Since ancient times, the consumption of fermented low-alcoholic beverages has enjoyed widespread popularity in various countries, because of their distinct flavors and health benefits. Several studies have demonstrated that light to moderate alcohol consumption is associated with beneficial effects on human health, mainly in cardiovascular disease prevention. Fermented beverages have different non-ethanol components that confer beneficial health effects. These bioactive compounds are mainly peptides that have often been overlooked or poorly explored in numerous fermented beverages. The aim of this review is to provide knowledge and generate interest in the biological activities of peptides that are present and/or released during the fermentation process of widely consumed traditional fermented beverages. Additionally, a brief description of the microorganisms involved in these beverages is provided. Furthermore, this review also explores topics related to the detection, isolation, and identification of peptides, addressing the structure–activity relationships of both antioxidant and angiotensin-converting enzyme inhibitory (ACE-I) activities.
El consumo de bebidas fermentadas de bajo contenido alcohólico – conocidas desde la antigüedad – se ha vuelto muy popular en muchos países debido a su sabor especial y beneficios para la salud. Varios estudios demostraron que el consumo ligero a moderado de alcohol está asociado a efectos beneficiosos en la salud humana, principalmente en la prevención de enfermedades cardiovasculares. Las bebidas fermentadas poseen diferentes componentes bioactivos distintos del etanol a los que se han atribuido los efectos beneficiosos mencionados; se trata, principalmente, de péptidos poco o nada estudiados a la fecha. El propósito de este artículo es proporcionar conocimiento sobre este conjunto de péptidos presentes en bebidas fermentadas y despertar interés en ellos. En ese contexto, también se presenta una breve descripción de los microorganismos involucrados en estas bebidas y de las técnicas analíticas para la detección, el aislamiento y la identificación de estos péptidos, junto con un análisis de las relaciones estructura-actividad que darían cuenta de su desempeño como agentes antioxidantes e inhibidores de la enzima convertidora de angiotensina (ECA-I).
In recent times, the key role of food and beverages has earned increasing recognition in disease prevention and treatment. Therefore, the production and consumption of functional foods have gained significant importance as they provide health benefits in one or more target functions in the body, in addition to their basic nutritional functions34, which benefits are mainly attributed to the presence of bioactive compounds45.
Fermented foods are commonly described as foods or beverages processed through controlled microbial growth and enzymatic changes of primary and secondary components of foods17. Fermented alcoholic beverages such as wine, beer, cider, among others, are the most commonly commercialized fermented products worldwide82, playing a prevalent role in many cultures. The scientific literature has widely addressed the benefits arising from moderate alcohol consumption. In this context, red wine and beer consumption has been associated with a reduced risk of coronary heart disease (CHD)42. Light to moderate consumption of red wine has been proposed as a possible explanation for the phenomenon known as the “French Paradox”52, which postulates that the French population shows relatively lower CHD incidence/mortality rate compared to other Western populations, despite their diets containing higher amounts of total fat and saturated fatty acids. Numerous studies provide evidence that light to moderate alcohol consumption is associated with higher levels of high-density lipoprotein cholesterol (HDL-C), a decreased risk of cardiovascular diseases, a lower incidence of type-2 diabetes (T2D), and a reduction in lipid oxidative stress25.
The fermentation process of these beverages is initiated by microorganisms, which involves the chemical transformation of primary compounds into new secondary metabolites that generate organoleptic changes in the product, thereby extending its shelf life83. During the process, primary and secondary metabolites are produced, including antibiotics, carbon dioxide, alcohol, vitamins, folates, organic acids, polyphenols and peptides, all of which are considered bioactive compounds17.
The health benefits associated with fermented foods and beverages are often attributed, among other biologically active compounds, to bioactive peptides (BPs) that may be synthesized during the microbial degradation of proteins in the fermentation process57. BPs can be produced through enzymatic hydrolysis or fermentation by proteolytic microorganisms22. Several authors have presented scientific evidence indicating that food peptides exhibit specific biological activities on health, aside from their nutritional value16. During the fermentation process, encrypted biological active peptides can be released from their inactive forms present in the structure of their precursor protein22. The type of microorganism used is crucial to obtain optimal peptide production and high bioactivity during microbial fermentation39. In this context, lactic acid bacteria (LAB), ubiquitous microorganisms involved in numerous fermentation processes, exhibit proteolytic activity on proteins from natural environments, contributing to the release of BPs from dietary proteins46. BPs are specific protein fragments that exert various beneficial effects on the human body, ultimately influencing health, depending on the structural properties, amino acid composition and sequences of the peptides15. They are short amino acid sequences, usually comprising 2–20 residues, with low-molecular weight (<6000Da) compared to the protein mass. Furthermore, they also have specific N-terminal and C-terminal amino acid residues74. BPs can be absorbed through the intestines, and enter the circulatory system in intact forms, enabling them to exert their biological activities. Consequently, they are potent candidates for the development of novel healthcare products and functional foods88.
This review reports the most recent progress, challenges and perspectives related to the biological activities of peptides derived from alcoholic fermented beverages and their potential health benefits.
Bioactive peptides in alcoholic fermented beveragesBioactive peptides in wineWine is a traditional alcoholic beverage that is typically made from fermented grape juice. The primary role of microorganisms involved in the vinification process is to metabolize the sugars found in grape juice into ethanol, reduce acidity and enhance flavor in the final product80. Moderate wine consumption has been associated with beneficial effects on human health, which are partially attributed to the presence of BPs43. Wine peptides originate from multiple sources, some of which come from grapes, while most of them develop during the different stages of winemaking, either through yeast autolysis2,85 or the proteolytic activity of LAB on wine proteins6–8.
The composition of peptides in wine is modified continuously during the vinification process due to multiple interactions between yeasts and bacteria in grape must21. During alcoholic fermentation (AF), the initial stage of winemaking, various yeast species, mainly Saccharomyces cerevisiae, develop and metabolize sugars from grape juice into ethanol and carbon dioxide. During this process, concomitantly with the increase in yeast population, peptides are assimilated along with free amino acids, consequently leading to a depletion of assimilable nitrogenous compounds in the medium71. However, in the final stages of this fermentation, when the assimilable nutrients become depleted and metabolites accumulate in the medium, there is an excretion of free amino acids and small peptides resulting from the yeast autolysis into the wine4, and also from the action of endo and exoproteases on proteins derived from yeast or grape juice60. This process is a crucial step in the production of sparkling wines.
The main constituents released by yeast lysis are peptides and, to a lesser extent, amino acids and proteins4. In this regard, other authors have demonstrated that after the autolysis of S. cerevisiae, a substantial increase in nitrogenous compounds released into the extracellular medium was evidenced2,4,9.
During the vinification process, at the end of AF, numerous LAB associated with wine can increase their population by utilizing residual sugars and nitrogen compounds released by yeasts. Oenococcus oeni is the main LAB found at this stage, due to its ability to survive the harsh conditions of wine (high alcohol content, low pH, and low nutrient levels)6. This bacterium can carry out malolactic fermentation (MLF) converting the l-malic acid from grape juice into l-lactic acid and carbon dioxide, a process considered favorable for bacterial growth and viability maintenance in wine conditions10. Additionally, MLF brings about beneficial effects on wine, improving its organoleptic characteristics and increasing its microbiological stability11. In general, wine provides a limited environment for bacterial growth due to the scarcity of available nutrients3. As LAB have complex nutritional requirements, the release of peptides and amino acids has an important role in sustaining O. oeni growth in natural media because this bacterium cannot synthesize several amino acids75. However, O. oeni can use small peptides containing up to eight amino acid residues to supply its nutritional requirements8,72. Aredes-Fernández et al.8 demonstrated that the replacement of essential amino acids by dipeptides results in a significant increase in the growth parameters of the microorganism. Consequently, O. oeni has developed complex enzyme systems that contribute to the production of small peptides and the release of free amino acids from the large peptides in its immediate environment. Manca de Nadra et al.53 reported the proteolytic activity of the X2L strain of O. oeni on the nitrogenous macromolecular fraction of white and red wines, which promoted the release of peptides. Moreover, under starvation conditions, the release of O. oeni protease into the extracellular medium is increased54. The exoprotease of O. oeni has been partially purified and characterized29. In addition, another study69 confirmed the presence of extracellular protease activity in the IOB84-13 strain of O. oeni, which was evidenced during the growth phase on a poor-nitrogen medium. Moreover, Folio et al.31 demonstrated the presence of extracellular proteins from O. oeni ATCC BAA-1163. One of these proteins, named EprA, was isolated and characterized for its hydrolytic activity against several proteins.
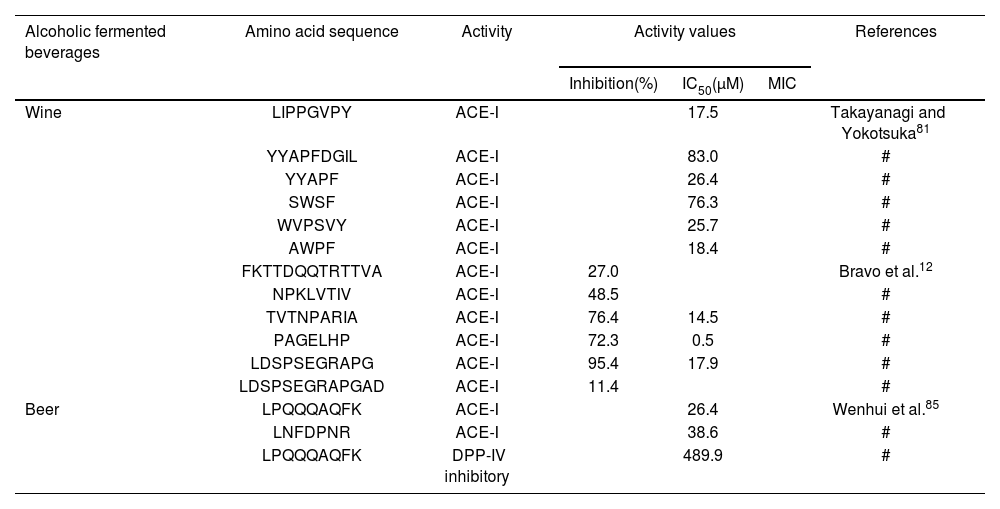
In this way, it has been documented that LAB associated with MLF can release BPs from yeast and wine proteins1,6,7,9,80. Numerous BPs with antihypertensive activity have been identified in wine. The pioneering study by Takayanagi and Yokotsuka81 first reported the ACE inhibitory activity (ACE-I) of six peptides, LIPPGVPY (IC50=17.5μM), YYAPFDGIL (83.0μM), YYAPF (26.4μM), SWSF (76.3μM), WVPSVY (25.7μM), and AWPF (18.4μM) (Table 1). Likewise, Pozo-Bayón et al.67 reported the antihypertensive activity of 41 wines, determining ranges from 10.3% to 95.4%, with the highest activity in red wine. However, these authors highlighted the possible contribution of polyphenols to this activity. In addition, Alcaide-Hidalgo et al.2 determined ACE-I activity in peptides released from S. cerevisiae in a model wine (Table 1). In this assay, there were no phenolic compounds, thus attributing ACE-I activity exclusively to yeast peptides. No et al.63 demonstrated that the increase in ACE-I activity takes place after AF, suggesting that the nature of the ACE inhibitors could be peptides. Nevertheless, other authors determined that ACE-I activity decreased after MLF, despite reaching its maximum value during aging with lees1. Additionally, the aging process is accompanied by yeast autolysis, which is responsible for the increased peptide concentration and ACE-I activity4.
Biological activities of isolates and identified peptides from alcoholic fermented beverages.
| Alcoholic fermented beverages | Amino acid sequence | Activity | Activity values | References | ||
|---|---|---|---|---|---|---|
| Inhibition(%) | IC50(μM) | MIC | ||||
| Wine | LIPPGVPY | ACE-I | 17.5 | Takayanagi and Yokotsuka81 | ||
| YYAPFDGIL | ACE-I | 83.0 | # | |||
| YYAPF | ACE-I | 26.4 | # | |||
| SWSF | ACE-I | 76.3 | # | |||
| WVPSVY | ACE-I | 25.7 | # | |||
| AWPF | ACE-I | 18.4 | # | |||
| FKTTDQQTRTTVA | ACE-I | 27.0 | Bravo et al.12 | |||
| NPKLVTIV | ACE-I | 48.5 | # | |||
| TVTNPARIA | ACE-I | 76.4 | 14.5 | # | ||
| PAGELHP | ACE-I | 72.3 | 0.5 | # | ||
| LDSPSEGRAPG | ACE-I | 95.4 | 17.9 | # | ||
| LDSPSEGRAPGAD | ACE-I | 11.4 | # | |||
| Beer | LPQQQAQFK | ACE-I | 26.4 | Wenhui et al.85 | ||
| LNFDPNR | ACE-I | 38.6 | # | |||
| LPQQQAQFK | DPP-IV inhibitory | 489.9 | # | |||
IC50: peptide concentration needed to inhibit 50% of the original ACE-I, antioxidant, and DPP-IV activities.
The symbol “#” in the reference column indicates that this reference is the same as the one in the previous line.
Aredes-Fernandez et al.9 reported the expression of bacterial proteolytic activity after the inoculation of O. oeni in a synthetic simil-wine medium under accelerated yeast autolysis conditions. These authors showed that this proteolytic activity causes a decrease in the concentration of proteins released by yeast, accompanied by a concomitant release of peptides, which simultaneously produces a significant increase in ACE-I as well as antioxidant activities.
Similar results were reported by Apud et al.6, who demonstrated that the strain m1 of O. oeni releases biologically active peptides from the protein-polypeptidic fraction with a molecular weight higher than 12kDa from four Argentinian wine varietals (Cabernet Sauvignon, Malbec, Tannat and Torrontés).
Stivala et al.80 evidenced that the proteolytic activity of the X2L strain of O. oeni in grape juice or in grape juice fermented with S. cerevisiae mc2 led to an increase in antioxidant activity (determined by FRAP and DPPH scavenging assays) and ACE-I activities. These authors also demonstrated that higher biological activities were evidenced in the yeast-fermented medium, an effect related to the expression of a stress exoprotease released under unfavorable environmental conditions, which enables peptide release with specific biological activities primarily from a protein substrate derived from yeast.
In the last decade, several by-products from the agri-food industry have emerged as an interesting alternative protein source. In this regard, Bravo et al.12 reported the ACE-I activity of peptides from wine lees hydrolysate using commercial endo- and exo-peptidases. These authors identified six antihypertensive peptides, with three of them showing ACE-I (IC50) values lower than 20μM (Table 1). Additionally, these peptides with antihypertensive effects were evaluated in vivo on spontaneously hypertensive rats, leading to a reductin in blood pressure.
The antioxidant properties of wine have been traditionally attributed to polyphenols18; however, various studies have reported that peptides released by the action of peptidases of O. oeni on proteins from yeast autolysis and grape juice may exhibit antioxidant activity2,6,9,80. In this context, Apud et al.6,7 found that peptides released by the proteolytic activity of O. oeni X2L on the macromolecular nitrogen fraction of wines showed antioxidant activity. Similar results were obtained by Aredes-Fernández et al.9 and Stivala et al.85, who demonstrated that the proteolytic activity of a strain of O. oeni isolated from Argentinian wines enables the release of antioxidant peptides from autolyzed yeast proteins and fermented grape juice by S. cerevisiae. These results are comparable with those observed in other fermented foods (fermented milk and derivatives, fermented meat and fermented soybean products), which reported antihypertensive peptides with radical scavenging (antioxidant) activity, suggesting the presence of multifunctional activity70.
Bioactive peptides in beerBeer is one of the most widely consumed alcoholic beverages worldwide and is traditionally brewed with raw materials including water, malt (usually barley), hops and yeast27. Briefly, the germination process of malt grains produces enzymes that hydrolyze starches into dextrins and fermentable sugars that will be used by yeasts during AF in the brewing process.
Despite its few starting ingredients, the different processing steps (malting, maceration, fermentation, among others) make beer a complex beverage rich in micronutrients and compounds with interesting biological activities. According to Cortacero-Ramírez et al.19, beer contains more than 800 compounds, which can be classified into volatile and non-volatile components. The former are primarily synthesized in the fermentation step and are responsible for the aroma of beer (alcohols, ethers, aldehydes, ketones, organic acids, phenolic compounds, among others). On the other hand, non-volatile compounds include inorganic compounds, carbohydrates, nitrogenous compounds, phenolic compounds, ethyl alcohol, vitamins, and other compounds such as lipids and organic acids present in lower concentration. Many of these components have been studied for their beneficial effects for the consumer.
Nitrogenous compounds mostly originate from cereals, and can be modified during each step of the brewing process. Beer contains between 0.2 and 0.6g/100ml of protein-derived material (mainly from barley) in the form of peptides and polypeptides19. Peptides present in beer result from protein degradation during the malting process and, to a lesser extent, from yeast metabolism and autolysis85. On the other hand, amino acids (except proline) are used by yeast during fermentation, with some of them being metabolized to produce aromatic compounds, esters, alcohols and aldehydes13. These nitrogenous compounds have been extensively studied for their impact on organoleptic and technological characteristics (foam stability, haze formation, color, palatability), as well as for their ability to cause allergic reactions or trigger autoimmune responses in individuals with celiac disease66. In addition to their nutritional role, the proteins and amino acids in beer can have other health effects. A study showed that beers with high protein content (5.7g/l) improved the lipid profile in experiments carried out with rats37. With regard to amino acids, the Trp present in beer is a precursor in the synthesis of melatonin, a peptide hormone that has also been found in wines. Melatonin exerts multiple benefits due to its anticancer, antioxidant, immunomodulatory and neuroprotective capacities56.
However, to date, there is limited information about peptides with beneficial biological activities in beer. In a study conducted with Australian beers86, a 10kDa peptide with antioxidant properties capable of scavenging reactive oxygen species was identified. However, the antioxidant properties of beer have been mainly attributed to its phenolic content25. Wenhui et al.85 obtained 50 peptides containing between 4 and 14 amino acids from Tsingtao draft beer and studied them based on their potential as angiotensin-converting enzyme (ACE) and dipeptidyl peptidase IV (DPP-IV) inhibitors. Eight peptides (ERFQPMFR, FPVGRGL, PPPVHDFNME, PMAPLPRSGP, LNFDPNR, LAQMEAIR, LPGELAK, and LPQQQAQFK) were selected using the Peptide Ranker program, comparing their amino acid sequences with previous literature. Among them, LNFDPNR and LPQQQAQFK were selected for their high potential for ACE and DPP-IV inhibitory activities (Table 1). Finally, the activities were further corroborated with in vitro assays, and their inhibition mechanisms were verified using a molecular docking model.
On the other hand, the by-products of the brewing industry (yeast and spent grain) have recently received the attention of researchers and have been extensively studied for their content of peptides with antioxidant, antihyperglycemic, antihypertensive, and lipid-lowering properties, among others65.
Given that yeasts are fundamental ingredients in beer, and numerous studies support their role in the presence of peptides with biological activity2,49, this review aims to encourage future research on BPs released by S. cerevisiae during beer fermentation that have not been explored to date.
Bioactive peptides in ciderCider is a beverage that results from the alcoholic fermentation of apple juice (AJ) by yeasts (mainly of the Saccharomyces genus), achieving a minimum alcoholic proof of 4% (v/v). When fermentation occurs spontaneously, LAB such as O. oeni can carry out MLF93.
The proteolytic activity of different O. oeni strains has been demonstrated against various substrates such as grape juice, yeast mannoproteins, casein, and more recently AJ proteins6,30,48. Recently, Kristof et al.48 have reported that cider fermented with O. oeni showed enhanced in vitro antihypertensive activity, suggesting that this activity could be related to the release of peptides during MLF. However, to date, there are no reports of BPs in ciders.
Thus, considering the potential of O. oeni to hydrolyze proteins31, it is likely that peptides with biological activities are released during cider fermentation, a behavior that has already been reported in wines1,6.
Bioactive peptides in sakeSake, a traditional Japanese fermented alcoholic beverage, is a transparent moderate-alcohol drink made from rice and water by a fermentation process involving a fungus (Aspergillus oryzae, named koji-mold) and yeast (S. cerevisiae)61. The alcoholic content of sake generally ranges between 13% and 17vol.%. In the manufacturing process, rice grains are fermented with water and koji-mold for 5–7 days. The amylase enzymes of koji-mold degrade the rice starch and the released glucose is then fermented by yeast for 7 days at 4°C, resulting in the production of ethanol33.
A. oryzae produces extracellular acid proteinases and acid carboxypeptidases that degrade the rice proteins and produce large amounts of amino acids and short-chain peptides42. Several studies have demonstrated that short-chain peptides in sake exhibit angiotensin-I-converting enzyme (ACE-I) inhibitory activity77 and exert antihypertensive activity when orally administered to spontaneous hypertensive rats76. Yamada et al.90 developed a method to breed a sake yeast that increases the concentration of peptides in the beverage, with the ACE-I inhibitory activity approximately increasing 3.6-fold. This strain is currently utilized in sake breweries.
Sake has numerous positive effects on human health, with several studies reporting anxiolytic effects41. In addition, sake concentrate, and its specific sugar (α-d-glucoside ethyl ester) have been reported to inhibit chronic alcoholic liver injury40. The compounds 2,5-diketopiperazines (DKPs), also known as cyclic dipeptides, have received considerable attention as bioactive compounds62. They can be formed from the N-terminal amino acid residues of a linear peptide or protein and have been identified in various foods and fermented beverages, such as beer, distillation residue of awamori, and sake. Sake may contain unique bioactive compounds, including short-chain pyroglutamyl peptides; however, there is limited information available on the pyroglutamyl peptides present in this beverage47.
Bioactive peptides in low alcohol and non-alcoholic fermented beveragesBioactive peptides in kefirKefir is a traditional fermented beverage with a low and variable alcohol content (0.5–2%). It is obtained from milk or water kefir grains, resulting in two types of this beverage: dairy and non-dairy kefir36. In this section, we focus on water kefir, also known as aqua kefir or sugary kefir. It is produced by the action of a consortium of yeasts, LAB and acetic bacteria, which exist in a symbiotic association, being Saccharomyces spp., Lactobacillus spp. and Acetobacter spp., respectively, the main microbial genera of water kefir grains50.
Throughout history, kefir has been recommended for the treatment of numerous diseases, including the clinical management of tuberculosis, cancer, gastrointestinal disorders, metabolic diseases, hypertension, ischemic heart disease, allergies and antimicrobial activity68. The health benefits of this beverage could be attributed to the presence of probiotic microorganisms and bioactive compounds yielded during the fermentation process, among them, peptides with beneficial properties73.
Water kefir is prepared using a solution of water and sugar, typically sucrose, as the substrate for yeasts during fermentation. Water kefir does not contain lactose or proteins; therefore, peptides with biological activities have not been identified yet14. Nevertheless, yeasts can autolyse and release low-molecular-weight compounds and peptides with beneficial properties. In this context, the release of BPs by S. cerevisiae during its autolysis in wine medium is well-known1,9.
Furthermore, the incorporation of fruits into water kefir elaboration is currently gaining popularity, increasing its health benefits45. The presence of proteins in fruits could release peptides with biological activities during the fermentation process; however, further studies are required to validate this hypothesis44.
Bioactive peptides in kombuchaKombucha is a fermented drink of Asian origin that is considered a non-alcoholic beverage with a low alcohol content (approx. 0.5% v/v)64. Despite its Asian origin, kombucha has gained popularity in the West due to its therapeutic, antimicrobial, antioxidant, anticancer, antidiabetic effects, and its potential in the treatment of gastric ulcers and high cholesterol levels28. This beverage is a tea that is mildly acidic, moderately sweet and slightly effervescent. During its production process, it is supplemented with sugar and flavors and undergoes fermentation with a symbiotic culture of yeasts (Saccharomycodes, Saccharomyces, Schizosaccharomyces and Pichia species, among others), acetic acid bacteria (Gluconobacter and Acetobacter species) and LAB (Lactobacillus and Lactococcus species)83.
The beneficial properties of kombucha are mainly attributed to the presence of live microorganisms, and also of proteins, amino acids, polyphenols, microbial enzymes, and a variety of micronutrients produced by microorganisms during fermentation32.
Although researchers have recently identified numerous bioactive food components, there is a need to further explore promising ACE-inhibitory peptides, given the antihypertensive potential of kombucha tea. This potential was evaluated through the inhibition of ACE activity and promising results were obtained28. Nevertheless, to date, there are no reports on BzP production or release during the kombucha production process.
Detection, isolation and identification of bioactive peptidesInitially, peptides were isolated from natural sources, including tissues or cell cultures cultivated in bioreactors. However, their fractionation and isolation is quite difficult due to their different physicochemical properties, such as size, charge, adsorption characteristics and solubility, being a challenging task even for experienced scientists. Consequently, numerous techniques have been developed over the decades to ensure their effective isolation.
Detection and identificationPeptides are commonly detected by absorbance at 200–220nm. However, numerous compounds present in wine may interfere with the ultraviolet detection of peptides when low wavelengths are used. Thus, for the analysis of these compounds, it is advantageous to utilize sensitive and selective detection methods. For this purpose, it is possible to form derivatives of peptides that can be detected at higher and more specific wavelengths. Fluorescence detection can also be employed to detect peptides containing fluorescence amino acids (tyrosine and tryptophan). For peptides lacking this property, the formation of derivatives using derivatizing agents has proved to be very useful59.
To identify and characterize BPs, mass spectrometry (MS) is widely used. The MS analysis is performed on individual peptides. Key steps in this strategy include sample preparation, enrichment of peptides of interest, and cleanup or desalting of the final peptide mixture prior to the MS analysis by either MALDI-TOF-MS/MS (matrix-assisted laser desorption/ionization-time of flight tandem mass spectrometry) or LC–ESI-MS/MS (liquid chromatography–electrospray ionization tandem mass spectrometry). In MS, an unknown peptide is subjected to fragmentation, and its fragments (ions) are recorded in a spectrum known as peptide mass spectrum. In MS/MS, these ions are known as precursor ions, breaking into two parts and generating one fragment containing the N-terminus of the original peptide sequence and a complementary fragment containing the C-terminus. Then, computational methods deduce the peptide sequence from its spectrum. An MS/MS spectrum is similar to an MS spectrum, with the difference that in the former, the peaks correspond to fragment ions of a peptide, whereas, in the latter, the peaks correspond to complete peptide ions20.
Nowadays, because of the development of science and technology, there are novel methods, combined with computer-aided design, online data and virtual screening techniques that can be used to investigate peptide activity and improve their identification85. In this context, virtual screening and molecular docking are used with the aim of confirming BP identification87. These methods are widely utilized by computational scientists due to their low cost, speed, and ability to explore large numbers of compounds.
Chromatographic separation techniquesHigh-performance liquid chromatography (HPLC) has become a widely used, well-established tool for the separation, identification, and purification of BPs. It offers numerous advantages, such as versatility, short analysis times, high resolution, effective separations, and suitability for automation procedures.
There are several mechanisms that could be employed in the chromatographic separation of peptides, such as those based on molecule size (gel filtration chromatography), charge (ion-exchange chromatography), hydrophobicity (reversed-phase and interaction chromatography), and even their combinations.
Gel filtration chromatography (GFC) is used to obtain specific BPs with molecular weights and higher biological activity. It is an accessible chromatography technique that is known for its high selectivity and resolution, and has been extended to a molecular weight range from 100 to 8×107Da. However, it also has a few drawbacks, such as high costs and time-consuming sample collection89.
Ion-exchange chromatography (IEC) is an efficient technique used for the purification of bioactive peptides. Its great advantage is the implementation of mass separation, which could save time and improve accuracy (Levison, 2003). However, its disadvantages include cost-effectiveness, complexity, and sensitivity to pH and metal ions89.
Reversed-phase high-performance liquid chromatography (RP-HPLC) is the most widely used technique, being capable of separating peptides with almost identical sequences based on their hydrophobic properties, especially in the study of peptide structures and their functional properties79. This technique is characterized by the use of a stationary phase and an aqueous mobile phase containing an organic solvent, either acetonitrile or an alcohol, with the most commonly mixture being water and acetonitrile under gradient conditions. The least used mixtures are those that use methanol, phosphate buffer and tetrahydrofuran59. However, this technique has disadvantages such as the use of large amounts of solvent, generating in specific cases, low yields and requiring long purification times, leading to increased production costs38.
Electrophoretic separation techniquesAlthough RP-HPLC remains the preferred peptide separation method, electrophoretic techniques are recognized for their effectiveness in separating, visualizing, and quantifying single proteins or peptides. Several of these approaches are automated, enabling the rapid processing of a large number of samples.
Capillary electrophoresis (CE) is considered successful in peptide separation, offering separation mechanisms similar to those observed in gel electrophoresis, but without the time-consuming gel preparation and staining methods. Detection occurs in real time, similar to HPLC, but with superior separation efficiency and resolution compared to HPLC35.
Structure–activity relationship of bioactive peptidesBioactive peptides derived from the fermented beverages described in this review mainly exhibit ACE-inhibitory and antioxidant activities. Although the structure–activity relationship (SAR) has not been well established yet for ACE-inhibitory and antioxidant peptides, these activities are significantly influenced by composition and orientation of amino acids in their sequences26.
Potent ACE-inhibitory peptides typically consist of short sequences with 2–12 amino acid residues because, generally, large peptides cannot bind to the active site of ACE23. However, the type of amino acids could be more relevant than the length of the peptide sequence. In this context, peptides containing highly acidic amino acids, such as Asp and Glu, have a net negative charge whose interaction with ACE could chelate zinc atoms in its active center and inhibit enzyme activity5. The presence of C-terminal tripeptides composed of aromatic amino acids such as Phe, Tyr and Trp or N-terminal tripeptides containing branched-chain aliphatic amino acids, such as Val, Leu and Ile, was found to exhibit high ACE-inhibitory activity and low IC50 values24. Furthermore, highly hydrophilic peptides are weak ACE-inhibitors because they cannot correctly fit into the active site of ACE. Nevertheless, hydrophilic–hydrophobic peptides can positively interact with the ACE active center to inhibit its activity91.
Antioxidative peptides are proton donors that have the ability to finish a radical chain reaction, with their amino acid residues being the primary targets for oxidants. Therefore, the transfer rate of radicals to amino acids and their stability significantly affect the effectiveness of amino acids as antioxidants92. Tyr, Met, Lys, Cys, and His are some examples of amino acids that exhibit antioxidant activity58. Peptides containing hydrophobic amino acids exhibit higher antioxidant activity than those with hydrophilic amino acids because they can improve the interaction between the peptides and hydrophobic targets such as the cell membrane, consequently, enhancing bioavailability and showing increased interaction with oxidants94. In a study, Wan et al.84 found that the presence of hydrophobic amino acids (Gly and Pro) and the -SH group of Cys as an effective hydrogen donor to free radicals, contributes to a high antioxidative property in the peptide. It is thought that peptides with an amphiphilic nature could increase antioxidant activity by enhancing peptide solubility and facilitating interaction and proton exchanges with oxidants94.
The positions of amino acid residues in peptide sequences, along with their spatial structure also play an important role in antioxidant activity51. It has been reported that peptides with a similar amino acid composition but minor differences in the sequence may not exert a similar biological effect94.
Computer-based molecular modeling techniques have been recently developed to determine the relationship between peptide activity and molecular characteristics55. In this context, quantitative structure–activity relationship (QSAR) and molecular docking are two standard tools employed in this field. QSAR is a model that mathematically determines the activity of peptides based on the quantitative modeling of molecular properties78. QSAR is conducted by collecting quantitative data of all amino acid sequences, using factors that describe specific structural properties (e.g., hydrophilicity and electronic properties) to build a mathematical model.
ConclusionIn conclusion, the beneficial properties of fermented foods are the object of many studies. Nowadays, these products are regarded not only as diet and nutrient sources but also as sources of bioactive compounds that may confer health benefits and inhibit the development of diseases. While alcoholic fermented beverages have been studied to a lesser extent, they also serve as sources of components with health-promoting effects, particularly biologically active peptides. These peptides exhibit various activities, mainly functioning as antihypertensive, antioxidant, antidiabetic, antiobesity and antimicrobial agents. To date, there has been scant or no research at all on the presence or liberation of peptides in fermented beverages, despite their wide consumption.
Existing evidence demonstrates that moderate alcohol consumption provides benefits against major chronic conditions such as cardiovascular diseases. In this regard, perhaps a synergistic effect between alcohol and the bioactive compounds of fermented beverages could be related to the prevention of several common chronic diseases. Nevertheless, clinical trials are needed to establish and elucidate the joint mode and mechanism of action of alcohol and BPs.
It will be interesting in the near future to conduct further research to expand the current knowledge of peptides from alcoholic fermented beverages, focusing on their potential biological properties on health, as well as more studies and new approaches for enhancing the oral bioavailability of these peptides.
Conflicts of interestThe authors confirm that they have no conflicts of interest regarding the work described in this manuscript.
The authors wish to thank the National Scientific and Technical Research Council of Argentina (CONICET).