Fumonisin occurrence was reported in wheat grains and F. proliferatum has been suggested to be the main contributor to its presence in wheat. Thus, a survey was performed in order to study the impact of four commercial fungicides used in Argentina for controlling Fusarium head blight disease (epoxiconazole+metconazole, tebuconazole, pyraclostrobin+epoxiconazole, and prothioconazole) on growth and fumonisin production of two F. proliferatum strains in relation to water activity (aW; 0.99, 0.97, 0.95) and temperature (15°C and 25°C). Most fungicides reduced growth rates when compared to the control (reduction increased as fungicide concentration increased), and reduced fumonisin production when they were used at high doses; however, most fungicides enhanced fumonisin production at sublethal doses, with the exception of prothioconazole. Thus, fungicides used for FHB management could enhance fumonisin production by F. proliferatum strains present in wheat grains.
En los últimos años se ha observado contaminación de granos de trigo con fumonisinas, y se ha postulado a Fusarium proliferatum como el principal responsable de la presencia de dicha micotoxina en este cereal. Por este motivo, se realizó un ensayo de manera tal de estudiar el efecto de cuatro formulaciones comerciales de fungicidas utilizadas para el manejo y control de la fusariosis de la espiga de trigo en el crecimiento y la producción de fumonisinas de dos cepas de F. proliferatum, en relación con la actividad de agua (aW; 0,99; 0,97 y 0,95) y la temperatura (15 y 25°C). La mayoría de los productos fueron capaces de reducir la velocidad de crecimiento de ambas cepas, con una mayor acción a medida que la concentración del fungicida aumentó, y también de reducir la producción de fumonisinas cuando fueron utilizados en altas dosis. Sin embargo, la producción de fumonisinas se elevó cuando estos fueron utilizados a dosis subletales, excepto cuando se aplicó protioconazol. Se concluye que los fungicidas comúnmente utilizados para el control de la fusariosis de la espiga de trigo en Argentina podrían estimular la producción de fumonisinas en cepas de F. proliferatum presentes en los granos de trigo, llevando así a la contaminación de este cereal con estas micotoxinas.
Fusarium head blight (FHB) is one of the most important diseases of wheat and other cereals in many areas of the world. In Argentina, Fusarium graminearum sensu stricto is the main pathogen associated with this disease, consequently this cereal could be contaminated with mycotoxins, deoxynivalenol (DON) being the predominant one26,39,44. DON occurrence in wheat grains is of concern because this toxin has been associated with adverse health effects in humans and animals41. Wheat in Argentina is often grown in rotation with maize, increasing the risk of fungal and mycotoxin accumulation. Fumonisin (FB) contamination of wheat grains and wheat-based products has recently been observed not only in Argentina, but also in other countries5,8,9,38. Moreover, Fusarium proliferatum and Fusarium verticillioides were isolated from wheat grains, and many authors have suggested that F. proliferatum could be the main contributor to FB occurrence in wheat2,18,36,38.
FBs are mycotoxins that can cause various adverse health effects in humans and animals. FB consumption has been associated in humans with esophageal cancer, neural tube defects, and stunting in children11,45. Fumonisin B1 (FB1) is the most significant in terms of toxicity and occurrence. It has been classified as a “2B” carcinogen by the International Agency of Research on Cancer20, and the Joint FAO/WHO Expert Committee on Food Additives has determined a provisional maximum tolerable daily intake of 2μg/kg body weight/day for FB1, FB2 and FB3 alone or in combination47.
As wheat is an important grain cereal used for human consumption its safety is of concern. In order to decrease FHB damage and mycotoxin contamination several strategies are used: combinations of agronomical practices, resistant cultivars and fungicides 14. The use of fungicides is a complementary control measure when weather conditions are conducive to fungal infection. Among the fungicides used, azole application is a primary method for FHB management40. Azoles block the ergosterol biosynthesis pathway by inhibiting sterol a-demethylase24. These compounds have been shown to reduce FHB symptoms and DON content in wheat; however, the effectiveness of azole compounds varies and is strongly dependent on different factors (disease severity, resistance level of the wheat cultivar, and spraying technology)22,24,35,40. Strobirulins are also used to manage FHB by blocking electron transport in the mitochondrial respiratory chain, reducing aerobic energy production, and inhibiting fungus growth46.
A significant focus has been laid on the development and use of fungicides to prevent and control the infection of pathogenic Fusarium spp. of small grain cereal crops, but less attention has been paid to the effect that those fungicide applications may have on mycotoxin production. Moreover, increasing evidence suggest that fungicides might not be as efficient at reducing toxin production, because in certain conditions they may act as stress factors resulting in the induction of toxin biosynthesis. Sublethal doses of some fungicides may lead to a stimulation of mycotoxin production by several Fusarium species15,16,19,23,32,33,43. It is surprising that most reports took no account of the interactions between the efficacy of fungicides and key environmental factors, while it has been demonstrated that the main factors that influence fungal growth and mycotoxin production are temperature, water activity (aW) and the presence of antifungal substances1,25,29,33,43.
As fungicide applications occur in cereal production systems, Fusarium species colonizing ripening cereals are exposed to them45. Thus, the balance among fungi (toxigenic and saprophytic) as well as the diversity of toxin producers and the final amount of mycotoxin contamination are affected by fungi sensitivity to fungicides28. Presuming that fungicides used for FHB management are active against all Fusarium species present in wheat grains, they could be effective against the accumulation of many Fusarium mycotoxins21. When Ramirez et al.43 analyzed some fungicide effects on F. graminearum growth and DON production, they concluded that further work is needed to understand the combined effects of environmental factors and fungicides on growth and mycotoxin production of fungal pathogens and saprophytic populations present in wheat.
Assuming that F. proliferatum strains are responsible for FB contamination of wheat, the aim of the present study was to analyze the impact of four commercial fungicides used in Argentina for controlling FHB on growth and FB production of two F. proliferatum strains in relation to water activity and temperature using a wheat-based medium.
Materials and methodsStrains. Two F. proliferatum strains (ITEM 15654; 15664) isolated from Argentinean wheat grains and characterized by molecular, biological and morphological methods were used. Their ability to produce FBs was analyzed38. These strains are registered at the Microbial Culture Collection of ISPA (Institute of Sciences and of Food Production), Italy, and are deposited at the Research Institute on Mycology and Mycotoxicology (IMICO, UNRC-CONICET). Cultures are maintained in 15% glycerol at -80°C.
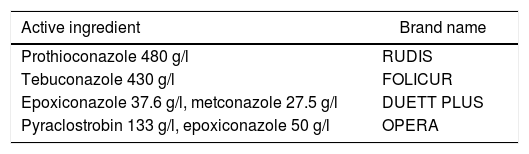
Fungicides and media preparation. Four fungicides were used (Table 1). Dilutions of the fungicides were prepared by mixing appropriate amounts of each fungicide in sterile distilled water and stored at 4°C until used. A 2% (w/v) milled wheat agar was used. The aW of the basic medium was adjusted to 0.995, 0.97 and 0.95 by the addition of different amounts of glycerol13. Media were autoclaved at 120°C for 20min before cooling to 50°C at which time the fungicides were added to obtain the required concentration (0.5, 2.5, 5, and 15μg/ml). Media without fungicide addition were used as control treatments per each aW. Flasks of molten media were thoroughly shaken, prior to pouring into 9cm sterile Petri dishes. The aW of representative media samples was checked with an Aqualab Series 3 (Decagon Devices, Inc.,WA, USA). Uninoculated plates were measured at the end of the experiment in order to detect any significant deviation of the aW.
Inoculation, incubation and growth assessment. Treatments and control plates were inoculated centrally with a 3mm diameter agar disk taken from the margin of a 7-day-old colony of each strain grown on synthetic nutrient agar17 at 25°C and transferred face down to the center of each plate. Inoculated plates of the same aW were sealed in polyethylene bags and incubated at 15 and 25°C for 28 days. A full factorial design was used where the factors were aW, temperature, fungicide concentration and strain. The experiments were carried out with three replicates per treatment.
Fungal growth assessment was performed every day during the incubation period and two diameters of the growing colonies were measured at right angles to each other until the colony reached the edge of the plate. Colony radii were plotted against time, and linear regression was applied in order to obtain the growth rate (mm/day) as the slope of the line. FB determination was performed after the incubation period.
Fumonisin extraction. For FB extraction Petri plates of each strain for every treatment condition were used. Toxins were extracted with 40ml of acetonitrile: water (1:1 v/v) by shaking the whole culture media (∼20g) and mycelia with the solvent for 30min in an orbital shaker (150rpm) and then filtering the extracts through filter paper (No.4; Whatman International Ltd., Maidstone, Kent, UK). An aliquot of the extracts (1000μl) was taken and diluted with acetonitrile: water (1:1 v/v) as necessary for the HPLC-MS/MS analysis.
HPLC-MS/MS analysis. Fumonisin detection was performed as described by Cendoya et al.7 with a Waters 2695 separation module (Waters Corporation, Milford, MA, USA) equipped with a 2695 autosampler and interfaced to a Micromass®156 –Quattro 157 Ultima™ Platinum tandem quadrupole mass spectrometer with electrospray ionization (ESI) source. A 158 XBridge™ C18 (3.5μm, 2.1 x 150mm) column with a XBridge BEH C18 Sentry Guard Cartridge (130 159Å, 3.5μm, 2.1 x 10mm). The mobile phase of the chromatographic procedure was a gradient of 160 aqueous 1% formic acid (solvent A) and methanol 1% formic acid (solvent B). The gradient program was performed as described by Cendoya et al., 7 The flow rate was 0.2ml/min. The temperature of the column was kept at 20°C. The nitrogen flow was adjusted to 109 and 726 l/h for cone and desolvation gases, respectively. Blank matrix extracts were investigated to confirm that no spectrometric interferences came from the matrix. Data acquisition and processing were performed using Mass Lynx V.4.1, Waters INC software. The interface was operated in a positive ion mode. Nebulization and desolvation temperatures were 150 and 200°C, respectively. The capillary voltage was 3.00kV. Multiple-reaction monitoring (MRM) was used for toxin determination. The precursor peak [M+H]+ and two product peaks monitored to accomplish both quantification and qualification criteria, as well as the retention times and the detector settings, are shown in Table 2. Trace m/z 722 >352 was used for the quantification of FB1, while 706> 336 was used for both FB2 and FB3, respectively. Aliquots of 10μl of sample extracts were injected into the HPLC unit. Four points of identification were used to identify FB1, i.e. retention time, the precursor ion [M+H]+ and two product ions (m/z 334 and 352). A calibration curve was obtained injecting 10μl of a different mixed standard solution (FB1+FB2) (0.25; 0.5; 1.0 and 2.0mg/ml). Good linearity was obtained for the calibration range with a correlation coefficient of 0.98 and 0.97, for FB1 and FB2, respectively. The calculated instrumental LOD (S/N=3) for FB1 and FB2 was 0.01 ng/g and LOQ (S/N=5) was 0.05 ng/g, and the relative within day and between-day standard deviations (% RSD) were 6.5.
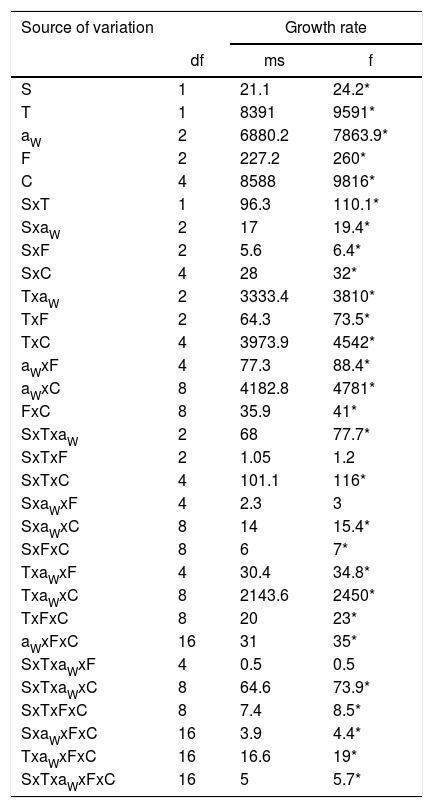
Analysis of variance of the effects of strain (S), water activity (aW), temperature (T), fungicide (F), and fungicide concentration (C) on growth rate in two Fusarium proliferatum strains on a wheat-based medium.
| Source of variation | Growth rate | ||
|---|---|---|---|
| df | ms | f | |
| S | 1 | 21.1 | 24.2* |
| T | 1 | 8391 | 9591* |
| aW | 2 | 6880.2 | 7863.9* |
| F | 2 | 227.2 | 260* |
| C | 4 | 8588 | 9816* |
| SxT | 1 | 96.3 | 110.1* |
| SxaW | 2 | 17 | 19.4* |
| SxF | 2 | 5.6 | 6.4* |
| SxC | 4 | 28 | 32* |
| TxaW | 2 | 3333.4 | 3810* |
| TxF | 2 | 64.3 | 73.5* |
| TxC | 4 | 3973.9 | 4542* |
| aWxF | 4 | 77.3 | 88.4* |
| aWxC | 8 | 4182.8 | 4781* |
| FxC | 8 | 35.9 | 41* |
| SxTxaW | 2 | 68 | 77.7* |
| SxTxF | 2 | 1.05 | 1.2 |
| SxTxC | 4 | 101.1 | 116* |
| SxaWxF | 4 | 2.3 | 3 |
| SxaWxC | 8 | 14 | 15.4* |
| SxFxC | 8 | 6 | 7* |
| TxaWxF | 4 | 30.4 | 34.8* |
| TxaWxC | 8 | 2143.6 | 2450* |
| TxFxC | 8 | 20 | 23* |
| aWxFxC | 16 | 31 | 35* |
| SxTxaWxF | 4 | 0.5 | 0.5 |
| SxTxaWxC | 8 | 64.6 | 73.9* |
| SxTxFxC | 8 | 7.4 | 8.5* |
| SxaWxFxC | 16 | 3.9 | 4.4* |
| TxaWxFxC | 16 | 16.6 | 19* |
| SxTxaWxFxC | 16 | 5 | 5.7* |
A recovery experiment was performed in triplicate by spiking 2% milled wheat agar at levels of 1 to 10μg/g of each fumonisin (FB1 and FB2). Mean recovery ranged from 95 to 98% for FB1 and FB2, respectively.
Statistical analysis. Growth rates and mycotoxin concentration were evaluated by analysis of variance (ANOVA) using SigmaStat for Windows version 2.03 (SPSS Inc., Chicago, IL). Statistical significance was determined at p<0.01.
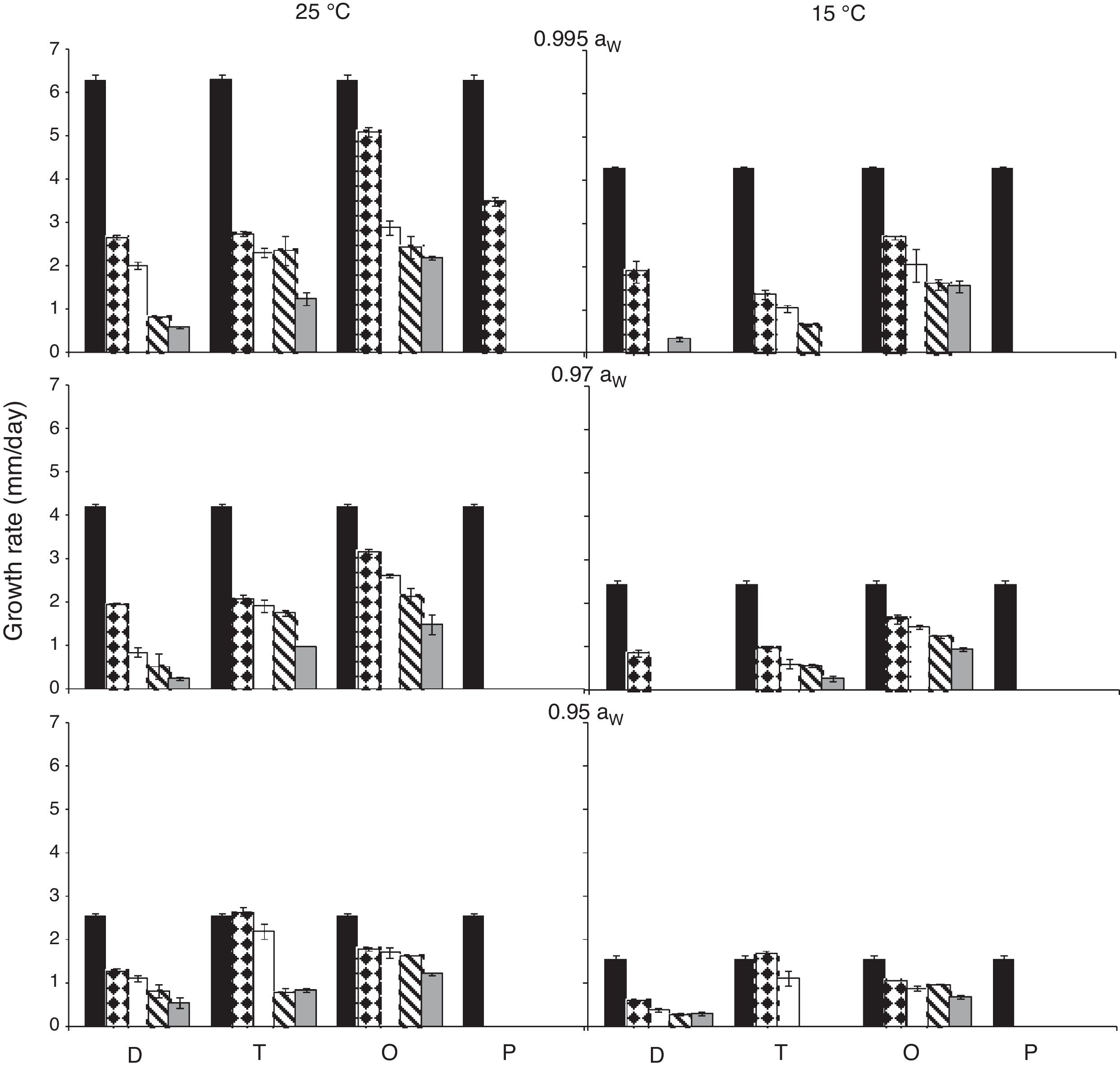
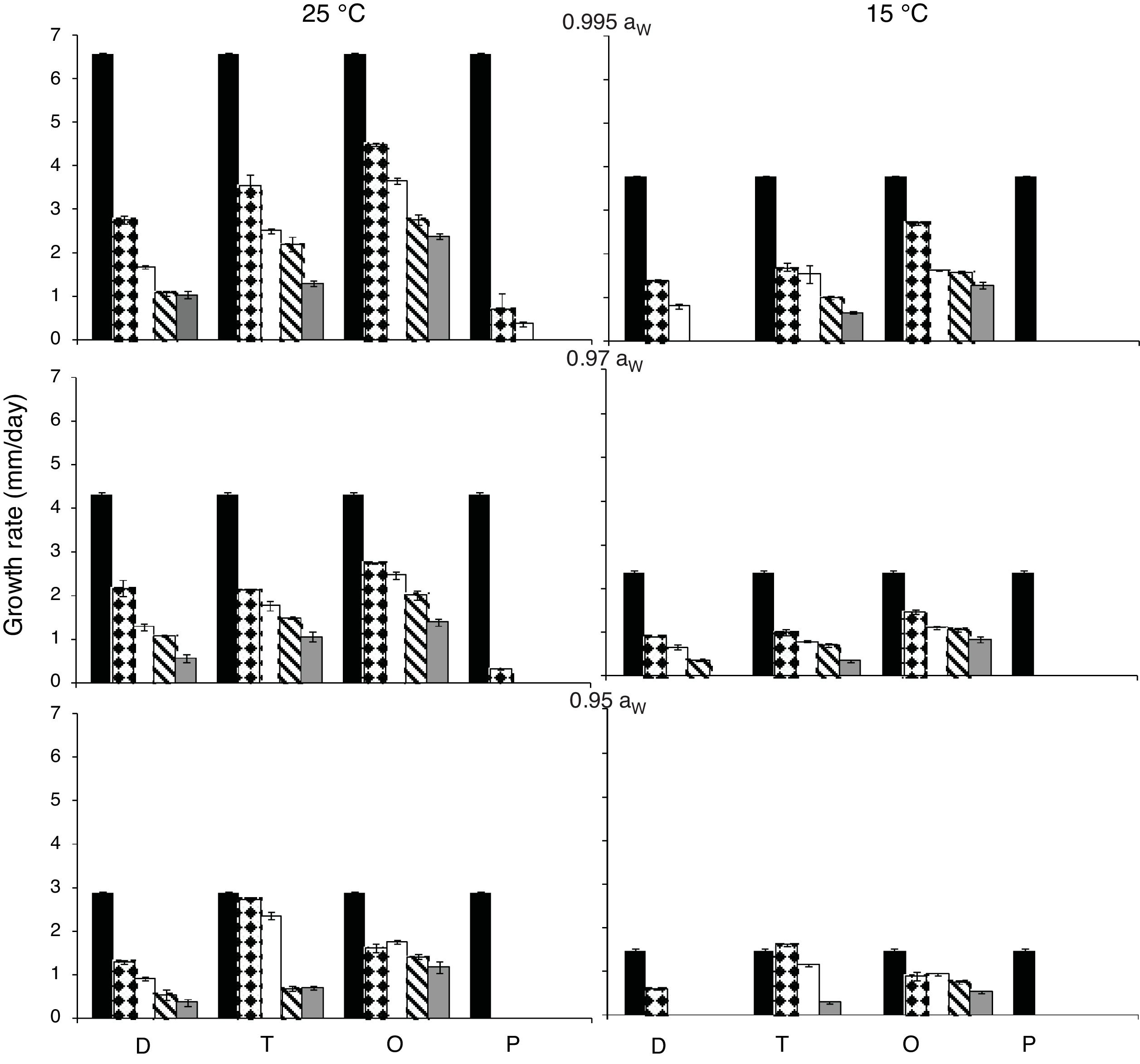
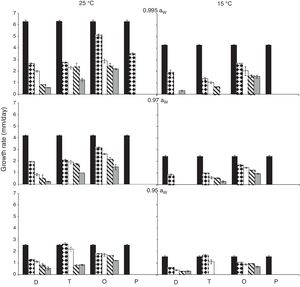
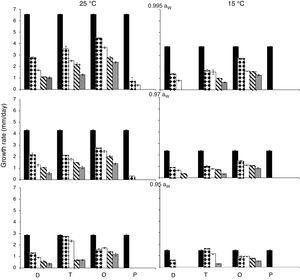
ResultsEffect of aW, temperature and fungicides on growth rate.Fig. 1 and Fig. 2. show the effect of aW, temperature, and fungicides on growth rates of both F. proliferatum strains on a wheat-based medium. Both strains showed a similar behavior in all tested conditions. Under control conditions, i.e. without fungicide addition, maximum growth rates were obtained at 25°C and 0.995 aW, and decreased as the aW of the medium and temperature were reduced. All fungicides were able to reduce the growth rates when compared to the control condition, with the exception of tebuconazole (T) at the lowest concentration used at 0.95 aW regardless of the temperature; however, the same fungicide in other conditions had a considerably effect on fungal growth (at least 50% reduction). This reduction increased as the fungicide concentration increased. Prothioconazole (P) was able to inhibit fungal growth at 15°C in all tested conditions, and at 25°C at 0.97 and 0.95 aW; at 0.995 the growth rate was observed just at 25°C and at the lowest fungicide concentration. The mixture expoxiconazole and metconazole (D) was able to reduce the growth rates of both strains by 50% (sometimes more than 50%) at the lowest concentration used. Pyraclostrobin+epoxiconazole (O) showed the lowest reduction effect in growth rates under all conditions in comparison with control conditions: at high concentrations the growth rate reduction reached 50% while at low concentrations the reduction was about 25%. The ANOVA of the effect of single variables and two- three- four- and five-way interactions revealed that all single variables and some interactions had a significant effect on growth rates. The most significant effect was given by fungicide concentration, followed by temperature, aW, and fungicide (Table 2).
Growth rates (mm/day) for F. proliferatum ITEM 15654 under different aW (0.995; 0.97; 0.95), temperatures (15; 25 ̊C), fungicides (D: epoxiconazole + metconazole; T: tebuconazole; O: pyraclostrobin + epoxiconazole; P: prothioconazole), and fungicide concentrations: 0 (
); 0.5 (); 2.5 (); 5 (); 15 () μg/mL.Growth rates (mm/day) for F. proliferatum ITEM 15664 under different aW (0.995; 0.97; 0.95), temperatures (15; 25 ̊C), fungicides (D: epoxiconazole + metconazole; T: tebuconazole; O: pyraclostrobin + epoxiconazole; P: prothioconazole), and fungicide concentrations: 0 (
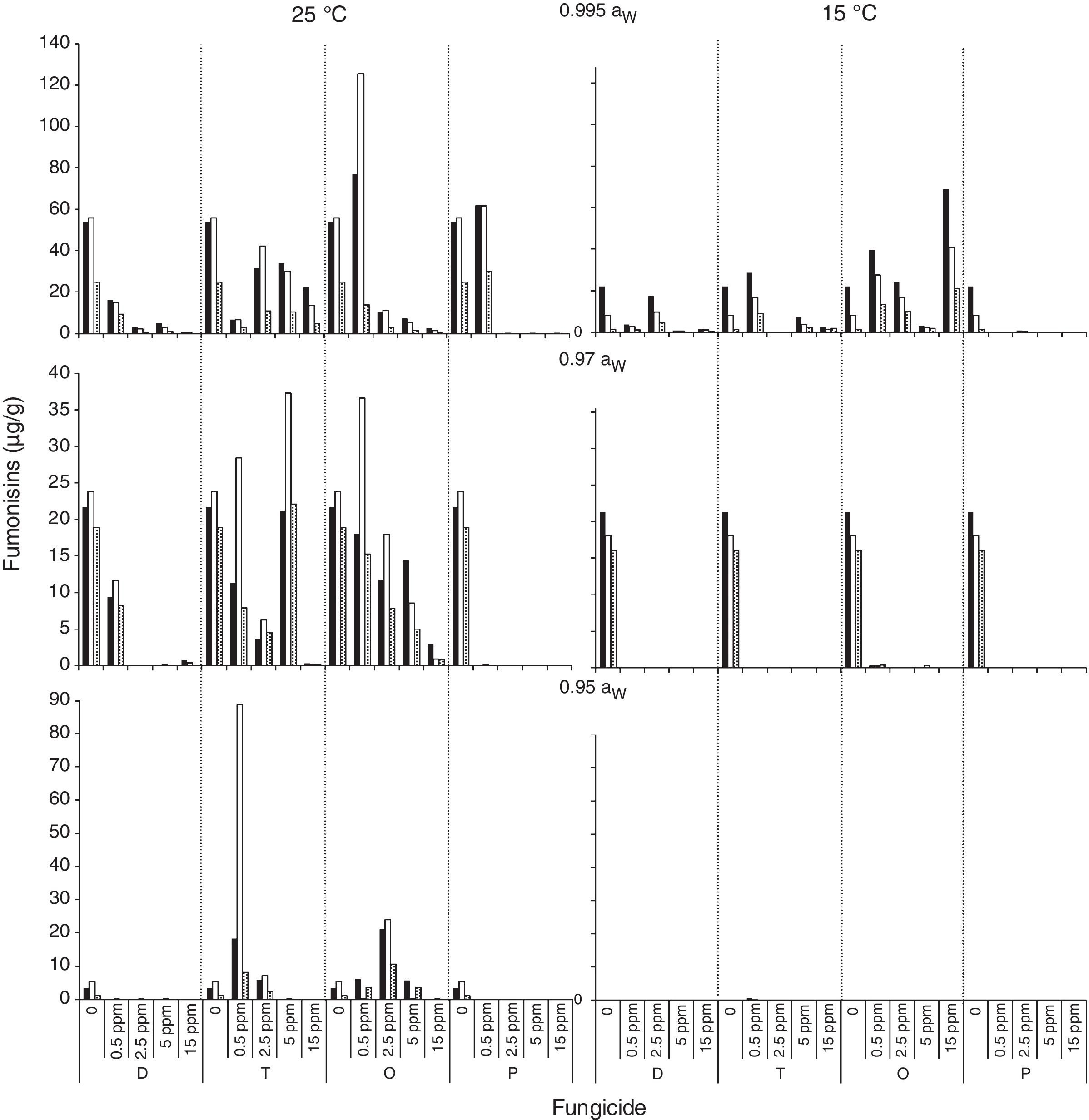
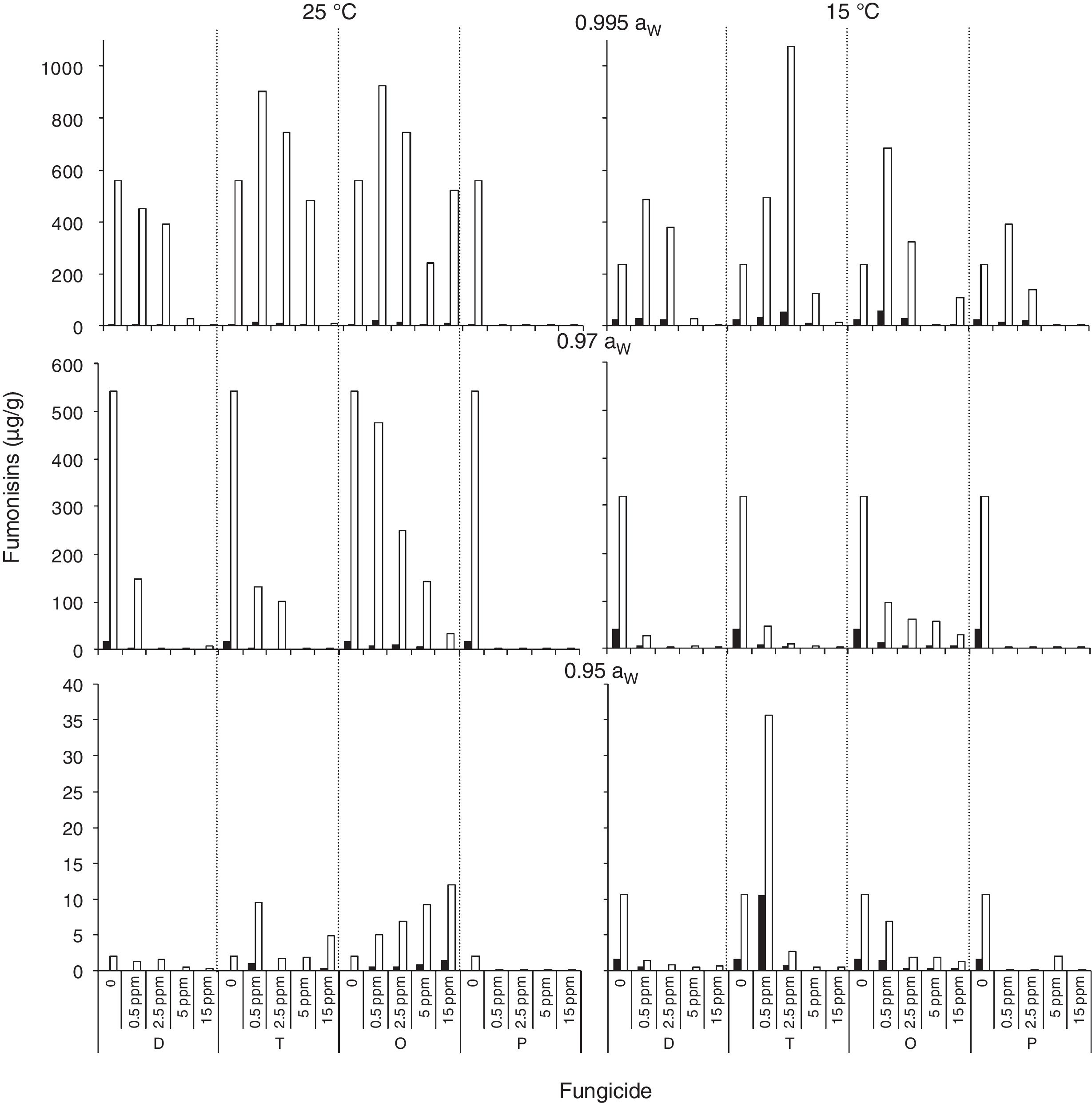
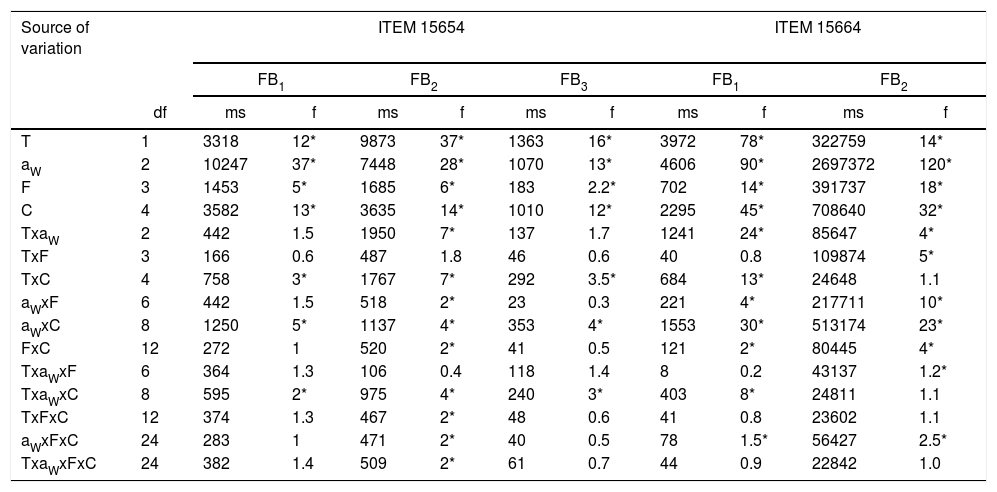
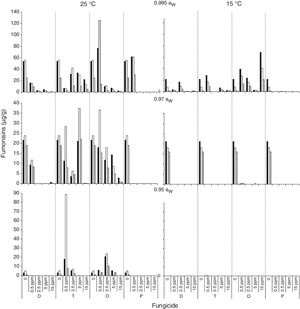
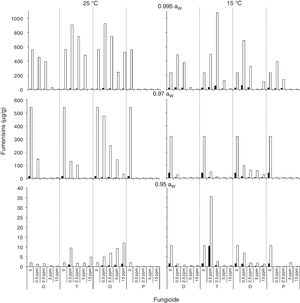
); 0.5 (); 2.5 (); 5 (); 15 () μg/mL.Fig. 3 and Fig. 4 show mean FB concentrations obtained on a wheat-based medium inoculated with two F. proliferatum strains in relation to aW, temperature and fungicide type and concentration. Strain ITEM 15654 produced FB1, FB2 and FB3. Minor amounts of FB3 were produced at all aW and temperature conditions tested, and higher amounts of FB1 than FB2 were produced at 25°C, while higher amounts of FB2 than FB1 were produced at 15°C. Strain ITEM 15664 produced just FB1 and FB2, and higher amounts of FB2 than FB1 under all conditions. For both strains as long as the aW of the medium and temperature were reduced, FB production was reduced. ITEM 15664 was able to produce higher amounts of FBs than ITEM 15654 under all tested conditions. When fungicides were applied, FB production was significantly reduced in comparison with the controls under some conditions, but in other conditions FB production significantly increased. Differences between strains were also observed for fungicide effects on FB production. Most fungicides used (tebuconazole, pyraclostrobin+epoxiconazole and expoxiconazole+metconazole) showed FB stimulation in at least one of the assayed conditions. The mixture pyraclostrobin+epoxiconazole and tebuconazole showed a higher stimulation effect. Prothioconazole was the only fungicide that was able to reduce FB production by both strains under all assayed conditions; with the exception of the lowest doses used (0.5μg/ml) at aw 0.995, and 15 and 25°C for ITEM 15664 and ITEM 15654, respectively. The mixture expoxiconazole+metconazole reduced FB production under all the assayed conditions for strain ITEM 15654, while for strain ITEM 15664, an increase in FB production was observed at 15°C and 0.995 aW when fungicide doses were low. Tebuconazole application reduced FBs for strain ITEM 15654 at 25°C and at 0.995 aW, while at the same temperature but lower aW levels, stimulation of FB production was observed when low doses (< 5μg/ml) of fungicides were applied. Using the same fungicide at 15°C, stimulation was observed just at the highest aW level when low fungicide concentrations were used. For the other strain, ITEM 15664 the same behavior was observed at both assayed temperatures: when aW was 0.995, low levels of fungicide (< 5μg/ml) stimulated FB production, while at lower aW levels a reduction in FB production was observed. The effect of the pyraclostrobin+epoxiconazole mixture on strain ITEM 15664 was the same at both assayed temperatures: reduction in FB production when aW levels were low, and stimulation at 0.995 aW and low fungicide doses. For the other strain the same fungicide stimulated FB production at all aW levels at 25°C and 0.995 aW at 15°C. Prothioconazole reduced FB production under all assayed conditions for both strains, with an exception at 25°C and 0.995 aW for strain ITEM 15654 where toxin production was similar to that observed in the control condition. Statistical analysis showed that all single source of variance and some, two-, three-, and four-way interactions significantly influenced FB production, aW being the most significant for both F. proliferatum strains, with the exception of temperature, which was the most significant for FB3 and FB2 production by F. proliferatum strain ITEM 15654 (Table 3).
Effect of fungicides (D: epoxiconazole + metconazole; T: tebuconazole; O: pyraclostrobin + epoxiconazole; P: prothioconazole), added to a wheat-based medium at different concentrations (0; 0.5; 2.5; 5; 15 μg/mL) on fumonisin production (μg/g): FB1 (
); FB2 () and FB3 () by F. proliferatum ITEM 15654, at different aW levels (0.995; 0.97; 0.95) and temperatures (15; 25 ̊C). Means of replicates in experiments.Effect of fungicides (D: epoxiconazole + metconazole; T: tebuconazole; O: pyraclostrobin + epoxiconazole; P: prothioconazole), added to a wheat-based medium at different concentrations (0; 0.5; 2.5; 5; 15 μg/mL) on fumonisin production (μg/g): FB1 (
) and FB2 () by F. proliferatum ITEM 15664, at different aW levels (0.995; 0.97; 0.95) and temperatures (15; 25 ̊C). Means of replicates in experiments.Analysis of variance of the effects of water activity (aW), temperature (T), fungicide (F), and fungicide concentration (C) on fumonisin production by two Fusarium proliferatum strains on a wheat-based medium.
| Source of variation | ITEM 15654 | ITEM 15664 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FB1 | FB2 | FB3 | FB1 | FB2 | |||||||
| df | ms | f | ms | f | ms | f | ms | f | ms | f | |
| T | 1 | 3318 | 12* | 9873 | 37* | 1363 | 16* | 3972 | 78* | 322759 | 14* |
| aW | 2 | 10247 | 37* | 7448 | 28* | 1070 | 13* | 4606 | 90* | 2697372 | 120* |
| F | 3 | 1453 | 5* | 1685 | 6* | 183 | 2.2* | 702 | 14* | 391737 | 18* |
| C | 4 | 3582 | 13* | 3635 | 14* | 1010 | 12* | 2295 | 45* | 708640 | 32* |
| TxaW | 2 | 442 | 1.5 | 1950 | 7* | 137 | 1.7 | 1241 | 24* | 85647 | 4* |
| TxF | 3 | 166 | 0.6 | 487 | 1.8 | 46 | 0.6 | 40 | 0.8 | 109874 | 5* |
| TxC | 4 | 758 | 3* | 1767 | 7* | 292 | 3.5* | 684 | 13* | 24648 | 1.1 |
| aWxF | 6 | 442 | 1.5 | 518 | 2* | 23 | 0.3 | 221 | 4* | 217711 | 10* |
| aWxC | 8 | 1250 | 5* | 1137 | 4* | 353 | 4* | 1553 | 30* | 513174 | 23* |
| FxC | 12 | 272 | 1 | 520 | 2* | 41 | 0.5 | 121 | 2* | 80445 | 4* |
| TxaWxF | 6 | 364 | 1.3 | 106 | 0.4 | 118 | 1.4 | 8 | 0.2 | 43137 | 1.2* |
| TxaWxC | 8 | 595 | 2* | 975 | 4* | 240 | 3* | 403 | 8* | 24811 | 1.1 |
| TxFxC | 12 | 374 | 1.3 | 467 | 2* | 48 | 0.6 | 41 | 0.8 | 23602 | 1.1 |
| aWxFxC | 24 | 283 | 1 | 471 | 2* | 40 | 0.5 | 78 | 1.5* | 56427 | 2.5* |
| TxaWxFxC | 24 | 382 | 1.4 | 509 | 2* | 61 | 0.7 | 44 | 0.9 | 22842 | 1.0 |
Since fungicides used for FHB control are active against all Fusarium species present in wheat, and it has been shown that F. proliferatum as well as FBs can be present in wheat, the effect of fungicides on F. proliferatum growth and FB accumulation on a wheat-based medium was analyzed. As a result, it was shown that complex interactions occur between abiotic factors (aW and temperature) and fungicides on growth and FB production by F. proliferatum strains.
Growth rate results observed under control conditions are in agreement with previous results reported using the same two strains grown in the same medium and also using irradiated wheat grains6,7,10.
With regard to the effect of fungicides on growth rates, all fungicide applications reduced growth rates (as fungicide concentrations increased, growth rates decreased) of F. proliferatum strains when compared to the control condition. Prothioconazole caused the greatest effect in both strains. Differences in fungicide efficacy are related to their action mechanism. Such information could be useful for the effective control of F. proliferatum growth and possible FB production on wheat grains. In the present study, little differences between the strains were observed, which is in agreement with other studies23. The differences in the efficacy of the same fungicide in inhibiting the mycelial growth of different strains of the same Fusarium species could be related to their genetic variability (higher or lower sensitivity of the strains)16 .The efficacy of the fungicides is dependent on the fungal species, strain, ecological conditions and interactions among all these factors33.
Fungicides used in the present study were included because they have been reported to reduce the severity of FHB losses in Argentina 4. Tebuconazole, metconazole+epoxiconazole and prothioconazole belong to the triazole group. The other fungicide used belongs to the strobilurin fungicide group: pyraclostrobin. Although the fungicides have different modes of action, during the present study they were all able to reduce fungal growth under almost all assayed conditions. Only tebuconazole showed growth stimulation when it was used at the lowest concentration and aW, 0.95. Marin et al.32 obtained similar results when studying the effect of tebuconazole on F. proliferatum and F. verticillioides strain growth, and observed that tebuconazole application in situations of high water stress may not be very effective. Furthermore, the authors noted that F. proliferatum was less susceptible to the fungicide than F. verticillioides. Tebuconazole is one of the most extensively used antifungal compounds applied in agriculture to control fungal pathogens in cereals and other crops and is considered to be very effective against Fusarium species15,37. Mateo et al.33 observed that azole-based compounds such as prochloraz and tebuconazole were more effective at 15°C than at 20- 25°C, which may be important as some Fusarium species are known to cause problems in cooler climatic regions.
The effect of fungicides on growth rates have been observed in many Fusarium species. Machado et al.27 determined the sensitivity of F. graminearum species complex strains to tebuconazole and metconazole, and concluded that regardless of the species, the isolates were around 4-fold less sensitive to tebuconazole compared to metconazole. Shin et al.46 analyzed F. subglutinans and F. temperatum growth in vitro with fungicide addition (tebuconazole, difenoconazole, fluquinconazole, azoxystrobin, prochloraz and resoxim-methyl), and concluded that azoles were more effective than strobirulin fungicides at inhibiting mycelial growth (prochloraz and tebuconazole being the most effective). As FHB epidemics are common in Croatia and wheat is frequently followed by maize in crop rotation, Ivic et al.22 studied the effect of carbenzamin, tebuconazole, flutriafol, metconazole and prochloraz on F. graminearum, Fusarium avenaceum and F. verticillioides growth, the last one being the most sensitive to the fungicides used (prochloraz and metconazole showed the greatest effect, followed by tebuconazole). However, several field studies recorded higher efficacy of tebuconazole compared to prochloraz in FHB management12,21,34. Thus, many authors remark that the effect of a fungicide in vitro may not reflect the efficacy of a product under practical conditions. Some authors observed that prochloraz was most active in decreasing fungal growth than tebuconazole32,33,43.
It has been stated that sublethal doses of some fungicides may lead to an increase in mycotoxin production by Fusarium species. Thus, when fungicides are used to control fungal diseases, the implications for mycotoxin production should be considered. FB production patterns and concentrations under the control conditions observed in the present study were similar to those previously observed using the same two F. proliferatum strains6,7,10. When fungicides were applied, different situations were observed. Prothioconazole reduced FB production under almost all assayed conditions. Contrary to our results, Audenaert et al.3 observed that sublethal concentrations of prothioconazole on F. graminearum cultures resulted in an increased accumulation of DON.
With respect to mycotoxin stimulation, when low doses of fungicides were applied, similar results were observed in other fungal species by other researchers23,30,31,33. With regard to the effect of fungicides on FB production by F. proliferatum and F. verticillioides, it has been previously observed by Marin et al.31 that tebuconazole treatment did not reduce FUM1 gene expression (good indicator of FB production). Moreover, FUM1 induction was higher in F. proliferatum than in F. verticillioides. They concluded that FUM1 induction with tebuconazole treatments might represent a potential additional risk of FB contamination. This fact should be remarked because tebuconazole is used for FHB management in wheat and F. proliferatum strains could be present in this cereal, consequently wheat grains could be contaminated with FBs as has been observed worldwide5. Marin et al.32 concluded that a direct effect of tebuconazole might exist at a transcriptional level of the toxin biosynthetic enzymes for FB production in F. proliferatum and F. verticillioides, which might enhance the induction level caused by environmental stress and the combination of those two situations might result in an undesirable increase of mycotoxin levels. Other fungicides (quintozene and fludioxonil+metalaxyl-M) increased mean FB1 levels when they were added to the culture medium of F. verticillioides16, suggesting the importance of focusing on the effect of fungicides on mycotoxin production as well as on phytopathogen control.
Similarly to tebuconazole, the mixture pyraclostrobin+epoxiconazole reduced FB production under some conditions, and stimulated production in other conditions, with differences between the analyzed strains. Those results are in agreement with previous in vitro studies mentioned above where mycotoxin production was enhanced when sublethal doses of fungicides were applied. Furthermore, DON production by F. culmorum was significantly increased at reduced aW in the presence of epoxiconazole and propioconazole19.
The mechanisms of fungicide stimulation on mycotoxin production are not completely elucidated and can vary according to fungal species and strains, climatic conditions during cropping and fungicide class16. Edwards and Godley15 suggested that mycotoxin production may be favored by fungicide applications by eliminating fungal species competitive to Fusarium spp., by affecting the balance between Fusarium species differing in fungicide sensitivity, and by a direct stress effect on mycotoxicogenic species increasing the enzyme activity involved in toxin biosynthesis; moreover, fungicides can affect the gene expression of toxin biosynthesis. Therefore, interactions between environmental conditions and fungicide treatments should be carefully considered. Furthermore, Popiel et al.42 suggested that increased mycotoxin contamination can be strongly influenced not only by the amount or the type of antifungal compound, but also the timing of fungicide exposure. They observed that even a very effective treatment (complete growth suppression) can still result in the induction of mycotoxin biosynthetic genes and increase mycotoxin accumulation.
There is one comparable study on the effect of fungicides used for FHB control and FB contamination in wheat grains21. In that study, the authors determined the relationship between fungicide effectiveness, FHB severity and FB1 and zearalenone (ZEN) contamination of wheat kernels. As a result, no significant differences between fungicide treatments were found between FB1 and ZEN levels in the analyzed grains. Additionally, no significant correlation was observed between FB content and FHB severity. The authors concluded that further research is needed to make hypothetical conclusions.
Our results showed that the stimulation or reduction in FB production in the presence of fungicides is influenced by complex interactions between abiotic factors and fungicide concentration in both F. prolifertum strains. It can be observed that fungicides used for FHB management have a profound effect on F. proliferatum growth and FB production, leading to FB contamination of wheat grains. However, this study was performed using a wheat-based medium, it would be interesting to perform a similar assay using irradiated wheat grains, in order to exactly reflect the ability of the same strains to grow and produce mycotoxins using a natural substrate. Moreover, field experiments are needed in order to prove the results obtained in this study.
Conflict of interestThe authors declare that they have no conflicts of interest.
FundingThis work was supported by the Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT-PICT-1253/2015) and Secretaría de Ciencia y Técnica, Universidad Nacional de Río Cuarto (SECYT-UNRC-18/453).
This work was supported by the Secretaría de Ciencia y Técnica, Universidad Nacional de Río Cuarto (SECyT-UNRC) and PIP-CONICET.