5, respectively (data not shown). All new selected strains exhibited high tolerance to bile after 4h incubation at 37°C (90% resistance). Although bile had influenced viability to some extent of all the strains tested, the number of viable cells of commercial probiotics remained constant after 1–4h of incubation. With respect to new selected strains, we recorded a significant increase in viable cell counts after 4h of incubation. In Figure 2, we showed the mean of viable cells counts determined at each hour of incubation.
The survival of LAB strains at the pH 2.5
| Strains | pH 2.5/Time of incubation (h) | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| L. collinoides UTNFa37 | 5.40±0.14 | 4.14±0.05a | 3.22±0.33 |
| L. lactis subsp. lactis UTNFa38 | 5.20±0.05 | 3.70±0.09 | 2.23±0.25b |
| L. brevis UTNFa39 | 5.05±0.27 | 3.52±0.05 | 1.48±0.22c |
| L. lactis subsp. lactis UTNFa40 | 5.83±0.30 | 3.63±0.45 | 2.92±0.45 |
| L. lactis subsp. lactis UTNFa41 | 5.43±0.33 | 3.55±0.43 | 1.92±0.43c |
| L. pentosus UTNFa8.2 | 5.75±0.04 | 3.57±0.24 | 3.03±0.10 |
| L. paracasei subsp. paracasei 3 UTNFa17.2 | 5.03±0.20 | 3.61±0.10 | 3.34±0.02 |
| L. paracasei subsp. paracasei 1 UTNFa19 | 5.15±0.15 | 3.52±0.15 | 3.13±0.25 |
| L. paracasei subsp. paracasei 1 UTNFa23 | 5.20±0.12 | 4.96±0.24a | 3.63±0.22 |
| L. paracasei subsp. paracasei 3 UTNFa33 | 5.50±0.34 | 3.48±0.20 | 1.30±0.32c |
| L. fermentum CNCM 1-2998 | 5.32±0.10 | 3.55±0.49 | 1.63±0.25c |
Results are means of 3 measurements±standard error of the mean.
a,b,c means within the column followed by different subscripts are significantly different p<0.05.
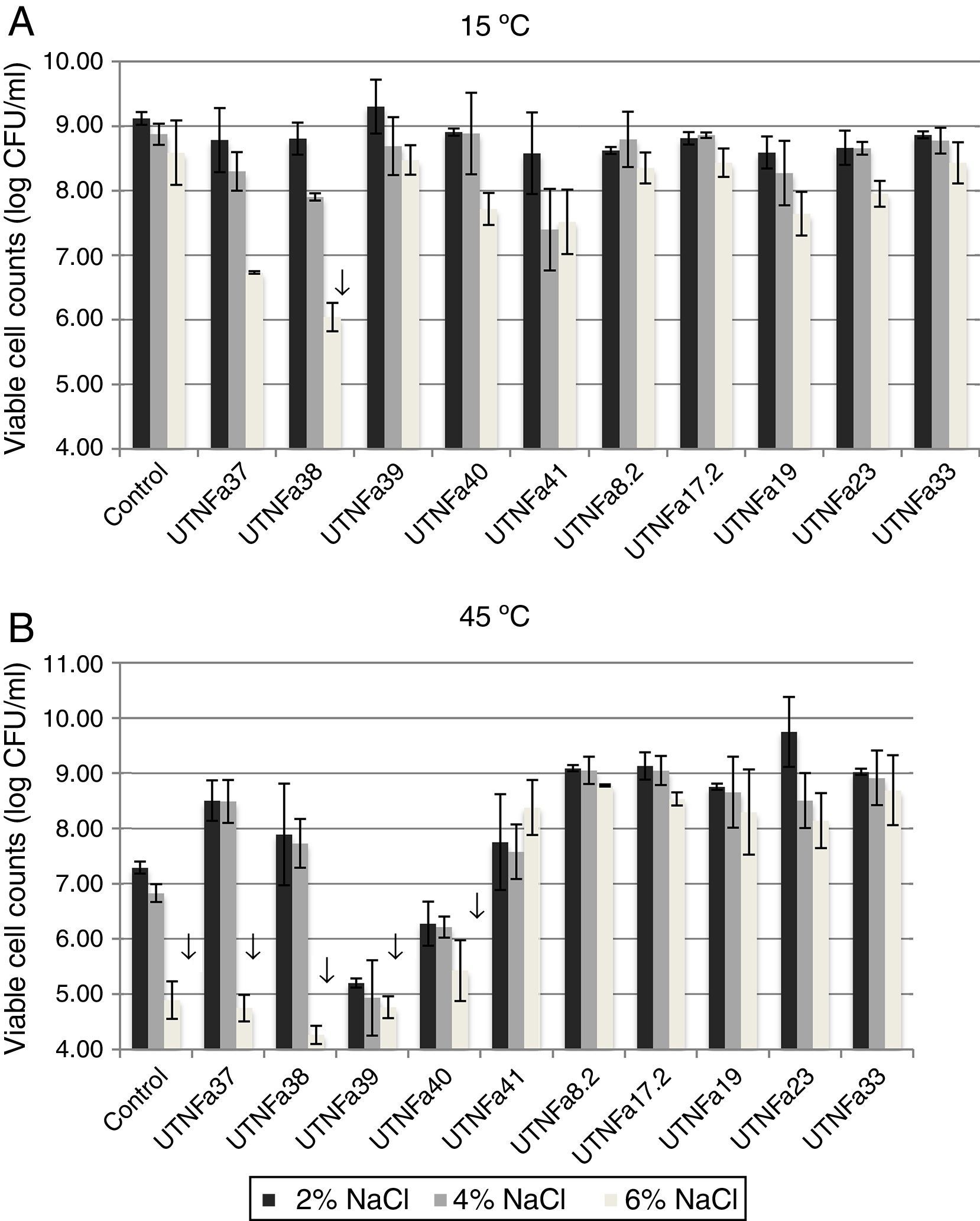
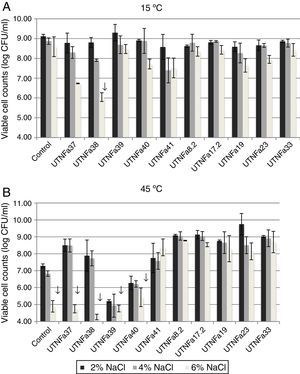
All selected isolates grew at temperatures of 15°C and 45°C and exhibited greater tolerance to sodium chloride. At 15°C and treatment with 2%, 4% and 6% NaCl, no significant differences among the isolates were recorded (Fig. 3A). A significant decrease in viable cell counts was observed for isolate UTNFa38 at 6% NaCl (p<0.05). However, the changes in viable cells were detected at 6% NaCl at both temperatures tested, although this was statistically significant at 45°C for UTNFa37, UTNFa38, UTNFa39, UTNFa40 strains as well as for the control strain (p<0.05) (Fig. 3B). The results suggested that the growth of 50% of the strains was neither influenced by the temperature nor by the percentage of NaCl added to the medium. Approximately half of the strains had a capacity to grow at higher concentrations of sodium chloride (6%) and at a temperature of 45°C. In two isolates, the growth was influenced by a higher temperature and all the sodium chloride concentrations (UTNFa39 and UTNFa40), while in three strains (UTNFa37, UTNFa38 and the control strain), the growth was only influenced by a higher sodium chloride concentration (Fig. 3B).
The effect of temperature and NaCl on growth of LAB isolates. (A) Viability at 15°C and 2%, 4% and 6% of NaCl. (B) Viability at 45°C and 2%, 4% and 6% of NaCl. Bars are means±standard error of the mean, bars with ↓ are statistically significantly different, p<0.05 according with Tukey. Control: L. fermentum CNCM 1-2998.
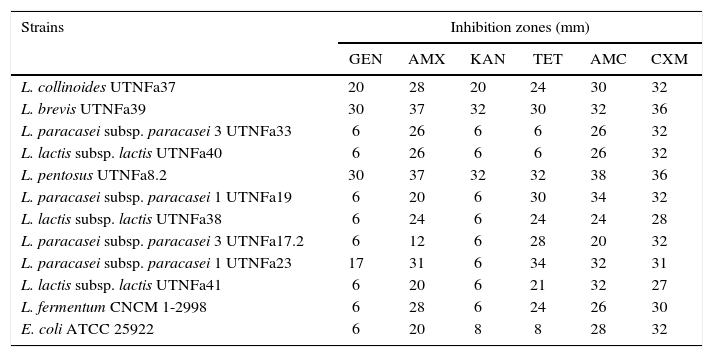
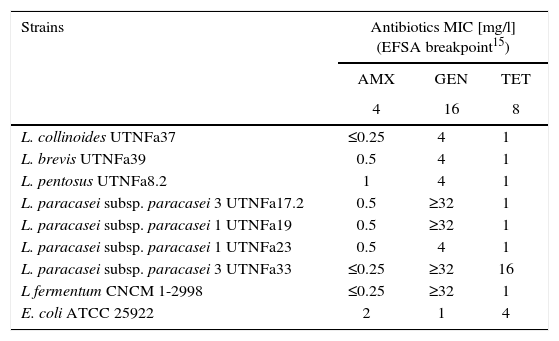
The LAB antibiotic susceptibility is shown in Table 2. Among the antibiotics, ampicillin, cefuroxime and amoxicillin/clavulanic acid did not show an inhibitory effect on any of the isolates tested, whereas gentamicin inhibited the growth of UTNFa33, UTNFa40, UTNFa19, UTNFa38, UTNFa17.2, UTNFa41 and tetracycline inhibited the growth of UTNFa33 and UTNFa40. The aminoglycoside antibiotic kanamycin inhibited the growth of UTNFa33, UTNFa40, UTNFa19, UTNFa38, UTNFa17.2, UTNFa23 and UTNFa41. The MIC value distribution for the tested ampicillin, gentamicin and tetracycline in the lactobacilli group is shown in Table 3. In the case of ampicillin, when the MIC breakpoint was ≥4mg/l, all lactobacilli strains were sensitive, while in the case of gentamicin, when the MIC breakpoint was ≥16mg/l, the L. paracasei strains (UTNFa17.2, UTNFa19, UTNFa33) and the probiotic control were resistant. In the case of tetracycline, only the UTNFa33 strain showed resistance (MIC ≥16mg/l).
Antibiotic susceptibility of the selected LAB strains
| Strains | Inhibition zones (mm) | |||||
|---|---|---|---|---|---|---|
| GEN | AMX | KAN | TET | AMC | CXM | |
| L. collinoides UTNFa37 | 20 | 28 | 20 | 24 | 30 | 32 |
| L. brevis UTNFa39 | 30 | 37 | 32 | 30 | 32 | 36 |
| L. paracasei subsp. paracasei 3 UTNFa33 | 6 | 26 | 6 | 6 | 26 | 32 |
| L. lactis subsp. lactis UTNFa40 | 6 | 26 | 6 | 6 | 26 | 32 |
| L. pentosus UTNFa8.2 | 30 | 37 | 32 | 32 | 38 | 36 |
| L. paracasei subsp. paracasei 1 UTNFa19 | 6 | 20 | 6 | 30 | 34 | 32 |
| L. lactis subsp. lactis UTNFa38 | 6 | 24 | 6 | 24 | 24 | 28 |
| L. paracasei subsp. paracasei 3 UTNFa17.2 | 6 | 12 | 6 | 28 | 20 | 32 |
| L. paracasei subsp. paracasei 1 UTNFa23 | 17 | 31 | 6 | 34 | 32 | 31 |
| L. lactis subsp. lactis UTNFa41 | 6 | 20 | 6 | 21 | 32 | 27 |
| L. fermentum CNCM 1-2998 | 6 | 28 | 6 | 24 | 26 | 30 |
| E. coli ATCC 25922 | 6 | 20 | 8 | 8 | 28 | 32 |
The data represents the diameter of the halo of inhibition determined from three measurements after disk diffusion method. Disk diameter: 6mm. GEN – Gentamycin; AMX – Ampicillin; KAN – Kanamycin; TET – Tetracycline; AMC – Amoxicillin/Clavulanic acid; CXM – Cefuroxime.
MIC distribution (mg/l) of antimicrobial agents for the Lactobacillus group
| Strains | Antibiotics MIC [mg/l] (EFSA breakpoint15) | ||
|---|---|---|---|
| AMX | GEN | TET | |
| 4 | 16 | 8 | |
| L. collinoides UTNFa37 | ≤0.25 | 4 | 1 |
| L. brevis UTNFa39 | 0.5 | 4 | 1 |
| L. pentosus UTNFa8.2 | 1 | 4 | 1 |
| L. paracasei subsp. paracasei 3 UTNFa17.2 | 0.5 | ≥32 | 1 |
| L. paracasei subsp. paracasei 1 UTNFa19 | 0.5 | ≥32 | 1 |
| L. paracasei subsp. paracasei 1 UTNFa23 | 0.5 | 4 | 1 |
| L. paracasei subsp. paracasei 3 UTNFa33 | ≤0.25 | ≥32 | 16 |
| L fermentum CNCM 1-2998 | ≤0.25 | ≥32 | 1 |
| E. coli ATCC 25922 | 2 | 1 | 4 |
AMX – ampicillin; GEN – gentamycin; TET – tetracycline.
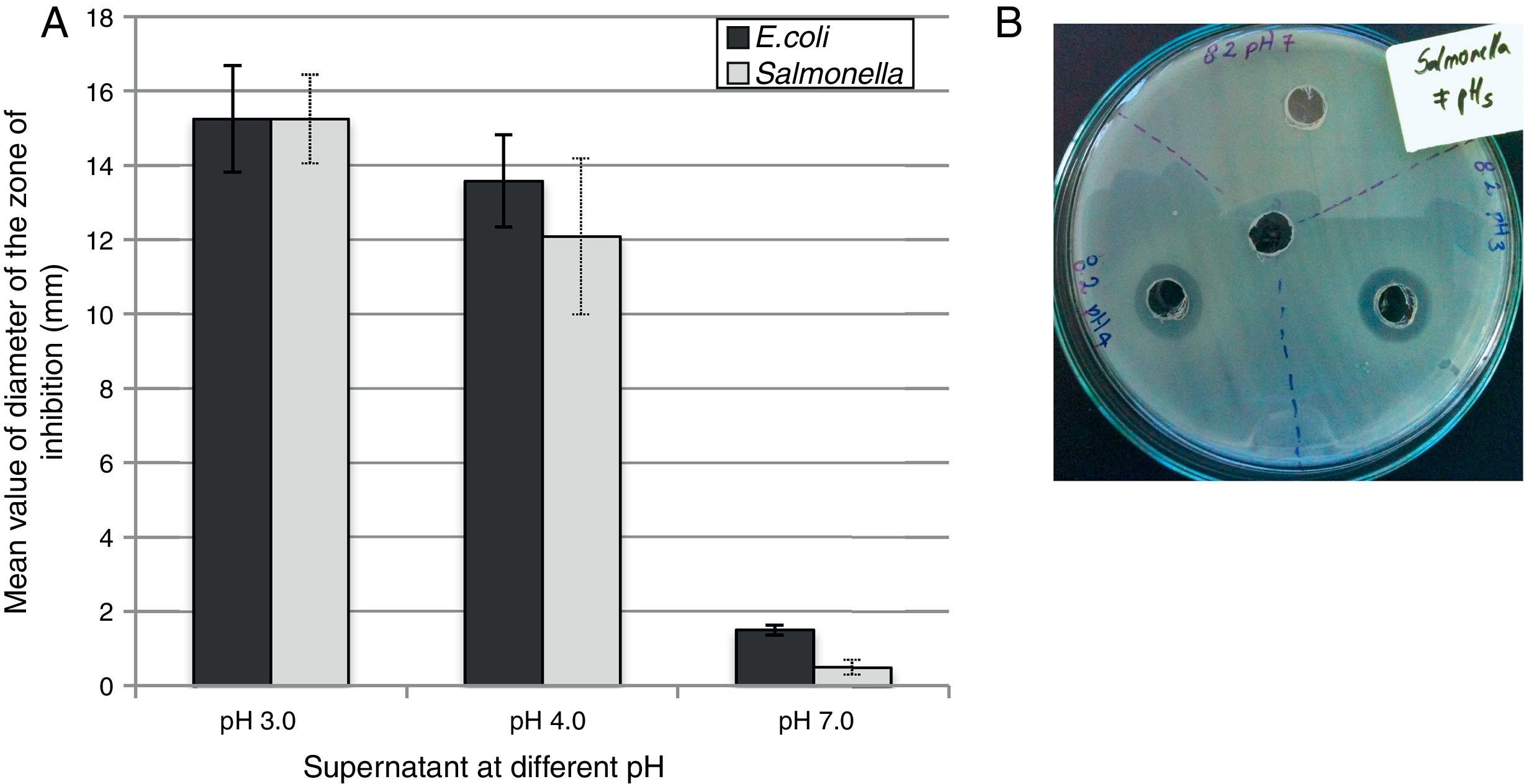
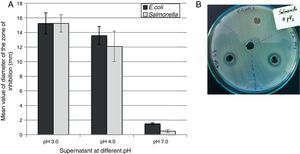
The selected isolates showed inhibitory activity against both foodborne pathogens. An elevated antimicrobial activity was observed when the pH of the supernatant was highly acid (pH 3.0) with a mean value of inhibition zone of 15.25mm (±0.5) against both pathogens (Fig. 4A). At pH 4.0, no significant difference were recorded for any of the samples tested against both pathogens, although the mean value of the inhibition zone diameter was 13.58mm (±1.24) for E. coli and 12.09mm (±2.04) for Salmonella Typhimurium after 48h of incubation. At pH 7.0 no inhibitory activity was recorded (the inhibition zone diameter was about 1–2mm). Figure 4B shows an example of a visualized inhibition zone formed at pH 3.0 and 4.0 but not at pH 7.0 of the UTNFa8.2 strain.
DiscussionDespite the numerous probiotic strains currently in the market, there is an ongoing need for the improvement of the LAB strains to be used as starter cultures; therefore, the LAB isolated from their natural environment (e.g. native fruits, flowers) might possess unusual characteristics including phenotypic differences and intraspecific variability compared to the well-known ones. In this study, we assumed the presence of acid tolerant bacteria as the fermentation of raw material overtakes at a pH of about 3.5. However, the LAB screening demonstrated the presence of Lactococcus (54%), predominantly found in immature fruits and flower inflorescences and Lactobacillus (46%) species most frequently found in mature fruits of subtropical natives niches of Ecuador. Based on their metabolic profile, ten selected strains were identified as L. lactis subsp. lactis (3 strains), L. collinoides (1 strain), L. brevis (1 strain), L. paracasei subsp. paracasei 1 (2 strains), L. paracasei subsp. paracasei 3 (2 strains) and L. pentosus (1 strain). In a similar way to our results, numerous lactobacilli species (i.e. L. paracasei, L. pentosus) were identified in different fruits and vegetables31. Moreover, the similarity among the isolates calculated according to the electrophoretic bands was plotted as a dendrogram showing five distinct groups. These results correlate with the cluster group of metabolic profile and were in agreement with early studies30,31.
It has been stated that a probiotic strain must present distinct characteristics such as, it should survive passage through the upper gastrointestinal tract, tolerate gastric acidity and bile toxicity, and be able to grow at different ranges of temperature12,24,34. In this study the selected LAB isolates grew at 15°C and 45°C, and half of them exhibited high tolerance to acidic conditions (pH 2.5) after 3h of incubation.
Our results indicated that the new selected strains exhibited high tolerance to bile in comparison with commercial probiotics. In agreement with the early data appointed by Bevilacqua et al.7 we observed that the lactobacilli continued to grow in a bile-containing medium 8h after inoculation, meaning that the bile might stimulate the growth of the new isolated strains. The results of the survival study demonstrated that bile stress did not have an inhibitory effect on the selected strains, while the commercial probiotics were less tolerant. These results were in agreement with those of Succi et al.34 Although the sampled material consisted of fruits having a pH of 3.5–6.0 and variable sugar content, it appears that the acid and bile tolerance is rather species-specific and might be influenced by the origin of the samples. Interestingly, strain UTNFa33 isolated from mature berries, exhibited higher tolerance to both bile and acid, suggesting that this strain might be able to survive the gastro-intestinal passage in vivo.
In the current study, all isolates tolerated different concentrations of sodium chloride (2%, 4% and 6%) at different incubation temperatures. The isolates that grew up to a concentration of 4% NaCl were considered to have the principal characteristics of a starter culture strain without being necessary for them to withstand higher sodium chloride levels. Nonetheless, some isolates were tolerant to 6% NaCl at all temperatures tested. Our data were in agreement with previous studies showing that sodium chloride tolerance might be strain-dependent7.
Antibiotic tolerance is considered another essential characteristic for in vitro selection of LAB to be used as health-promoting probiotic ingredients in food and pharmaceutical preparations10. The antibiotic tolerance might be advantageous to maintain the natural balance of intestinal microflora while administrating antibiotics6,13,20. However, each LAB strain exhibited a particular antibiotic susceptibility profile, with all isolates being ampicillin-susceptible and some isolates gentamycin and tetracycline-resistant. The antimicrobial effect exerted by the LAB strains is related to the production of lactic acid, the pH reduction, and the inhibitory compound1,3,26 and has recently attracted much attention and was attributed as an important selection criterion for probiotic microorganisms14,17,28,35. In the present study, the supernatant of all ten selected isolates at the acidic condition of pH 3.0 and 4.0 showed elevated antagonistic activity against two foodborne pathogens often present in the local food market, while at pH 7.0 no activity was recorded, suggesting that the antimicrobial activity might be less effective under basic conditions. Overall all selected LAB isolates inhibited the growth of both pathogens; however, the efficiency and nature of this antimicrobial activity have to be investigated. Recently, the role of bacteriocin produced by L. pentosus strain ST712BZ isolated from boza in the preservation of beverage products has been shown36. In other investigation, another bile-resistant strain of L. pentosus displaying bacteriocin activity against a wide range of spoilage and pathogen bacteria was isolated27. Similarly, we showed that isolate UTNFa8.2 assigned as L. pentosus displayed elevated inhibitory activity as well as bile resistance, allowing to further explore its biotechnological properties.
To date this is the first report describing the presence of LAB in the unexploited native ecological niches of Ecuador. Taken together the results of the in vitro study indicated that the novel LAB isolates had distinct advantageous probiotic characteristics. Among them, L. penstosus UTNFa8.2, L. paracasei subsp. paracasei 1 UTNFa23, and L. paracasei subsp. paracasei 3 UTNFa17.2 exhibited desirable features demonstrated by their capacity to tolerate bile at physiological concentration and acidic conditions, tolerance to sodium chloride, strong antimicrobial activity against foodborne pathogens, and unique antibiotic profiles, hence, they could be further exploited for their validation from an industrial perspective. A further functional characterization would help us gain better knowledge for the improvement of current commercial probiotic strains and to exploit these autochthonous bacteria for other probiotic bioactivities that would result in food industry benefits.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis work was supported by the Prometeo Project of the Secretary for Higher Education, Science, Technology and Innovation (SENESCYT) and Technical University of the North (Grant No. 01388) of the Republic of Ecuador.
Conflicts of interestThe authors declare that they have no conflicts of interest.
GNT was sponsored by the Prometeo Project of SENESCYT. We are grateful to Dr. Miguel Naranjo Toro for technical support. We thank to Oscar Rosales for helping us with the samples mapping.