The aim of this work is to study the oregano essential oil (OEO) composition from Northwestern Argentinean regions and to evaluate its effect on the lactic starter cultures. The oregano used, Origanum vulgare var hirtum, was obtained from Andalgalá, Catamarca. The essential oil presented high amounts of α-terpinene (10%), γ-terpinene (15.1%), terpinen-4-ol (15.5%) and thymol (13.0%) as the main components. No negative effect on growth or metabolic activity of lactic acid bacteria Streptococcus thermophilus CRL 728 and CRL 813, Lactobacillus delbrueckii subsp. bulgaricus CRL 656 and CRL 468, and Lactococcus lactis subsp. lactis CRL 597 up to the maximum concentration (200μg/g) assayed was observed. No differences in the organoleptic characteristics of semi-hard cheeses flavored with oregano essential oil (200μg/g) and homemade cheeses flavored with oregano leaves were found. With respect to the microbiological quality of the products, neither enterobacteria nor mold and yeast were detected during ripening in essential-oil flavored cheese compared to control cheese (enterobacteria 2×103UFC/g) and cheese flavored with oregano leaves (mold/yeast 4×104CFU/g). Our results showed that the use of oregano essential oil and lactic starter culture considerably improved cheese quality.
El objetivo de este trabajo fue estudiar la composición del aceite esencial de orégano recolectado en el noroeste argentino y evaluar su efecto sobre algunos fermentos lácticos. El orégano recolectado correspondió a la especie Origanum vulgare var. hirtum proveniente de Andalgalá, Catamarca. En su aceite esencial (obtenido por arrastre con vapor de agua) se detectó principalmente α-terpineno (10%), γ-terpineno (15,1%), terpinen-4-ol (15,5%) y timol (13,0%). El aceite esencial no tuvo efecto inhibitorio (máxima concentración ensayada 200μg/g) sobre el crecimiento ni sobre la actividad metabólica de Streptococcus thermophilus CRL 728 y CRL 813, de Lactobacillus delbrueckii subsp. bulgaricus CRL 656 y CRL 468, y de Lactococcus lactis subsp. lactis CRL 597. No se observaron diferencias en las características organolépticas de quesos semiduros aromatizados con el aceite esencial (200μg/g) comparados con quesos artesanales aromatizados con hojas de orégano. Respecto de la calidad microbiológica de los productos, no se detectaron enterobacterias ni hongos o levaduras durante la maduración en los quesos aromatizados con el aceite esencial de orégano comparados con los quesos control, que presentaron desarrollo de enterobacterias (2×103UFC/g), y con los quesos elaborados con hojas de orégano, en los que hubo desarrollo de hongos/levaduras (4×104CFU/g). Los resultados obtenidos demostraron que el uso del aceite esencial de orégano y del fermento láctico incrementó la calidad general de los quesos artesanales.
Cheese is one of the most consumed dairy foods in the world. Artisanal cheeses, produced in much smaller quantities compared to commodity cheeses like cheddar and mozzarella, continue to grow because of the increasing sophistication and multiculturalism of consumers. An example of such homemade cheeses is the flavored cheese. Nowadays, various types of flavored cheeses are produced in the highland farms of the province of Tucumán in North-Western Argentina (NOA) by using traditional techniques and incorporating different species of native aromatic herbs, mainly leaves and fruits. Oregano (Origanum spp., Lamiaceae family) is one of the most important commercial spices; the crop covers more than 80% of the country cultivation area generating export markets of 1200tons/year with values about $2.5 million US dollars10. It is particularly used in culinary art due to its aroma, which is related to the essential oil. Cheeses flavored with oregano leaves are usually as semi-hard ones with short ripening periods of less than 3 months and have very good marketing potential1. However, these products are nowadays commercialized through informal market which plays an important role in dairy farmers’ economy. To reach the Protected Designation of Origin status, both the fermentation process and the microbiological quality need to be controlled. The main concern of this economic sector is the high microbial load in the surface of herbal leaves6, which reduces the microbiology quality and shelf life of the cheeses flavored with oregano leaves. Lactic starter culture and oregano essential oil could be useful to standardize the characteristics of flavored cheeses and to reduce the contaminating microflora present on the leaves. However, the antimicrobial properties of some essential herb oils is well known; for that reason, their possible effects on starter organisms need to be considered3,9. Therefore, the aim of this work was to study the oregano essential oil obtained from North-Western Argentina and to evaluate its potential inhibitory effect on the lactic starter culture.
Materials and methodsPlant materialAerial parts (leaves, stems and inflorescences) of an oregano plant were harvested from Andalgalá and Catamarca in Northwestern Argentina. The dry plant material was identified in the Laboratory for Plant Morphology at Miguel Lillo Institute by botanic experts.
Essential oil isolation and identificationSamples of leaves and flowers were hydrodistilled for 5h in a Clevenger-type apparatus to obtain the oregano essential oil (OEO). The light green colored oil obtained (0.86% yield) was dried over anhydrous sodium sulfate and stored under a nitrogen atmosphere in sealed vials at −18°C until use. The chemical composition of OEO was analyzed by the GC–MS technique using a Hewlett–Packard gas chromatograph (Model 6890) coupled with a quadrupole mass spectrometer (Model HP 5973) and a Perkin Elmer Elite-5MS capillary column (5% phenylmethylsiloxane; length 30m×inner diameter 0.25mm×film thickness 0.25μm). The injector, interphase, ion source and selective mass detector temperatures were maintained at 280°C, 230°C and 150°C, respectively. Helium (He) was used as carrier gas at a flow rate of 1.0ml/min. The oven temperature was programmed as follows: 60°C for 1min, then increased to 185°C (rate 1.5°C/min) and held for 1min, and increased from 185 to 275°C (rate 9°C/min) and held for 2min. The components were identified and compared on the basis of the retention index and mass spectra. The computer matching was done with the National Institute of Standards Technology (NIST 3.0) libraries provided with the computer controlling GC–MS systems. The retention indexes were calculated using a homologous series of n-alkanes C8–C1813.
Lactic starter cultureFive lactic acid bacteria (LAB) strains, Streptococcus thermophilus CRL 728 and CRL 813; Lactobacillus delbrueckii subsp. bulgaricus CRL 656 and CRL 468; and Lactococcus lactis subsp. lactis CRL 597 were obtained from the Culture Collection at the Centro de Referencia para Lactobacilos (CERELA, Tucumán, Argentina). The strains grown in LAPTg broth (g/l: 15, peptone; 10, tryptone; 10, glucose; 10, yeast extract; 1, Tween 80) were used to inoculate (1% v/v) reconstituted skim milk (RSM, 10% w/v) as single or mixed cultures. Single milk cultures were incubated at 40°C for thermophilic (lactobacilli and streptococci), and at 30°C (lactococci). The mixed strain starter culture was formulated as follows: CRL 728 (0.12% v/v), CRL 813 (0.12% v/v), CRL 656 (0.12% v/v), CRL 468 (0.12% v/v) and CRL 597 (0.52% v/v). All the culture had final counts of 8logUFC/ml. OEO was added to the milk cultures at different concentrations (50, 100, 150 and 200μg/g) and incubated at 38°C. Milk cultures without OEO were used as control. The samples were taken at 0, 2, 4, 8, 12 and 24h to determine cell viability (pour plate dilution method in LAPTg agar), pH (Altronix-TPX1, Saen, Argentina) and titratable acidity (potentiometric method with Dornic solution using phenolphthalein as an indicator). The effect of OEO on the fermentative activity of the strains was determined by impedimetric methods. The strains were harvested, washed with potassium phosphate buffer (10mM, pH 7) and suspended in milk to appropriate dilutions for the assay. The two components of impedance (I), capacitance (C) and conductance (G) were measured using a Bactometer® model 64 (Biomerieux 69289 Marcy-l’Etoile Inc., France) that determines impedance detection time (IDT) automatically. The maximum rate of conductance change (μmax) was obtained from the conductance curves [microsiemens (μS) vs time]. This method is based on the principle that bacterial growth converts uncharged or weakly charged compounds into charged end-metabolites whose accumulation increases the conductance (G) of the medium and capacitance (C) at the electrode–medium interface19.
Microbial quality of oregano leavesThe oregano leaves were suspended in saline sterile solution (NaCl 0.85% w/v) under vigorous agitation for 15min. Then, serial dilutions were made and inoculated on specific media to determine aerobic mesophilic bacteria (plate count agar medium, PCA), enterobacteria (McConkey agar) and yeast/molds (YMB medium; 0.9% yeast extract, 5.0% glucose, 0.5% triptein, 0.5% meat extract, 0.21% magnesium sulfate, 0.2% mono potassium phosphate, 0.005% diastase, 0.005% thiamine, 0.0026% bromocresol green sodium salt, 1.5% agar). The plates were incubated at 37°C for 48h (McConkey agar plates) and at 30°C for 4 days (PCA and YMB agar). The results were expressed as logCFU/g leaves.
Manufacture of prototype cheesesCow milk obtained from dairy producers of Tucumán, Argentina was analyzed with an automated equipment (Ekomilk, Milkania KAM98-2A) to determine total protein, milk solids, fat, adulteration (added water), freezing point and density. The acidity of the raw milk was determined by measuring pH (pHmeter) and the titratable acidity expressed as Dornic Grade (1°D represents 0.01g lactic acid in 100ml of milk). Pasteurization was carried out at 63–65°C during 30min; the pasteurized milk was rapidly cooled down to 38°C (manufacturing temperature) and supplemented with calcium chloride (0.2g/l CaCl2) and different concentrations of OEO (50, 100, 150 and 200mg/kg of pressed curd) dissolved in 5ml of ethanol. The mixed strain starter culture was formulated as follows: CRL 728 (0.12% v/v), CRL 813 (0.12% v/v), CRL 656 (0.12% v/v), CRL 468 (0.12% v/v) and CRL 597 (0.52% v/v). All the culture had final counts of 8logUFC/ml. This mixed strain starter was incubated at 38°C for 16h to reach pH 5.2. Afterwards, the pasteurized milk was inoculated with this active starter culture (1.0% v/v) and incubated at manufacturing temperature for approximately 40–60min in order to increase the acidity in 5°D. When the acidity condition was reached, rennet was added (1.0ml/l of rennet per liter of milk) and the temperature was maintained at 38°C for 40–60min until milk coagulation. Then, the curd was cut into small cubes and the temperature was raised up to 45°C (cooking process) to help curd draining. The remaining whey was discarded, the curd pressed, mixed and kneaded. Finally, the aromatized curd was packed in perforated molds under pressure for 24h. The salting process was carried out with brine (18.8% w/v salt; pH 5.2, 6°D and 1.15g/ml density) supplemented with CaCl2 (6g/l). Control groups were made with ethanol or with oregano leaves (10g/kg cheese, according to artisanal manufacturing data). Cheeses were ripened at 12°C at 75% humidity for 30 days.
Microbiological control during cheese ripeningThe slices were obtained at different ripening times: 5, 15 and 30 days. Portions of 10g of cheese were handled under sterilized conditions and homogenized with 90ml of physiological saline sterile solution (NaCl 0.85%, w/v) in a Stomacher Blender 400 (SEWARD). LAB were counted on LAPTg agar medium, aerobic mesophilic bacteria on PCA, enterobacteria on McConkey Agar and yeast/molds on YMB medium. LAPTg and McConkey agar plates were incubated at 37°C for 48h, mean while, PCA and YMB agar at 30°C for 4 days.
Sensory analysisTo evaluate consumer acceptance based on inner and outer appearance, flavor, and texture among the following types of cheese: 1) with different concentrations of OEO (from 50 to 200μg/g); 2) with oregano leaves (10g/kg cheese); 3) without OEO or leaves as a control cheese, the panel used a qualitative 5-point hedonic scale: 1 “very good”, 2 “good”, 3 “indifferent”, 4 “dislike slightly”, 5 “dislike very much”18,25. Previously, all panelists were trained (from INTI Lácteos-Rafaela-Argentina) to carry out a descriptive analysis and were involved in developing the descriptive vocabulary for the cheeses. The cheeses were cut into several dices of 2.0cm and were carefully placed in plates sealed with plastic film and held at room temperature (24°C). Each participant received five cheese samples and two additional samples belonging to a control without OEO and with oregano leaves, to compare the organoleptic properties. Between samples, panelists rinsed their mouth with mineral water. With the results obtained, the acceptance rate of each treatment was determined as the ratio of the total score and the maximum attainable score in accordance with the following equation: Acceptance rate (%)=(Sample score/number of judges×5)×100.
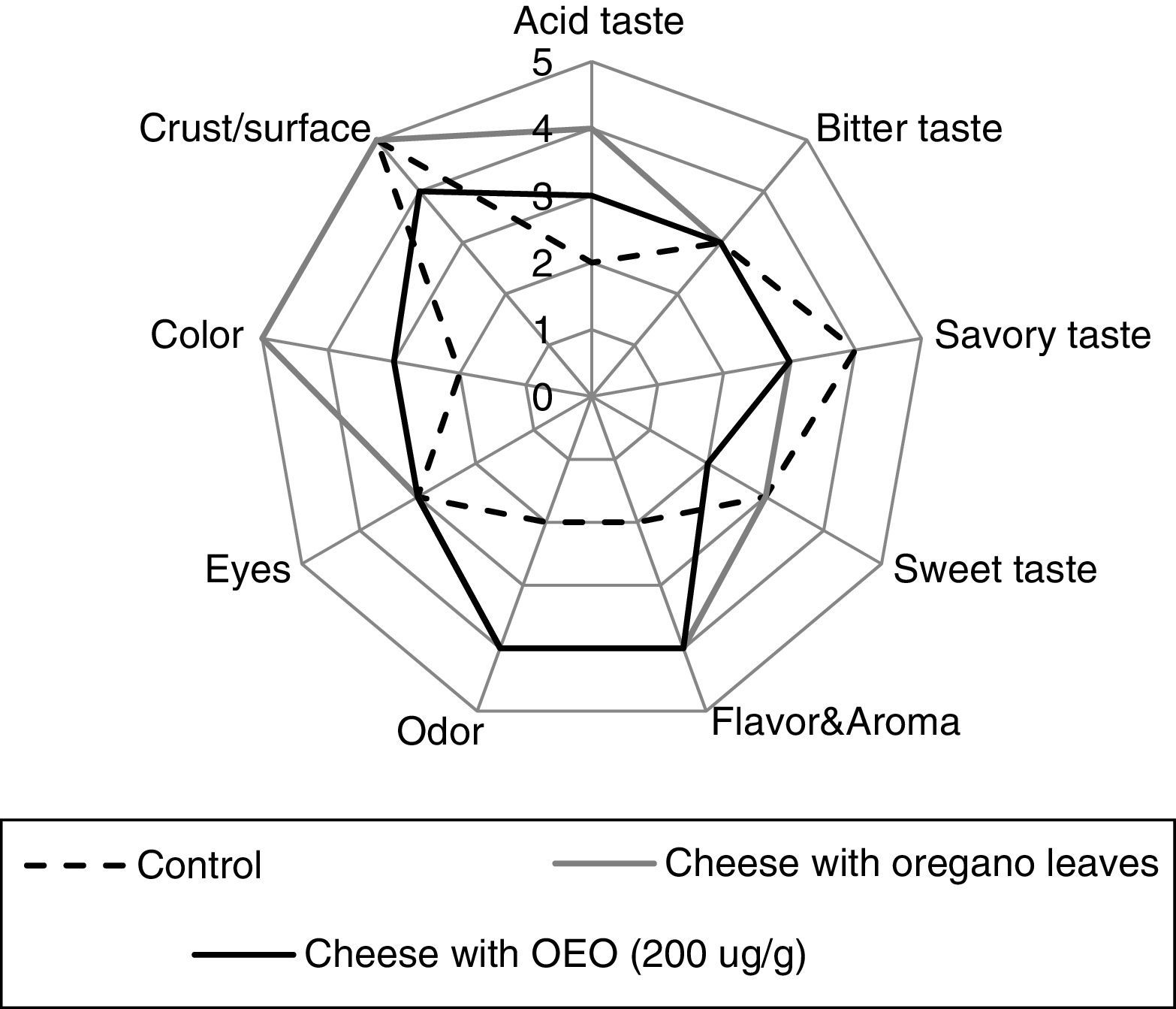
Subsequently to the consumer acceptance test, they proceeded to the evaluation of the sensory attributes (taste, flavor, odor, eyes, color, crust/surface) of the following cheeses: (i) with OEO (200μg/g); (ii) with oregano leaves (10g/kg cheese); (iii) without OEO or leaves as a control cheese. The practice methodology was similar to that of the consumer acceptance test described previously, but using a 5-point intensity scale ranging from less intense to more intense for most of the attributes.
Data analysisData corresponded to at least three independent assays and are reported as mean values with standard deviation. The analysis of variance (ANOVA) and LSD Fisher post tests were performed using the InfoStat 2008p software. All statistical analyses were performed at a significance level of p≤0.05.
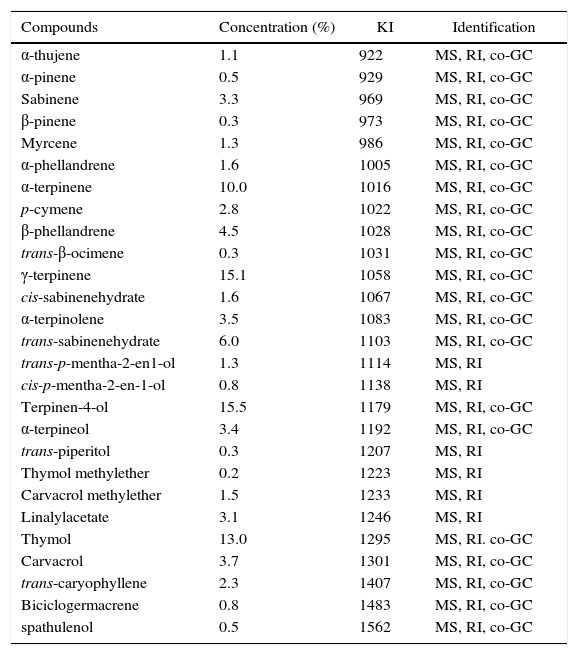
Results and discussionOEO isolation and identificationAerial parts (leaves, stems and inflorescences) of an oregano plant were harvested from Andalgalá, Catamarca, NOA. The parts allowed to identify the species as Origanum vulgare L. var hirtum (Lamiaceae) based on the comparison with different samples of Origanum spp. housed at the Institute Miguel Lillo Herbarium. Plant samples were hydrodistilled for obtaining OEO and the chemical composition was analyzed by GC–MS/FID. Only those compounds present in amounts higher than 0.5% are listed in Table 1. The species O. vulgare L. ssp. hirtum presented high amounts of γ-terpinene (15.1%), terpinen-4-ol (15.5%) and thymol (13.0%). Recently, Asensio et al.4 reported the chemical profile of Argentinean OEO, where trans-sabinene hydrate (17.9–28.12%) and thymol (12.1–18.6%) were the most relevant terpenes. Dambolena et al.8 also reported that the main components of OEO were the monoterpenes trans-sabinene hydrate and thymol, with lower amounts of terpinene, limonene, cis-hydrate sabinene, terpinen-4-ol, and carvacrol. These compounds were found in different oregano species such as O. vulgare L. var hirtum (Greece), O. vulgare (Ireland); O. floribundum (Italy), O. compactum (Morocco) and O. scambrum (Greece) but with some exceptions in O. vulgare var vulgare (Turkey) and O. microphyllum (Greece), where the chemical profile was totally different2,12,14–16.
Chemical composition of essential oil extracted from Oreganum vulgare L. var hirtum analyzed by GC–MS
| Compounds | Concentration (%) | KI | Identification |
|---|---|---|---|
| α-thujene | 1.1 | 922 | MS, RI, co-GC |
| α-pinene | 0.5 | 929 | MS, RI, co-GC |
| Sabinene | 3.3 | 969 | MS, RI, co-GC |
| β-pinene | 0.3 | 973 | MS, RI, co-GC |
| Myrcene | 1.3 | 986 | MS, RI, co-GC |
| α-phellandrene | 1.6 | 1005 | MS, RI, co-GC |
| α-terpinene | 10.0 | 1016 | MS, RI, co-GC |
| p-cymene | 2.8 | 1022 | MS, RI, co-GC |
| β-phellandrene | 4.5 | 1028 | MS, RI, co-GC |
| trans-β-ocimene | 0.3 | 1031 | MS, RI, co-GC |
| γ-terpinene | 15.1 | 1058 | MS, RI, co-GC |
| cis-sabinenehydrate | 1.6 | 1067 | MS, RI, co-GC |
| α-terpinolene | 3.5 | 1083 | MS, RI, co-GC |
| trans-sabinenehydrate | 6.0 | 1103 | MS, RI, co-GC |
| trans-p-mentha-2-en1-ol | 1.3 | 1114 | MS, RI |
| cis-p-mentha-2-en-1-ol | 0.8 | 1138 | MS, RI |
| Terpinen-4-ol | 15.5 | 1179 | MS, RI, co-GC |
| α-terpineol | 3.4 | 1192 | MS, RI, co-GC |
| trans-piperitol | 0.3 | 1207 | MS, RI |
| Thymol methylether | 0.2 | 1223 | MS, RI |
| Carvacrol methylether | 1.5 | 1233 | MS, RI |
| Linalylacetate | 3.1 | 1246 | MS, RI |
| Thymol | 13.0 | 1295 | MS, RI. co-GC |
| Carvacrol | 3.7 | 1301 | MS, RI, co-GC |
| trans-caryophyllene | 2.3 | 1407 | MS, RI, co-GC |
| Biciclogermacrene | 0.8 | 1483 | MS, RI, co-GC |
| spathulenol | 0.5 | 1562 | MS, RI, co-GC |
KI: Kovacs index. Estimated from an analogous Alkanes series (C9–C18).
The quality of lactic starter cultures is defined by the concept of biological activity, which includes cell viability and physiological state, and as the ability to acidify a certain medium11,20. These properties of lactic starter cultures could be affected by food additives. Due to the fact that the OEO had been generally used as antimicrobial agent with good results in food technology, the effect thereof on lactic starter cultures was evaluated. The presence of OEO did not affect the growth and acidifying activity of LAB in milk (data not shown). S. thermophilus CRL 728 and CRL 813 in the presence of OEO at 200μg/g (highest concentration evaluated) exhibited similar growth (8.0–8.2logCFU/ml at 24h) and acidifying activity (5.7–5.4°D/h) with respect to the control without OEO. Both strains presented a lag phase of 4h, indicating no adverse effects before their activation. Likewise, the OEO did not affect the growth or acidifying activity of L. lactis CRL 597 (9.1–8.8logCFU/ml, 3.4–4.4°D/h), L. bulgaricus CRL 656 (8.5–8.3logCFU/ml, 11.87°D/h) and CRL 468 (8.1–8.5logCFU/ml, 6.8°D/h) as well as the mixed-strains starter culture (9.4–9.2logCFU/ml, 5.7–5.4°D/h).
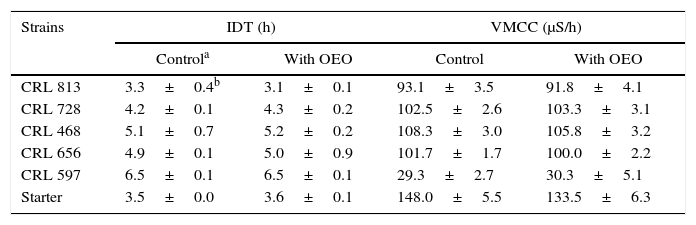
In addition, the effect of OEO on the fermentative activity of cultures was also determined by impedimetric methods. The impedance detection time (IDT) and maximum rate of change of conductance (VMCC) for the starter culture in the presence of OEO extracted from O. vulgare L. var hirtum at a concentration of 200μg/g is shown in Table 2. The minimum and maximum values of IDT were obtained by S. thermophilus CRL 813 (3.3h) and L. lactis CRL 597 (6.5h), respectively, while L. bulgaricus CRL 468 and CRL 656 had similar IDT (4.9–5.1h). With respect to VMCC values, the strains with high values of IDT showed lower values of VMCC, i.e., L. lactis CRL 597. The mixed strain starter culture had lower IDT value (3.5h) and high VMCC value (148.05μS/h), displaying a good fermentative activity. The results observed for culture media supplemented with OEO were similar to control media (without OEO), which corroborates data obtained previously (growth and acidifying activity). Based on these results, OEO could be used in cheese manufacture without significantly altering the lactic starter.
Values of impedance detection time (IDT) y maximum rate of change of conductance (VMCC) of LAB and lactic starter in presence of oregano essential oil (200μg/g) obtained from Oreganum vulgare L. var hirtum
| Strains | IDT (h) | VMCC (μS/h) | ||
|---|---|---|---|---|
| Controla | With OEO | Control | With OEO | |
| CRL 813 | 3.3±0.4b | 3.1±0.1 | 93.1±3.5 | 91.8±4.1 |
| CRL 728 | 4.2±0.1 | 4.3±0.2 | 102.5±2.6 | 103.3±3.1 |
| CRL 468 | 5.1±0.7 | 5.2±0.2 | 108.3±3.0 | 105.8±3.2 |
| CRL 656 | 4.9±0.1 | 5.0±0.9 | 101.7±1.7 | 100.0±2.2 |
| CRL 597 | 6.5±0.1 | 6.5±0.1 | 29.3±2.7 | 30.3±5.1 |
| Starter | 3.5±0.0 | 3.6±0.1 | 148.0±5.5 | 133.5±6.3 |
During cheese manufacture, no significant differences (p≥0.05) in acidification (from 18–20 to 60–65°D) and viability (an increase from 2.0 to 2.5 log units) of the LAB strains in the different cheeses (with and without OEO) were observed. The clotting and cutting times were 40 and 60min, respectively, for all types of cheese. Elaborated cheeses were ripened during 30 days and were subjected to microbiology and sensoryl analysis. The consumer acceptance test was conducted by hedonic evaluation of the cheese samples. The best score was reached by the cheese elaborated with 200μg/g of OEO or with oregano leaves (58 and 65% of acceptance, respectively) while cheese control without additives obtained a lower score (35%). The panelists did not detect any differences between cheeses elaborated with OEO until a concentration of 150μg/g and the cheese control (without OEO). The relationship between the consumer acceptability of the cheeses flavored with oregano leaves or OEO and their descriptive sensory attributes was not investigated previously by other authors7,17. The consumer acceptance of cheddar cheese is dependent on many factors such as odor and flavor, and rubbery and grainy texture.
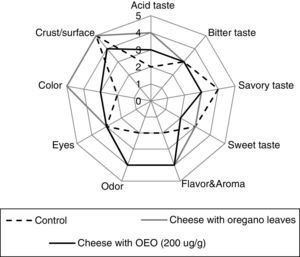
The consumer acceptance test was followed by the analysis of the sensory attributes (taste, flavor, odor, eyes, color, crust/surface) of cheeses with OEO (200μg/g), with oregano leaves or without additives (control). Spider web diagrams of the mean scores of the sensory attributes of cheese are depicted in Figure 1. The sensory characteristics of the cheese with 200μg/g OEO were similar (p≥0.05) to those of the aromatized cheese with oregano leaves while cheese control without additives obtained a lower score. Both the odor (4 points) and flavor (4 points) of cheese containing oregano leaves or OEO were considered quite higher than in the indifferent valuation (punctuation of 3.0). Moreover, the three cheeses evaluated did not show any significant differences (p≥0.05) in bitter or sweet taste, eyes and crust/surface.
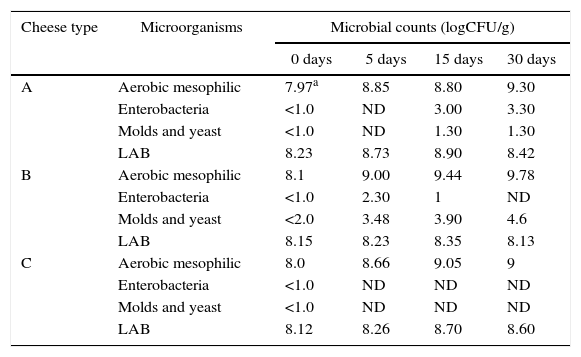
The microbiological characteristics of artisanal cheese without additives (A), elaborated with oregano leaves (B) or 200μg/g of oregano essential oil (C) are shown in Table 3. Furthermore, it was possible to observe the evolution of microbial growth throughout the period of maturation. LAB counts were similar (8.1–8.6logCFU/g) during the maturation of the different cheeses. Enterobacterial growth (2logCFU/g cheese) was detected in the control cheeses at 15 days of maturation and was not detected in the cheese with OEO. In all samples, the number of total aerobic mesophilic bacteria slightly increased (1–1.5logCFU/g). The level of LAB was constant throughout the maturation period. Increases in microbial counts up to five logarithmic cycles have been reported for semi-hard cheeses23.
Microbiological characteristic of artisanal cheese without additives (A), elaborated with oregano leaves (B) or 200μg/g of oregano essential oil (C) during ripening
| Cheese type | Microorganisms | Microbial counts (logCFU/g) | |||
|---|---|---|---|---|---|
| 0 days | 5 days | 15 days | 30 days | ||
| A | Aerobic mesophilic | 7.97a | 8.85 | 8.80 | 9.30 |
| Enterobacteria | <1.0 | ND | 3.00 | 3.30 | |
| Molds and yeast | <1.0 | ND | 1.30 | 1.30 | |
| LAB | 8.23 | 8.73 | 8.90 | 8.42 | |
| B | Aerobic mesophilic | 8.1 | 9.00 | 9.44 | 9.78 |
| Enterobacteria | <1.0 | 2.30 | 1 | ND | |
| Molds and yeast | <2.0 | 3.48 | 3.90 | 4.6 | |
| LAB | 8.15 | 8.23 | 8.35 | 8.13 | |
| C | Aerobic mesophilic | 8.0 | 8.66 | 9.05 | 9 |
| Enterobacteria | <1.0 | ND | ND | ND | |
| Molds and yeast | <1.0 | ND | ND | ND | |
| LAB | 8.12 | 8.26 | 8.70 | 8.60 | |
The initial yeast and mold counts were low in all cheeses (≤1logCFU/g cheese), remaining throughout maturation. On the contrary, the flavored cheese with oregano leaves had a significantly higher initial count (2logCFU/g cheese) and increased to a maximum of 4.6logCFU/g cheese after 30 days of maturation. Therefore, during the ripening process visible mold growth (4 days) was observed on the crust surface of the cheese elaborated with oregano leaves. The high initial value of molds/yeasts in this cheese may be attributed to contamination caused by the native flora of oregano leaves named phyllosphere (the leaf surface as a habitat). Thus, a microbiological study on oregano leaves was carried out. The microbiological analysis of oregano leaves showed a significant number of microorganisms: 3.61 and 3.65logCFU/g leaves of aerobic mesophilic bacteria and mold/yeast, respectively. Enterobacteria or LAB were not detected in oregano leaves.
Some authors reported that native herbs have antimicrobial activity1,9. The microbiological analysis of cheese samples suggests that the OEO was able to suppress the growth of either spoilage bacteria or molds/yeasts during ripening. This was confirmed by the comparison of microbial counts between control and flavored cheeses containing OEO. Asensio et al.5 showed that the essential oil obtained from four oregano-types from Argentina have significant inhibitory effects against yeast. Furthermore, other studies have shown a strong bactericidal effect (total elimination of the microbial initial inoculum) of Oreganum vulgare extracts21 due to the functional compounds of OEO that play an important role for anti-microbial activity22. On the other hand, phenolic components are capable of disrupting the microbial membrane, penetrating inside the cell, where they interact with cellular metabolic mechanisms, exerting the antimicrobial activity. Several studies have shown an association between the chemical composition and the anti-yeast activity of essential oils24. The essential oil O. vulgare L. var. hirtum evaluated in this work was rich in phenolic compounds such as thymol and carvacrol and other monoterpenes such as α-terpinene and γ-terpinene, which could be responsible for its antimicrobial activity.
This study showed that the essential oil of Origanum vulgare L. var. hirtum from Andalgalá, Argentina have similar chemical compounds to those of other oregano types. The addition of OEO in cheese elaboration protected the cheeses from the growth of spoilage organisms but did not affect the viability of the LAB starter culture necessary to carry out the ripening process. Furthermore, the OEO gives to the cheese a similar taste to that of the cheese aromatized with oregano leaves. The use of OEO (200μg/g) and the LAB starter culture allows the manufacture of a cheese with optimal sensory and microbial characteristics, and prevents contamination by the native flora of oregano leaves (a typical problem of artisanal cheese).
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors declare that they have no conflicts of interest.
The authors acknowledge the financial support of Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) from Argentina.