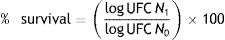The health benefits attributed to probiotics generate interest in the search of competent strains adapted to several ecological niches, especially those related to traditional beverages and foods of each country. Pineapple tepache, a traditional Mexican fermented beverage, was used for the isolation of lactic acid bacteria with probiotic potential, one of which withstood the in vitro tests. The isolated strain AB-05, which exhibited the tested probiotic functional properties, was designated as Lactobacillus pentosus ABHEAU-05. The sequence was registered in GenBank under access code MK587617. This study is the first report of a lactic acid bacterium with in vitro digestion resistance isolated from pineapple tepache. The survival of L. pentosus ABHEAU-05 in a symbiotic medium was proven using fermented milk enriched with inulin. The in vitro digestion-resistant probiotic activity of lactobacilli was measured through analysis of pH and proteolysis. Results showed that L. pentosus grew properly in fermented milk; therefore, this microorganism could be used in the manufacture of this kind of products. The concentration of L. pentosus reached up to 8.5logCFU/ml after 40h of fermentation. In addition, the production of peptides and the decrease in pH indicated the vigorous and active metabolic state of the lactic acid bacterium tested. The activity and the concentration of this microorganism were maintained during refrigeration. The results of this research conclude that L. plantarum ABHEAU-05 is an in vitro digestion-resistant microorganism that can be used as a starter culture for the production of functional foods of dairy origin.
Los beneficios a la salud atribuidos a los probióticos generan interés en la búsqueda de cepas competentes adaptadas a varios nichos ecológicos, especialmente los relacionados con bebidas y alimentos tradicionales de cada país. En este estudio, se aisló del tepache de piña, una bebida fermentada tradicional mexicana, una bacteria láctica resistente a la digestión in vitro. Entre 5 bacterias aisladas, una de ellas soportó las pruebas simuladas de digestión gastrointestinal. Se analizó la resistencia a sales biliares, a condiciones ácidas y al ataque enzimático con pepsina. La bacteria aislada, que exhibió las propiedades funcionales probióticas referidas, fue identificada como Lactobacillus pentosus y designada como L.pentosus ABHEAU-05. La secuencia fue depositada en GenBank (acceso MK587617). Se comprobó la supervivencia de L.pentosus ABHEAU-05 en una leche fermentada adicionada con inulina y su resistencia a la digestión in vitro mediante el análisis del pH y la proteólisis. Los resultados muestran que la leche fermentada es una matriz adecuada, donde L.pentosus ABHEAU-05 se desarrolla sin inconvenientes, alcanzando un título de 8,5logufc/ml después de 40h de fermentación. Además, la producción de péptidos y el descenso del pH indicaron el estado metabólico vigoroso y activo del microorganismo probiótico. Se concluye que L.pentosus ABHEAU-05 es un microorganismo resistente a la digestión in vitro, que puede servir como cultivo iniciador para la producción de alimentos de origen lácteo. Este es el primer informe acerca del aislamiento de una bacteria ácido láctica resistente a la digestión in vitro a partir del tepache de piña.
Tepache is a Mexican traditional fermented drink prepared from pineapple and brown sugar49. In Mexico, it is consumed as a refreshing drink and, despite the lack of reliable data; it is known that its origin goes back to pre-Hispanic times24. The microorganisms associated with the production of tepache include various species of lactic bacteria, acetic bacteria and yeasts, being part of a microbial consortium that turns this drink into a microbiologically complex system32. Several lactic acid bacteria (LAB) of interest for human health have been identified from some traditional fermented beverages15. Lactic acid bacteria are a broad group of microorganisms that have been considered an essential part of food biotechnology. These microorganisms are important because of their production of secondary metabolites during the fermentation processes as well as for their effect on human health55. Currently, research on LAB applications has been increasing on a daily basis; with the broadest approach in the area of probiotics, especially those belonging to the genera Lactobacillus and Bifidobacterium.
LAB potential use in foods as an ingredient or supplement is directed to the development of products with multiple benefits to consumer health50,38. Because consumers have become more aware of health issues and the effects that food has on it, there has been an increase in research related to the discovery of new probiotic microorganisms, mainly those that are isolated from matrices other than milk and dairy products59,44,26,1. Isolated LAB have been used as ingredients and starter cultures in the elaboration of functional foods1,30.
Probiotic cultures have been used successfully in different food matrices such as dairy, meat, beverages, fruits, vegetables, cereals and bakery products53. However, there are several factors that can affect the viability of probiotics in the food such as the strain, pH, presence of hydrogen peroxide and oxygen supply, concentration of metabolites, mainly lactic acid and acetic acid, buffer capacity of the medium, storage temperature, origin of added ingredients and matrix, among others10,14,48,7.
Survival in the gastrointestinal ecosystem and adhesion to the mucosa are considered fundamental requirements for probiotic bacteria to exert their activity11. For this reason, the in vitro tests used for the evaluation of susceptible microorganisms to be characterized as probiotics are based mainly on their ability to survive gastrointestinal transit4. These tests evaluate the capacity of microorganisms to tolerate the hostile conditions imposed by the gastrointestinal tract, such as extremely acidic pHs and the detergent action of bile salts present in the intestine. The ability to adhere to the epithelial surface, the antagonistic activity against pathogenic microorganisms and the potential to colonize the intestine temporarily are also tested through in vitro tests17,22,9. The analysis of those abilities can be qualitative or quantitative and will allow to correlate the strains with their probiotic capacity in vivo51. The objective of this research was to isolate and identify a microorganism with resistance of in vitro digestion from a Mexican traditional drink and to prove its survival in fermented symbiotic milk during refrigeration storage.
MethodologyPreparation of tepacheTepache was prepared according to the methodology reported by Corona et al.6 with some modifications. The beverage was elaborated using ripe pineapple peels (unwashed) and a previously pasteurized solution of 13% (w/v) of panela (brown cane sugar), in a pineapple/panela solution in the ratio 1:2 (w/w). The container was covered and sealed with self-adhesive plastic to simulate semiaerobic conditions. The fermentation was conducted in duplicate in 5l-glass containers at 22°C, for 54h with an initial pH of 4.5.
Sampling and measurement of pH and acidity changesAliquots of 25ml of tepache were taken at 2-h intervals from time 0 to 54h. For the total pH and acidity measurement, a volume of 24ml was used; the remaining milliliter was used for the viable lactic acid bacteria count. The pH was measured using a potentiometer and total acidity was determined by volumetry and results were expressed as mg of lactic acid per milliliter25.
Determination of lactic acid bacteria viability during fermentationThe viable lactic acid bacteria count was carried out by the microdrop technique in the Man, Rogosa and Sharpe medium (MRS)42. Seeding was performed from consecutive dilutions of the sample from 1×10−1 to 1×10−5 in peptone water solution. Plates were incubated at 37°C for 18–24h.
Isolation and macroscopic characterization of LABLAB strains were selected from the plates used for the viable count. Subsequently, the morphological differentiation was carried out based on criteria such as color, consistency, shape, size and type of edge previously described by Winn and Koneman60. The selected colonies were isolated on MRS agar by the streaking technique. Using this same technique, the developed LAB strains were transferred to the M17 medium to differentiate lactic bacilli from lactococci and streptococci. In order to corroborate the gram positive character of the isolated strains, as well as to identify their microscopic morphology and verify the purity of the culture, stained smears under the Gram technique for each identified colony were performed.
In vitro digestion resistance testsThree continuous in vitro tests (tolerance to bile salts, resistance to acidic pH and resistance to the action of pepsin) were carried out to test the survival capacity of the selected LAB strains under conditions imposed by the gastrointestinal tract. Lactobacillus casei Shirota was used as the reference strain.
Bile salt toleranceBile salt tolerance of the isolated strains was determined by the method proposed by Lee et al.33 with slight modifications. MRS broth was enriched with 0.3% (w/v) of bile salts and adjusted at pH=7.0 with 0.1N NaOH. One hundred (100)μl of the fresh culture were inoculated into the enriched medium and incubated at 37°C for 2h. To know the initial count, 100μl of fresh culture of each strain were inoculated in MRS broth without enrichment (control). The seeding of both samples was performed by the microdrop method on MRS agar and they were incubated for 24h at 37°C. Subsequently, the counting of each plate was carried out.
Resistance to acid pHThe resistance to acid pH was determined according to Park et al.47 with some modifications, inoculating 100μl of fresh culture into a culture tube containing 10ml of MRS broth previously adjusted to pH 2.0 with concentrated HCl. Then, it was incubated at 37°C for 2h. Additionally, an initial culture count was conducted from a control tube containing 100μl of fresh culture in MRS broth without acidification. To confirm the survival of the microorganisms after the incubation period, viable cells were counted by the microdrop technique.
Resistance to pepsin actionThe in vitro capacity of isolated LAB to resist pepsin action was measured by inoculating 100μl of culture into MRS broth supplemented with 1000IU of pepsin and adjusted to pH 2.554. The medium was incubated for a period of 2h at 37°C. The count of viable cells after incubation and the initial count were carried out on MRS agar using the microdrop technique.
Determination of survival rateThe number of viable cells was determined by plate count on MRS agar. The survival percentage was calculated using the following equation5:
whereN1 refers to the number of viable cells after treatments
N0 represents the initial number of LAB inoculated
The identification of isolated LAB was performed using the 16S rRNA sequence. After 24h of incubation, the reaction broths were centrifuged and total bacterial DNA extraction was carried out using the Wizard Genomic DNA Purification Kit (Promega) following the manufacturer's instructions. Genomic DNA was stored at −20°C for an additional molecular study. The variable region V1–V3 of the 16S rRNA gene (approximately 510bp) was amplified by the PCR technique using primers 4F and pD29. PCR reactions were carried out in a total volume of 50ul in a thermocycler (Biorad). The reaction mixture consisted of 10ng of genomic DNA, 1U of Taq polymerase, Taq buffer (1×), 200μM dNTP, 10μM of each primer (forward and reverse) and 2mM MgCl2. The thermocycler protocol was an initial denaturation at 95°C for 5min, followed by 30 cycles of 95°C for 1min, 56°C for 1min and 72°C for 1min with a final extension of 5min to 72°C. The amplicons were purified using the Gel DNA Extraction Kit (Promega) and sequenced in an ABI PRISM 3100 AVANT. The variable region of 16S sequences obtained was sent to the BLAST algorithm with the aim of comparing them to the database of the National Biotechnology Information Center (www.ncbi.nih.gov). For the phylogenetic analysis, the sequences were aligned using the CLUSTAL X software23. Based on the implemented algorithm, a phylogenetic tree was constructed using the maximum likelihood method based on the General Reversible model. The stability or accuracy of each inferred topology was assessed by bootstrap analysis with 1000 replicates. The Staphylococcus epidermidis L22 strain was used as an external group (access number KU922485.1). Molecular Evolutionary Genetics Analysis Version 7.0 (MEGA7) was used to infer the maximum probability in order to generate the phylogenetic Tree31.
Survival evaluation of Lactobacillus pentosus ABHEAU-05 in fermented milkIn order to assay the survival of L. pentosus ABHEAU-05 and to evaluate its behavior as a starter culture in fermented milk, this microorganism was grown in a solution of skimmed milk powder at 12% (w/v) (Dairy Gold®) added with 2% of inulin (E NATURE®) following the technique reported by Jaimez-Ordaz et al.28. The prepared milk solution was pasteurized at 90°C for 10min in autoclave, inoculated with 106CFU/ml and incubated at 37°C for 28h. Samples were taken every 2h, during fermentation until the end of the logarithmic phase (beginning of deceleration). At the end of the fermentation, the plate count was performed on MRS-agar by the microdrop technique. The fermented milk was stored at 4°C for 21 days. Sampling was performed at 7, 14 and 21 days to measure the pH decrease and the proteolytic capacity, which was monitored through the analysis of free amino groups by the TNBS technique2. Both analyses were performed during lactic fermentation and during the refrigerated storage of fermented milk. The survival of L. pentosus ABHEAU-05 in refrigerated fermented milk was verified through plating on MRS medium by the microdrop technique.
Results and discussionFermentation of tepacheChanges in pH, acidity and viable LAB count were monitored. The initial pH of the fermentation system was 4.4 and at the end of the process the pH reached 3.3, which is within the pH range (3.22–3.66) obtained by Moreno-Terrazas40 in a study on four tepache samples elaborated in Mexico City. The changes in pH during tepache fermentation are probably directly related to the production of organic acids, mainly lactic and acetic acid, which are generated by the native microbial consortium of this beverage (yeast, lactic and acetic bacteria), from available fermentable sugars58. With regard to acidity, values between 0.34 and 0.48% were obtained, which shows a significant increase, since the initial values were between 0.027 and 0.03%. Similar to pH, titratable total acid values obtained at the end of fermentation are in agreement with those reported by Moreno-Terrazas40.
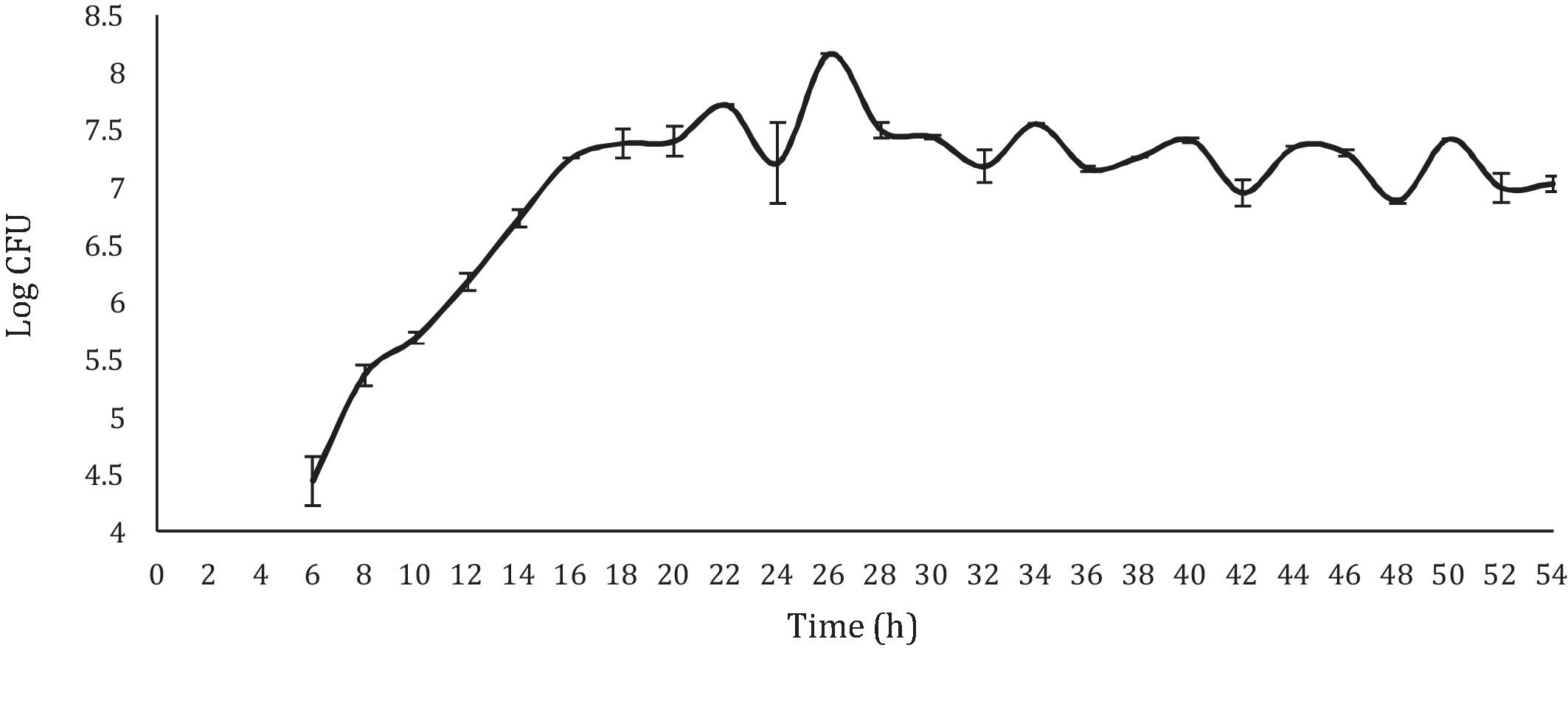
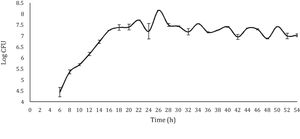
With respect to the viable LAB count, the results obtained showed a curve similar to the typical bacterial growth curve (Fig. 1). In the stationary phase, a straight line was not observed (which would respect the ideality of a typical bacterial growth curve). On the contrary, the points showed increases and decreases throughout this stage, approximately between 6 and 8logCFU/ml; with a final viable account of 7.29logCFU/ml. Despite the observed variations, the values observed during the stationary phase and the final viable account were similar (6.37–8.56logCFU/ml) to those obtained by Moreno-Terrazas40. The observed variations are due to competition for the substrate or to a metabolic imbalance caused by the depletion of fermentable sugars, since each metabolic reaction is regulated by the concentration of nutrients in the environment21.
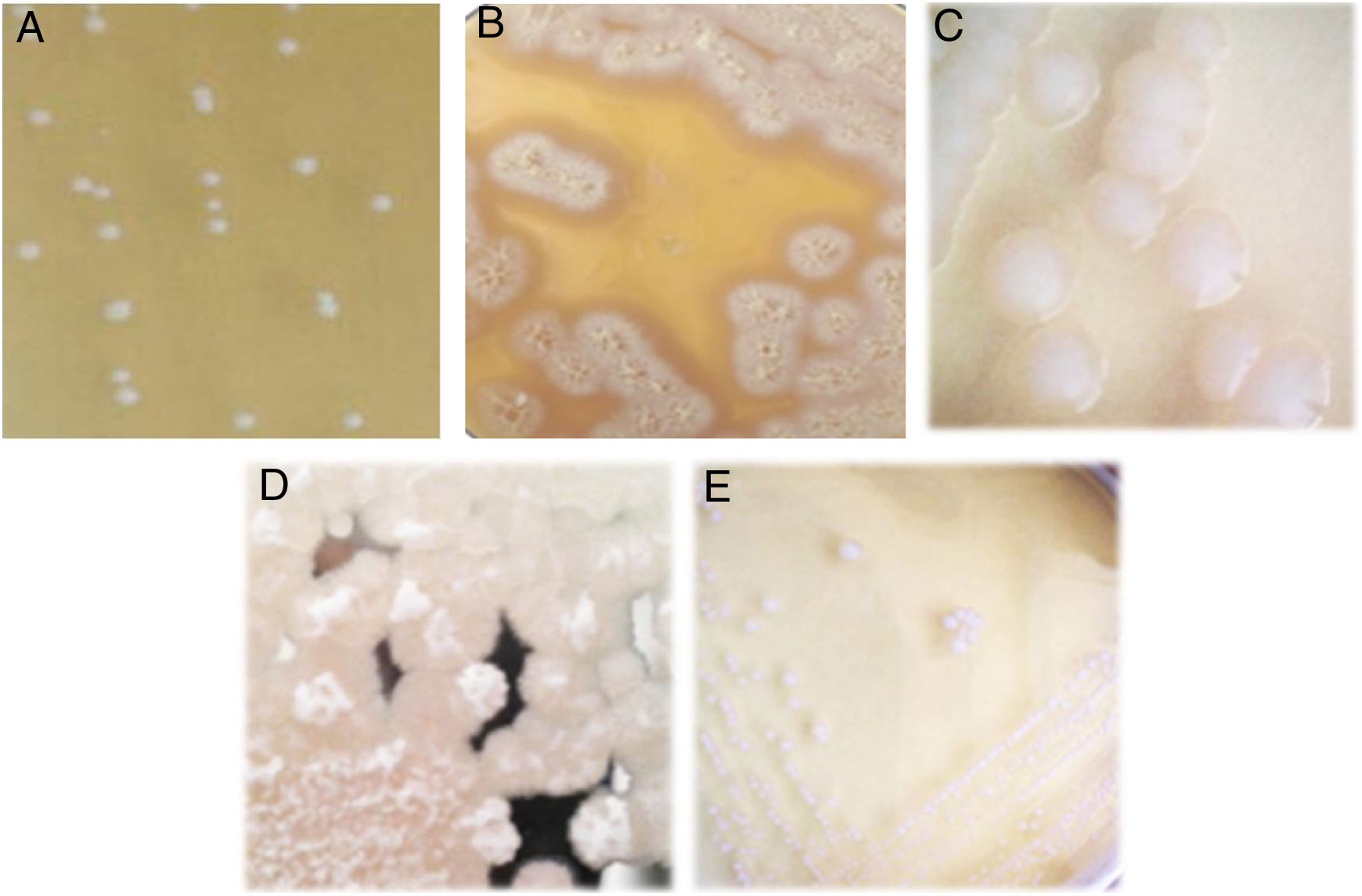
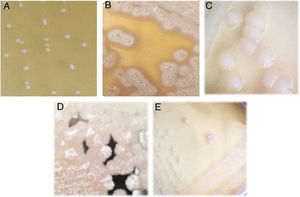
Isolation of lactic acid bacteriaFive lactic acid bacteria were isolated from tepache (AB-1 to AB-5). Only isolated strain AB-1 showed growth in both culture media (MRS and M17). Its morphology on MRS medium was slightly white, circular, with defined edges, convex, 2mm of approximate diameter and creamy consistency (Fig. 2A). The morphology observed for isolated strain AB-1 on M17 medium was similar to that described above, only differing in colony size (3mm) and the intensity of the white color. Maximum growth for this isolated bacterium was observed between 28 and 36h after fermentation.
Isolated strains AB-2 and AB-3 only grew on MRS agar. They showed colonies with a large extension on the surface of the plate due to the production of exopolysaccharides. Isolated strain AB-2 presented extended and filamentous colonies of beige color and a crystalline halo (Fig. 2B) while isolated strain AB-3 showed rhizoid acuminate white colonies, with dry consistency (Fig. 2C). Isolated strain AB-2 had a maximum prevalence between 36 and 46h of fermentation. In contrast, isolated strain AB-3 grew during all the fermentation process from the first 4h showing minimal and constant concentration compared to isolated strains AB-1 and AB-2.
Similar to isolated strains AB-2 and AB-3, isolated strains AB-4 and AB-5 only developed on MRS agar (Fig. 2D and E). Isolated strain AB-4 exhibited colonies of approximately 4mm in diameter, irregular shape, flat, white, with a transparent halo around it, exopolysaccharide production, creamy consistency and development only in the first hours of fermentation. In the case of isolated strain AB-5, the colonies measured approx. 4–5mm in diameter, were circular in shape with a defined edge, convex, exhibited intense white color, creamy consistency and its prevalent development occurred during the last hours of fermentation.
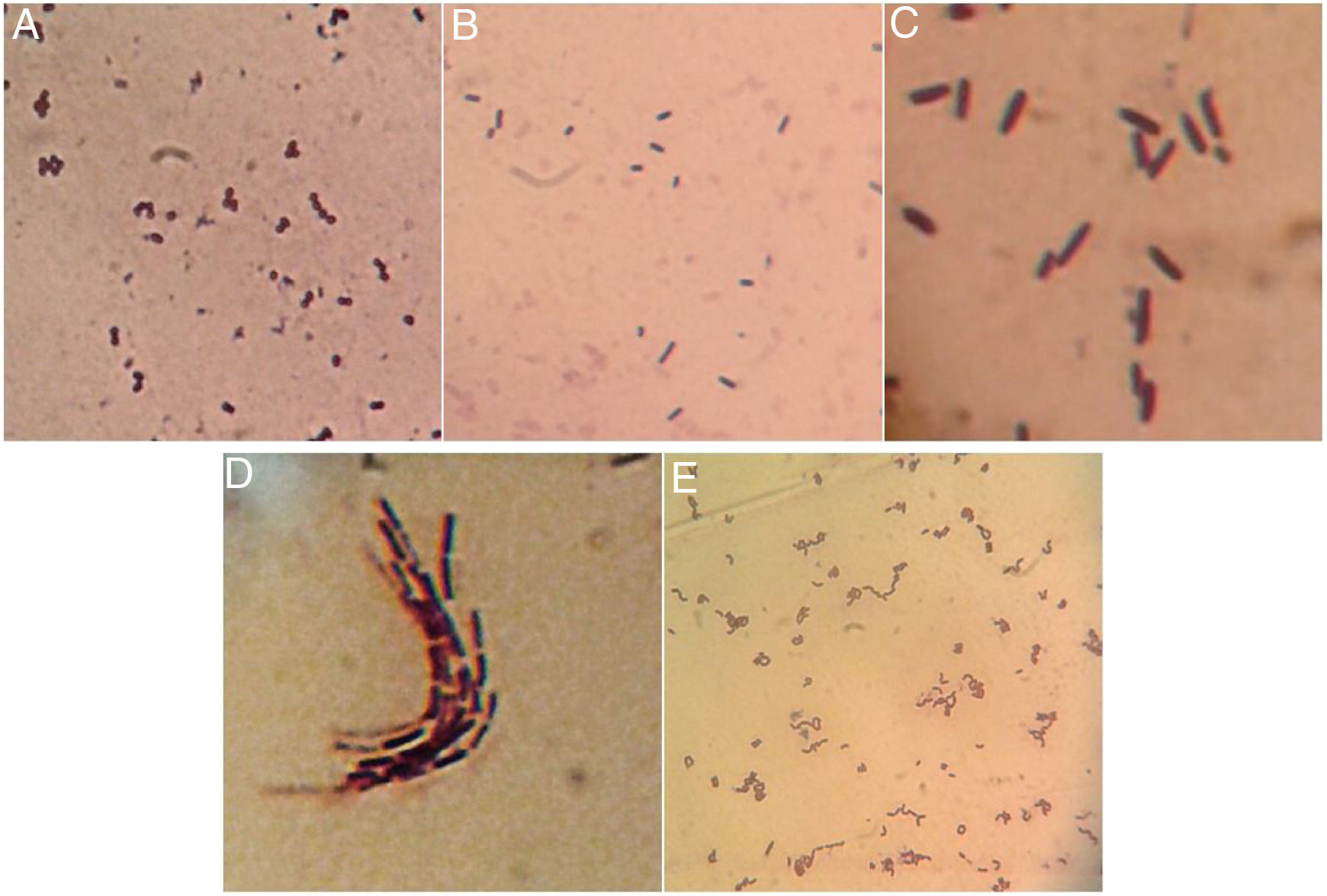
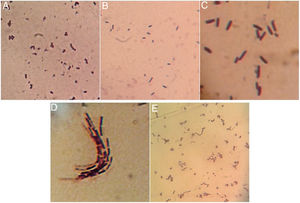
Once isolated on MRS medium, the five isolated bacteria obtained from tepache were analyzed microscopically by the Gram staining technique, with positive results. Using the observed morphology, an isolate corresponding to lactic streptococci and four bacillary isolates (Fig. 3) were identified. Strain AB-1 exhibited a morphology characterized by small cocci grouped in a chain (streptococci) (Fig. 3A) while strain AB-2 showed short bacilli with rounded edges (Fig. 3B). On the other hand, strain AB-3 presented a peculiar microscopic morphology in which it was possible to observe long bacilli, grouped in laterally joined chains. In addition, this isolate presented a halo around the cells due to the production of exopolysaccharides (Fig. 3C). Strain AB-4 showed long bacilli (Fig. 3D), for strain AB-5 the bacilli observed were of medium size, slightly curved and grouped in pairs at the ends. Some groups showed bacilli with curvatures so pronounced that appeared to join to form a circle (Fig. 3E).
Comparing the morphology described by several authors with the isolated bacteria in this investigation, it was found that isolated strains AB-3 and AB-5 presented morphological similarities to Lactobacillus species15,12. Isolated strain AB-3 also showed morphological similarities to a dextran-producing Leuconostoc species isolated from pulque samples57. On the other hand, isolated strains AB-2 and AB-4 were morphologically similar to 3 species of gram positive bacilli also isolated from pulque while isolated strains AB-1, AB-2 and AB-4 presented similarities to the gram positive cocci isolated from white pozol and with gram positive bacilli isolated from sotol12,43.
Several native microorganisms, including lactobacilli species have been isolated from traditional Mexican fermented beverages such as pulque, pozol, sotol, aguamiel and tepache15,12,43,13. For these revealed facts, tepache has been a traditional beverage to research for the isolation and identification of novel potential probiotic species49.
Several traditional Mexican fermented beverages such as pulque, pozol, sotol, aguamiel and tepache have been used for the isolation and identification of novel probiotics, including lactobacilli species15,12,43,13,49.
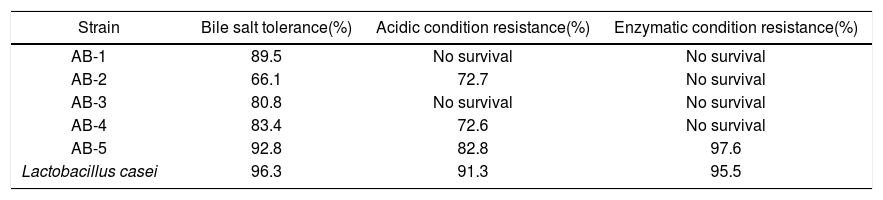
In vitro digestion resistance testsTable 1 shows the survival percentage of the five lactobacilli isolated from tepache, elaborated for the in vitro capacity tests conducted. These results were compared to those obtained for L. casei which was used as reference.
Percentage of viable cells measured by the in vitro probiotic capacity test.
| Strain | Bile salt tolerance(%) | Acidic condition resistance(%) | Enzymatic condition resistance(%) |
|---|---|---|---|
| AB-1 | 89.5 | No survival | No survival |
| AB-2 | 66.1 | 72.7 | No survival |
| AB-3 | 80.8 | No survival | No survival |
| AB-4 | 83.4 | 72.6 | No survival |
| AB-5 | 92.8 | 82.8 | 97.6 |
| Lactobacillus casei | 96.3 | 91.3 | 95.5 |
For bile salt treatment, the survival rates of the isolated bacteria were comparable to those of the reference probiotic, except for isolated strain AB-2. It is known that the ability to resist the bactericidal action of bile salts is directly related to the synthesis of hydrolases that cause their deconjugation, thus preventing the dissolution of cell membranes41. The isolated bacteria from tepache demonstrated strong tolerance to 0.3% bile salts, showing survival rates >80%, except for isolated strain AB-2 (Table 1). That concentration has been reported as critical for the evaluation of bile salt-resistant strains18. Bile salt-resistance of the isolated bacteria was similar to that reported for potential probiotic bacteria isolated from fermented food and assayed under the same experimental conditions including a L. pentosus strain isolated from mustard pickles59,44,26,4,17,22,9,35,52,39,51.
Resistance to acid pH and pepsin actionNone of the isolated bacteria, except for AB-5, showed resistance to the pepsin treatment, although isolated strains AB-1 and AB-4 were able to grow under acidic conditions (pH 2). It has been reported that each LAB species and even each isolate, exhibit different tolerance to acid conditions52. The results observed for the abovementioned isolated strains are in agreement with those reported in studies carried out with LAB isolated from traditional Mexican beverages such as pulque13, pozol, sotol as well as aguamiel49. Those LAB have demonstrated their ability to survive to acidic conditions. The behavior observed for isolated strain AB-5 coincides with that shown by several species of L. pentosus isolated from green table olives39, one lactobacillum isolated from pulque51 and some lactic acid bacteria isolated from fermented food9,39,51,56. Some of those microorganisms also showed resistance to the in vitro treatment with artificial gastric juices (different combinations of acidic conditions and enzymes such as pepsin).
The resistance to low pH (2.0–3.0) is a critical factor for the selection of a probiotic due to the fact that microorganisms must be able to survive unfavorable conditions in the stomach during transit39.
It is known that the ability of a microorganism to survive acidic conditions (which varies from one species to another) depends directly on the concentration of hydronium ions that accumulate inside the cell27, which may affect the Mitchell's chemiosmotic mechanism8, as well as the source and activity of H+-ATPase37. Survival under acidic conditions is positively affected by adaptation to low pH, a behavior known as the acid-tolerance response8.
With regard to resistance to pepsin, isolated strain AB-5 was the only one that was able to grow under the conditions of the in vitro tests conducted, showing a similar behavior to that of the L. casei strain used as reference. These results are also in agreement with viability reported for other lactobacilli species in the presence of pepsin at pH 2.0 for 3h34,36,37. The action of pepsin consists in the selective hydrolysis between hydrophobic amino acid bonds with certain specificity for some of them3, so that the resistance of each bacterial species to its proteolytic action is related to the content of amino acids of its membrane proteins.
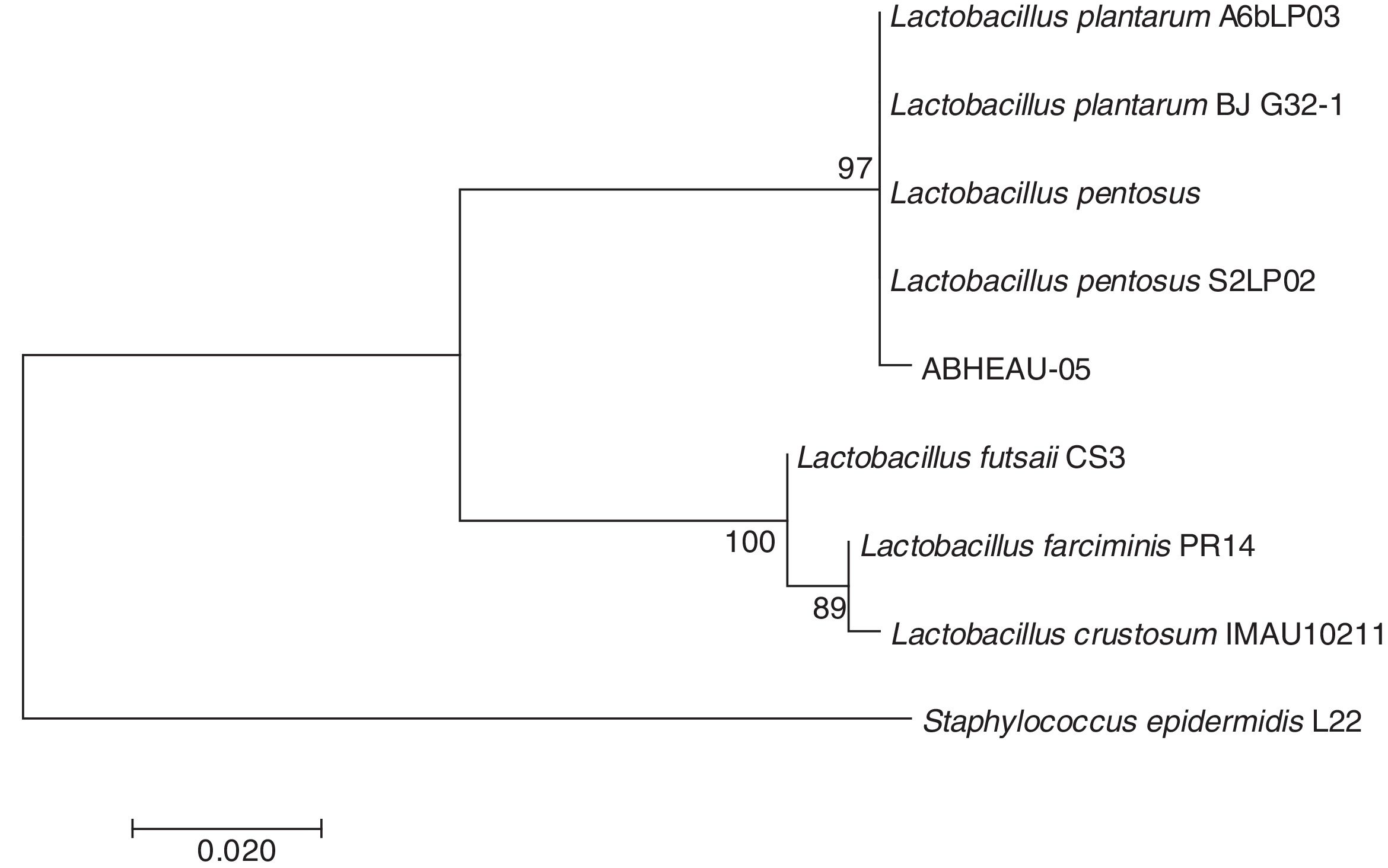
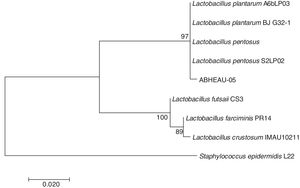
Molecular identification of the selected LABConsidering the in vitro digestion resistance of isolated LAB AB-5, which showed desirable characteristics compared to those of a commercial probiotic, its identification was performed using the 16S rDNA analysis sequence. The 16S rDNA sequence of the isolated LAB showed 99% similarity with several L. pentosus species available from GenBank (Fig. 4) (National Center for Biotechnology Information (NCBI)). Therefore, isolated strain AB-5 was designated as L. pentosus ABHEAU-05. The sequence was deposited in the GenBank under access number MK587617.
This study is the first to report the isolation of L. pentosus as a lactic acid bacterium resistant to in vitro digestion from a traditional Mexican fermented drink (tepache) prepared under standardized conditions. The results encourage the research on fermented beverages in Mexico as a potential source of probiotics of non-dairy origin.
Survival evaluation of L. pentosus ABHEAU-05 in symbiotic fermented milkThe pH decrease during milk fermentation demonstrated the activity of L. pentosus ABHEAU-05 during the process. The symbiotic fermented milk reached a pH of 5.0 after 28h. In this kind of system, the pH decrease is directly related to the conversion of lactose into lactic acid46.
With regard to survival, a two logarithmic cycles higher concentration of viable cells (8.68±0.007logofCFU/ml) was observed compared to the initial. It has been shown that some probiotic microorganisms that are developed in symbiotic milk as a fermentation medium reached similar values45.
Similar to the pH, the proteolytic capacity decreased through the fermentation process demonstrating the activity of the microorganism. The concentration of free amino groups reached a maximum of 269±2.2mg/l after 18h of processing, getting a final value of 123.16±0.83mg/l at 28h. This behavior has already been described by some authors16,20.
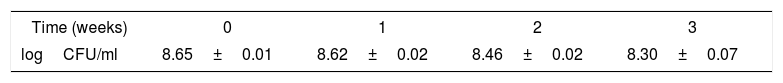
Concerning the survival of L. pentosus ABHEAU-05 under refrigerated storage of fermented milk, there was no significant difference in the concentration of viable cells after 21 days at 4°C (Table 2). The concentration of L. pentosus ABHEAU-05 remained at higher levels than those recommended for a probiotic in fermented milks (1×106CFU/ml). These results are comparable to those reported by several authors for probiotic and non-probiotic lactic acid bacteria10,28,35,19.
The evaluation of the survival of L. pentosus ABHEAU-05 during fermentation and refrigerated storage of a symbiotic fermented milk demonstrated that this microorganism isolated from a medium other than milk has the ability to grow, develop and remain viable under hostile conditions.
ConclusionThis is the first report of L. pentosus (ABHEAU-05) isolated from tepache, a Mexican traditional beverage, which has exhibited resistance to in vitro digestion, one of the preliminary analyses in order to select probiotic strains. However, further studies are required to consider this microorganism a potential promising starter culture in the manufacture of fermented dairy products in order to obtain a symbiotic functional food.
Conflict of interestThe authors have no conflict of interest to declare regarding the publication of this article.