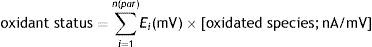This work demonstrates that the addition of metronidazole together with a ubiquitous quinone compound reduces adherence of Lactobacillus acidophilus to ovine vaginal cells.
Spectrophotometric and voltammetric studies have shown that neoformed compounds were observed in these systems; there were also changes in their electroactive composition, and the oxidant status had a significantly higher value compared to the control (p<0.05). Based on reduction potential (E; mV), the distribution of electroactive compound concentrations suggests that the compounds with low reduction potential induce this behavior, which would indicate that the addition of metronidazole with a ubiquitous quinone compound to the vaginal system might increase the reductive capacity of these systems.
This work shows that the study of behavior and fluctuations of the redox compounds that compose the vaginal environment, in terms of concentration and species of redox molecules, must be hierarchized in order to better understand the early stages of colonization by microorganisms.
Este trabajo demuestra que la incorporación de metronidazol conjuntamente con un compuesto quinónico ubicuo disminuye la adherencia de Lactobacillus acidophilus a células vaginales ovinas.
Los estudios espectrofotométricos y voltamétricos mostraron que en estos sistemas aparecieron compuestos neoformados y que hubo modificaciones en la composición electroactiva; asimismo, el estatus oxidante presentó un valor significativamente superior con respecto al control (p<0,05). Según los potenciales de reducción (E; mV), la distribución de las concentraciones de los compuestos electroactivos muestra que los compuestos con bajos potenciales de reducción inducen este comportamiento. Esto indicaría que la incorporación de esta mezcla al sistema vaginal aumentaría su capacidad reductora.
El trabajo muestra que el estudio del comportamiento y las fluctuaciones de los compuestos redox que componen el ambiente vaginal, en términos de concentración y especies moleculares, debe ser jerarquizado para comprender mejor las primeras etapas de la colonización de este ambiente por parte de los microorganismos.
Microbial adhesion to surfaces is the first step of the early processes not only in beneficial but also in pathogen microorganism installation in many diverse ecological niches24. At this first stage of bacterial adherence processes there are physicochemical conditioners acting in non-specific interactions (such as electrostatic repulsion and Van Der Waals’ force)23,24. Moreover, it has been shown that in cell–cell adhesion processes there is intervention of molecules that can undergo intra- and inter-molecular interactions with molecules or ubiquitous functional groups, such as H2O2 or thiols, through chemical reactions as well as redox reactions5,9,20. Based on these facts it is evident that the factors affecting these physicochemical variables must be especially considered in order to characterize this biological scenario8,13. Studies on bacterial adherence in bovine vaginal cells showed that the presence of oxidant compounds, such as periodate ions, may affect this process11, which suggests that the redox status of an extracellular environment, defined in terms of intensity (reduction potential; Eh) and capacity (number of electroactive compounds)14, might be a significant proximity factor13 in microorganism adherence to the vaginal mucosa.
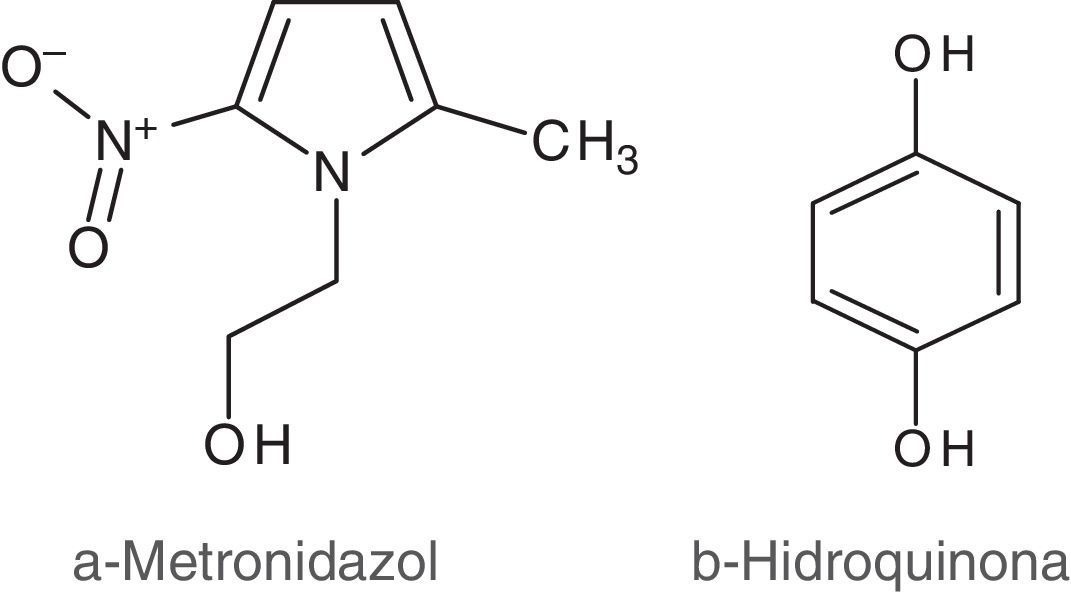
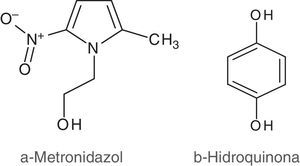
Metronidazole (MTZ) (Fig. 1a) is an antibiotic used in the digestive, reproductive and skin systems. The mechanism of action consists in inhibiting the synthesis of nucleic acids. MTZ and other nitroimidazole derivatives are active redox compounds which perform a significant antibiotic activity based on intracellular reduction from a nitro group to a nitro radical (R–NO2+e−→R–NO2•−)2, which is the reason why they are widely used in various microbial ecosystems inside the animal's body, including the vaginal environment10,19.
Quinones are molecules that play important roles in living organisms, such as the e− transfer in photosynthesis and in vitamin K. They are mostly benzoquinones, naphthoquinones and anthraquinones. Their important feature for this work is their ability to act reversibly in redox processes. Currently, natural and synthetic quinone derivatives are being strongly studied because of their chemotherapeutic activity against diseases such as cancer; furthermore, they have antimicrobial, anti-inflammatory and antioxidant properties1,21.
Independently of its antibiotic nature, when MTZ is in the vaginal-mucus-physicochemical-environment, it may act as a redox effector interacting with other ubiquitous redox compounds such as quinines (Fig. 1b), and in biological processes that are affected by redox conditions, for example in microbial adherence to vaginal cells.
This work aimed to assess the effect of adding MTZ alone and MTZ with hydroquinone (H2Q) (as a model of ubiquitous redox compounds in biological systems) on Lactobacillus acidophilus adherence to ovine vaginal cells suspended in simulated vaginal fluid, in order to hierarchize the study of the electrochemical scenario as a systemic view.
Materials and methodsOvine vaginal cellsOvine vaginal epithelial cells (VECs) were taken with a sterile swab from the vaginal cavity of experimental animals. This technique consists in separating the lips of the vulva, introducing the sterile swab into the dorsal commissure bypassing the clitoral fossa in dorsocraneal direction and rotating it in order to extract the cells.
Samples were put inside tubes containing 10ml Minimum Essential Medium Eagle (MEM) (Sigma®). After agitating the mixture for 2min with a Vortex agitator, the swab was removed and VECs remained refrigerated (4°C) until they were used. Before carrying out the trials, cells in the MEM medium were centrifuged at 200g for 10min. The sediment was resuspended in sterile saline solution (SS) and washed twice (200g; 10min) to eliminate native bacteria11. Finally, cells were resuspended in 3ml synthetic vaginal fluid (SVF) to give a final concentration of 104cells/ml (Thoma counting chamber).
Sterile swabs were used to obtain ovine vaginal fluid from the experimental animals (the same technique described to obtain the cells was used). Swabs were put in 4ml SS, agitated in Vortex, and filtered with 0.2μm (Millipore®). Synthetic vaginal fluid was also used, and it was prepared using the formula suggested by Owen and Katz12 modified (glucose: 10g/l; glycerol: 0.16g/l; lactic acid: 2g/l; acetic acid: 1g/l; urea: 0.5g/l; NaCl: 5.5g/l; KOH: 1.4g/l CaOH: 0.22g/l; yeast extract: 3g/l; pH: 6)4.
The strain used in this study was L. acidophilus ATCC 314 (USA), which was kept in suspension with skimmed milk at −20°C until it was used. Bacteria were activated in modified LAPT broth (proteose peptone: 2%; yeast extract: 1%, glucose: 1% Tween 80: 0.1%; pH: 6.5) at 37°C for 24h with two picks every 12h.
Bacteria were harvested by centrifugation at 3500×g for 15min at room temperature. Sedimented cells were washed twice with SS and resuspended in sufficient solution to give a final concentration of 107cellsml/l (Thoma counting chamber), corresponding to 106UFC/ml (MRS agar). This bacterial suspension was used for the adherence assays, named bacteria.
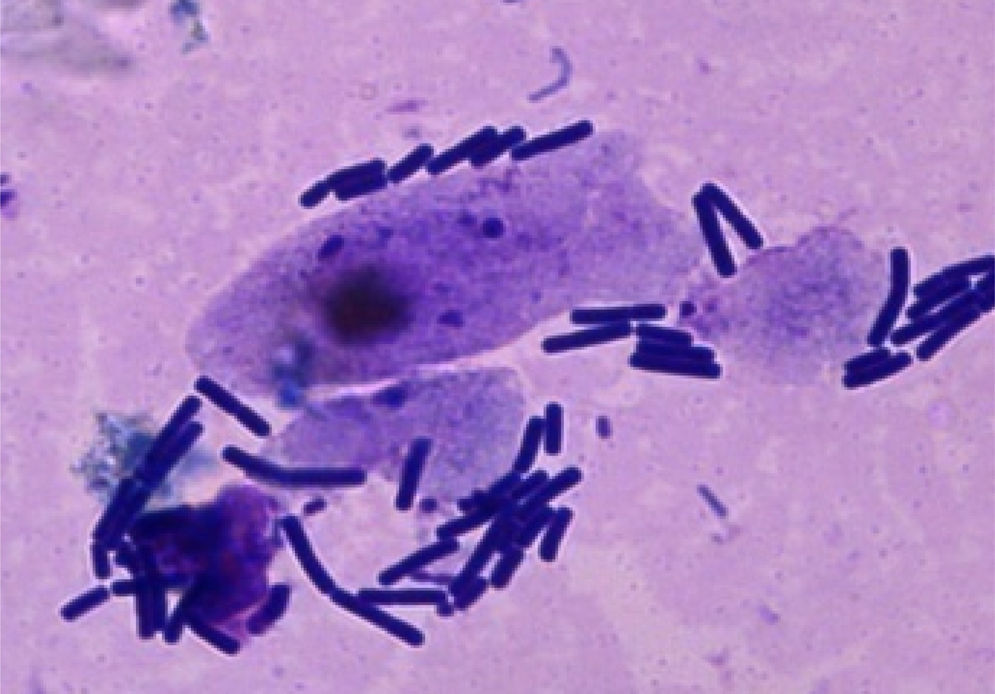
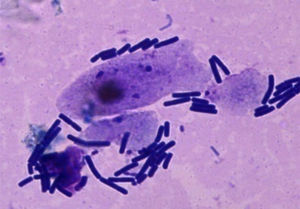
Adherence assaysIn order to incubate VECs with L. acidophilus in various treatments, 0.5ml of bacteria was added to 0.5ml VEC suspension. The systems were incubated during 1h at 37°C. After incubation, the treatments were centrifuged at 70×g for 10min, then washed in accordance with the protocol suggested by Prats et al.16, and were resuspended in SS. Following the incubation period, aliquots of the resulting suspension were stained with Tinción 15 (Biopur®), then placed on microscope slides, and cells and bacteria were counted under the microscope (Axiolab Zeiss Jena Gm).
The MTZ solution (0.125ml; 0.14mmol/l) and H2Q solution (0.125ml; 0.01mol/l) were used for the experiments.
The treatments were: (i) bacteria and cells (Control), (ii) bacteria and cells supplemented with MTZ, (iii) bacteria and cells supplemented with H2Q, (iv) bacteria and cells supplemented with MTZ and H2Q. All the experiments were carried out in triplicate.
The results were expressed as an adhesion percentage (%), defined as the number of VECs with adhered bacteria, divided by the total number of VECs observed and multiplied by 10011.
Molecular fractionationMolecular fractionation of ovine vaginal fluid suspensions was done by putting 0.4ml aliquots in a matrix of Sephadex G25® gel (Pharmacia). Gel calibration was carried out with Blue Dextran (MW: 2.106Da; CAS No. 115-39-9) and Bromophenol Blue (MW: 669.97Da; CAS No. 87915-38-6) following Cooper's technique (1984)3. Using a fraction collector (Roucaire, Retriever II, France), 26 fractions were recovered (2.8ml each), which were measured for absorbance at 200nm (Hitachi U, 1500 France).
Voltammetric measurementsCyclic voltammetry (CV) and differential pulse voltammetry (DPV) were used to characterize the electroactive components in the studied systems. A POL 150 (Radiometer Analytical, France)17 equipment was used to record current/potential scans. The experiments were carried out with no oxygen after purging the system with O2-free N2 for 3min before performing each voltammetric scan. In every case, the supporting electrolyte used was KCl 0.04mol/l. The working electrode was a vitreous carbon electrode; the reference electrode was a Pt foil, and every potential was measured in relation to a calomel standard electrode (CSE). Each sample underwent five repeated voltammetric scans until a reproducible cycle was reached, and the average for this value was found using the Trace-Master 5 software (Radiometer Analytical). CV scans started in the negative direction at +0.700V up to −1.900V with 0.025V/s scan speed.
In order to study the electrochemical changes in the systems of VECs and bacteria supplemented with H2Q and MTZ, DPV scans were performed. Scans were performed between +0.500V and −1.300V (step duration: 0.4s; width: 5mV). The voltammetric cell contained 900μl of analyte (constituted by bacteria in SS (0.5ml; 107cells/ml), the VEC suspension in SVF (0.5ml), 0.125ml of 10−2mol/l H2Q and 0.125ml of 1.5×10−4mol/l MTZ), 2000μl of dimethyl sulfoxide (DMSO), 2000μl O2 – free distilled H2O and 100μl of 2mol/l KCl as supporting electrolyte. In every case, final MTZ and H2Q concentrations in the voltammetric cell were, respectively, 2.5×10−6mol/l and 1.7×10−4mol/l. In DPV, the current unit/potential unit (nA/mV) quotient was recorded for each reduction potential value. These values are linearly dependent on element concentration (Radiometer, 2002) and were used to determine the system-oxidant-status, following the expression used by Pidello14, defining the variable by adding the reduction potential (intensity factor) (E; Volts) multiplied by the electroactive compound concentration (capacity factor) in the various redox pairs in a given system:
MTZ, H2Q, DMSO and KCl compounds were proanalysis reactive and were used without additional purification.
Statistical analysisThe Chi-squared distribution by means of the test of independence was used to analyze frequency in bacterial adherence studies. The t-Student test15 was used to evaluate the differences between treatments in the voltammetric studies.
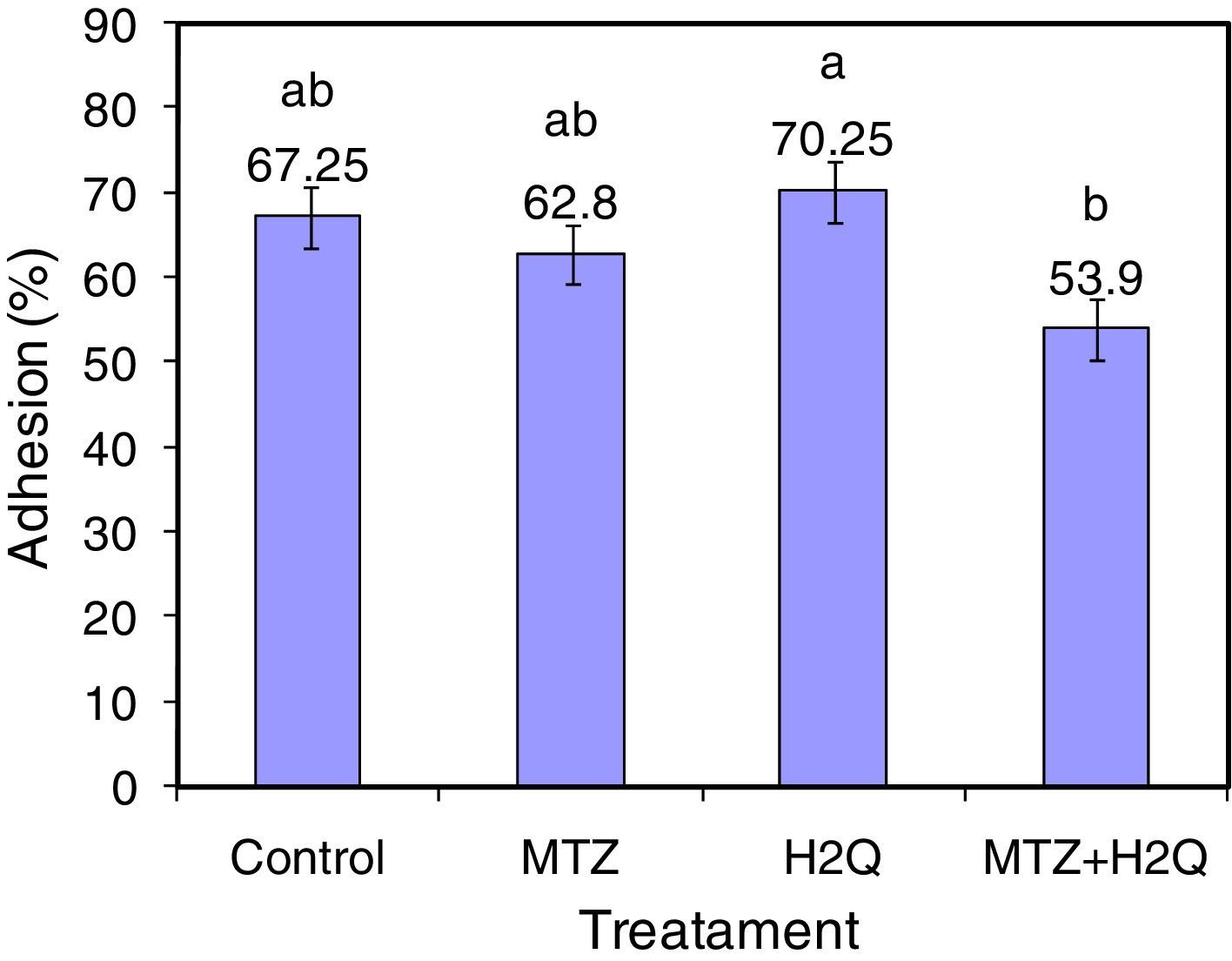
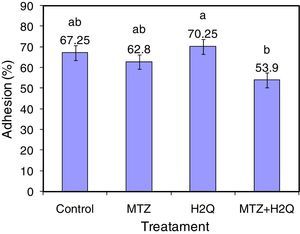
ResultsAdhesion of L. acidophilus ATCC 314 to ovine vaginal cellsFigure 2 shows adhesion of bacterial cells in the studied system. Adhesion studies were conducted with aliquots of VEC suspension and L. acidophilus cells both unsupplemented and supplemented with MTZ, H2Q or MTZ and H2Q. Figure 3 shows the results of adding L. acidophillus to ovine vaginal cells, expressed as an adherence percentage as described in Materials and Methods.
The results show that adhesion of L. acidophillus ATCC 314 to ovine VECs with H2Q was significantly affected by the supplementation with MTZ plus H2Q (p<0.0001). In the latter case, adhesion decreased significantly. Moreover, differences were found between Control and systems supplemented with MTZ or with H2Q alone (p>0.05).
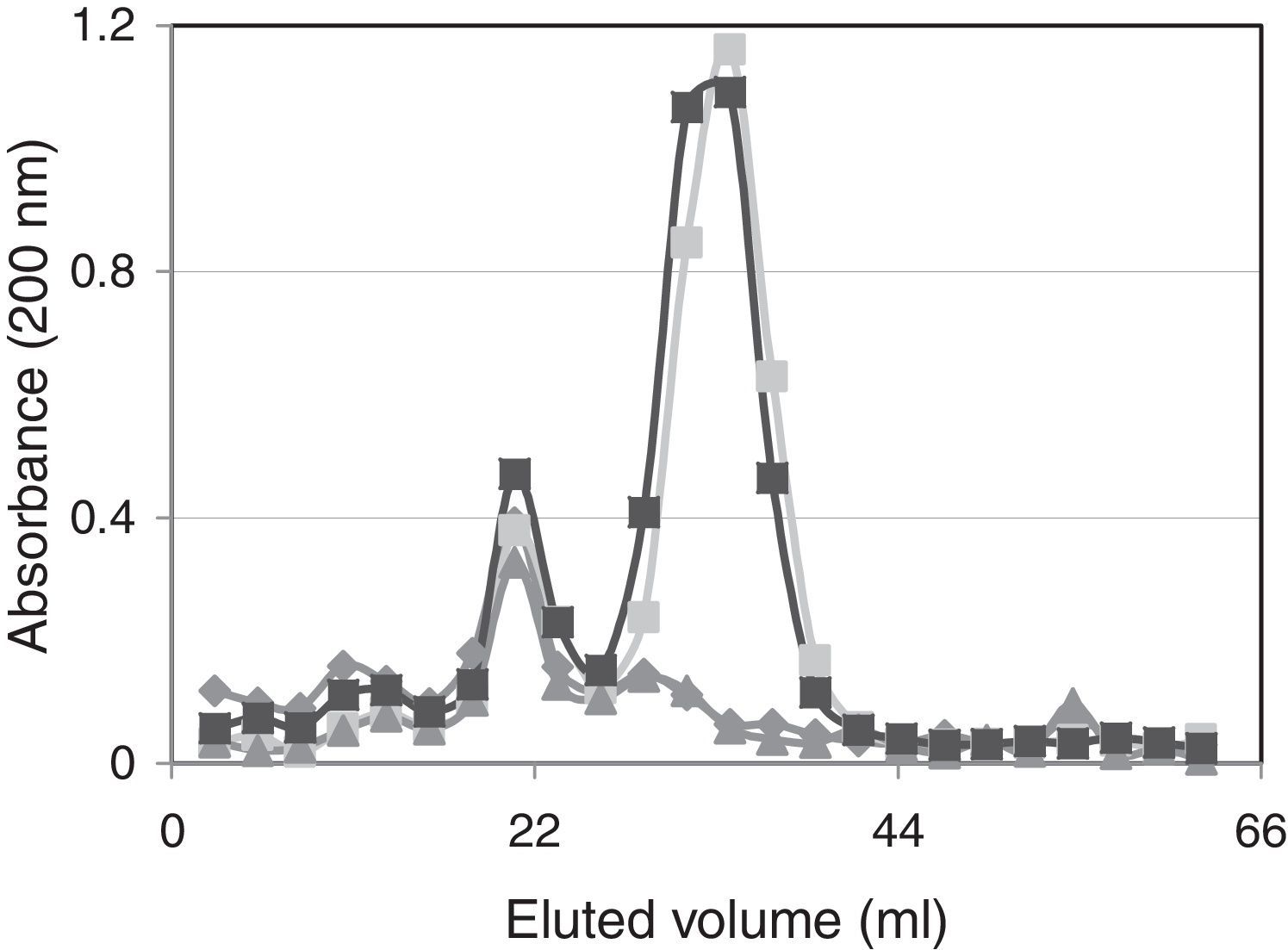
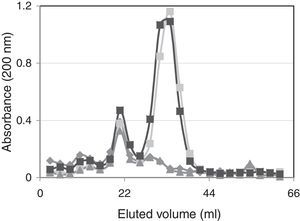
Spectrophotometric behavior of the studied suspensionsFigure 4 shows the spectrophotometric behavior of eluted solutions of unsupplemented and supplemented ovine vaginal fluid after fractionation with Sephadex G25. Absorbance values, measured at 200nm, indicate that the fraction with greater molecular weight (excluded fraction) in the ovine vaginal fluid samples was only slightly increased when it was supplemented with H2Q or MTZ (13%). The absorbance values of the solution containing H2Q (0.1l; 0.01mol/l) had a very well-defined signal at 200nm, which was observed in the fractions eluted with 30–40ml, with an approximate 1.2 absorbance value, coinciding with the behavior observed in previous studies25.
MTZ molecules (0.14mmol/l; 0.1ml) were eluted with 20–30ml, and these values coincided with the elution volume of ovine-vaginal-fluid-components, which showed a signal at wavelength 200nm. Although the MTZ absorbance signal under these fractionation conditions linearly responds to higher concentration (data not shown), with the amounts used in these experiments this signal was not different from the absorbance of ovine vaginal fluid alone (Fig. 3).
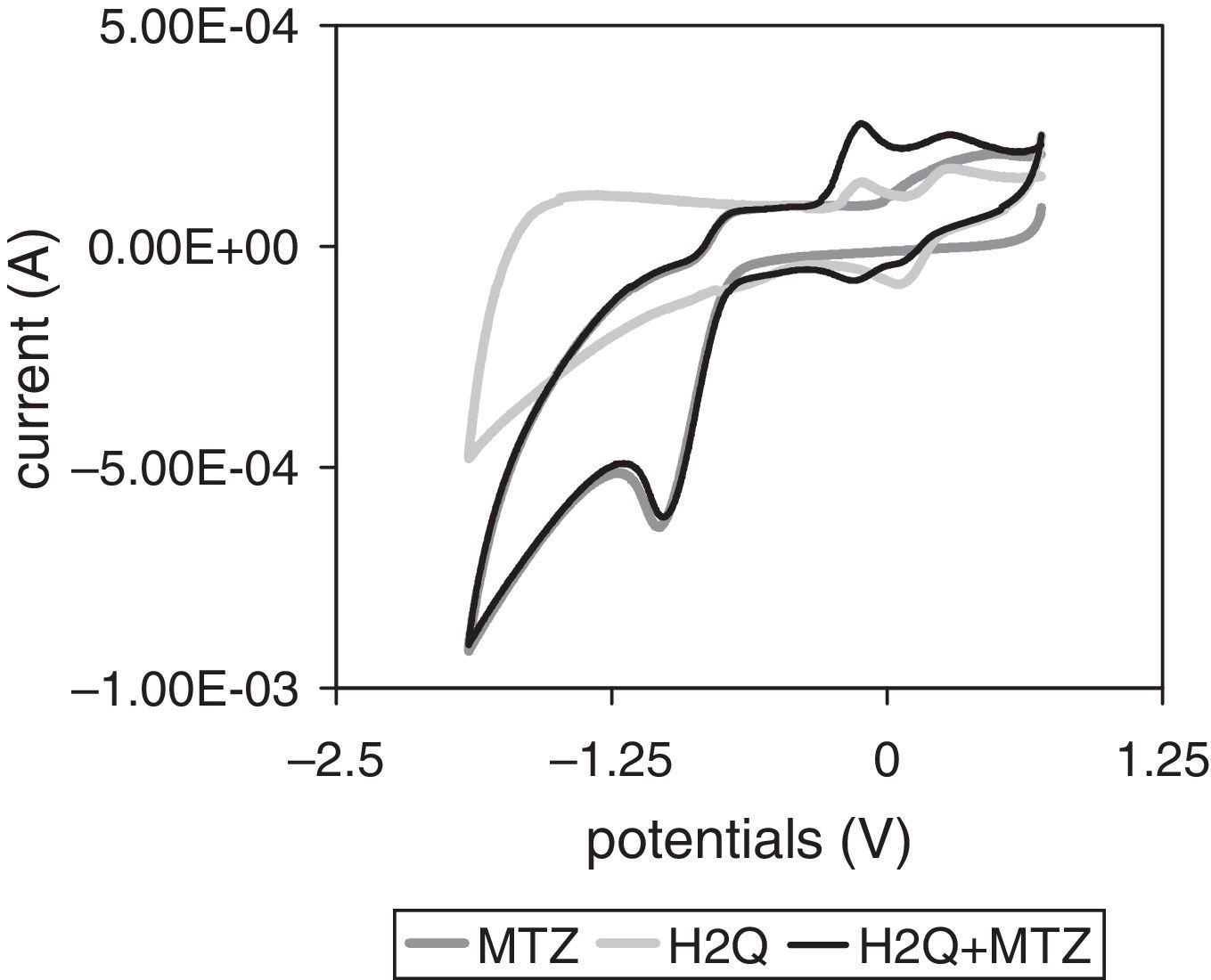
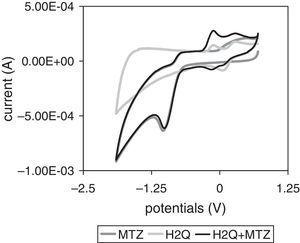
Voltammetric behaviorMTZ and H2Q aqueous solutionsFigure 5 shows the result of CV scans performed between +0.700 and −1.900V of the MTZ at a 4×10−3mol/l final concentration with deoxygenated distilled water or with an H2Q 7×10−4mol/l aqueous solution, and H2Q aqueous solutions (7×10−4mol/l). The solution containing MTZ alone had a single reduction peak (−1.034V) and its corresponding oxidation peak (−0.758V), with E1/2=−0.890V and ΔEp=0.276 and, therefore, electron transfer was irreversible under these conditions. The solution containing H2Q alone showed a single peak in the cathodic wave (+0.086V), whereas in the anodic wave there were two peaks corresponding to oxidation occurring in two stages of one electron each (−0.103V and +0.275V), which is characteristic of quinone behavior in unbuffered aqueous systems18. When two compounds constituted the same solution, MTZ did not change its electroactive behavior, while H2Q increased the intensity of anodic peaks and showed two signals corresponding to complementary cathodic peaks.
Cyclic voltammetry (CV) scans of metronidazole (MTZ), hydroquinone (H2Q), and metronidazole plus hydroquinone solutions. Every potential was measured against the CSE. Voltammograms started from a negative direction at +0.700V with 0.025Vs−1 scan speed. For further details see Materials and Methods.
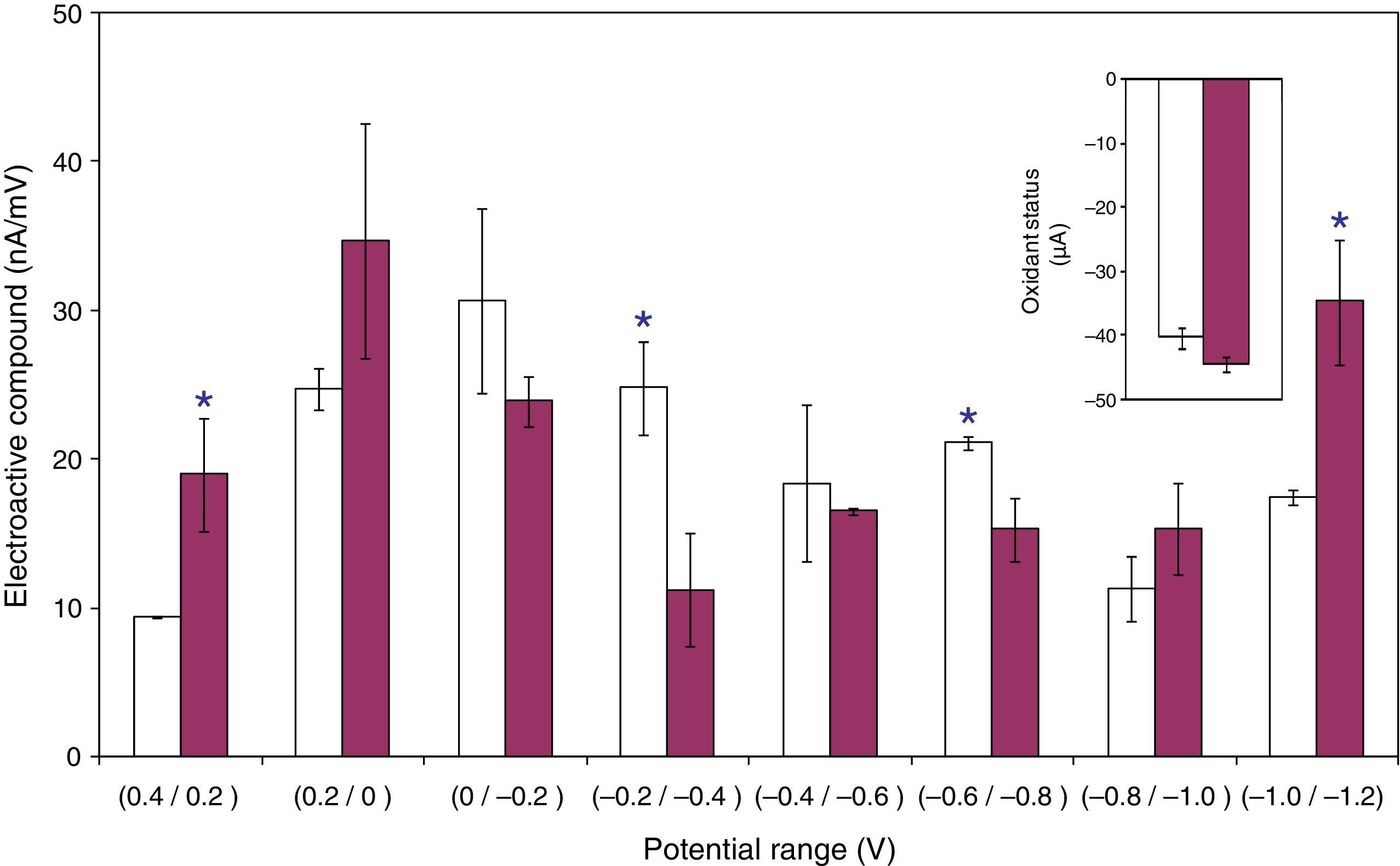
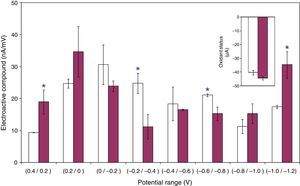
Based on the adherence percentages shown in “Adhesion of L. acidophilus ATCC 314 to ovine vaginal cells” section and the results observed in “MTZ and H2Q aqueous solutions” section, in order to study the electrochemical characterization of systems, only the systems supplemented with H2Q, and MTZ plus H2Q were retained. DPV scans indicate that these systems showed a total concentration of electroactive compounds (nA/mV) of 158.2±4.7 and 166.7±11.2 respectively, and these values did not differ statistically if compared between each other (p<0.05). However, when analyzed by potential reduction ranges, the treatments differed significantly at various potential values (p<0.05). While the systems supplemented with H2Q alone had greater electroactive compound concentrations in the −0.2/−0.4V and −0.6/−0.8V ranges, the systems supplemented with MTZ and H2Q had greater concentrations in the 0.4/0.2V and −1.0/−1.2V ranges (Fig. 6). The calculation of oxidant status, including only the potential ranges which differed significantly, showed −40.2±1.5 and −44.3±1.1 values for the treatments supplemented with H2Q or MTZ plus H2Q respectively, which also differed significantly when they were compared (p<0.05) (Fig. 6, inserted figure).
Number of electroactive compounds (nA/mV) in suspensions formed by ovine vaginal cells and Lactobacillus acidophilus ATCC 314 supplemented with hydroquinone (□) and metronidazol plus hydroquinone (
), determined from DPV scans within +0.4 and −1.2V potential ranges. Values were grouped at 0.2V intervals. The inserted figure shows the oxidant status in each of the systems considered. Bars on the columns show the standard error of the mean. An asterisc over the columns indicates that there are significant differences at p<0.05. For further details see Materials and Methods.Given the fact that the redox status is a conditioner of bacterial adherence, in this work we studied systems where: (i) there was an intention to modify adherence by adding an imidazole molecule (MTZ), which is a potential effector of the redox status due to its chemicals structure, and (ii) there was also an intention to modify speciation of this molecule by adding another redox effector (H2Q).
Under certain conditions and concentrations, MTZ inhibits Lactobacillus spp. growth (from 1000μg/ml), while under other conditions it stimulates growth (between 128 and 256μg/ml)22. In this work, 25μg/100ml of MTZ were employed, which would be innocuous for the strain used22. This fact was confirmed in this work, showing that adherence does not differ significantly when the control and the system supplemented with MTZ are compared. Furthermore, it was observed that adherence is not different in the control compared to the system supplemented with the quinone compound used (H2Q) to modify MTZ electrochemical behavior. However, the results showed that L. acidophillus ATTC 314 adherence to ovine VECs changed by the presence of the MTZ and H2Q combination. This result suggests that the chemical composition and redox status20 of the system were modified.
The spectrophotometric characterization of the various mixtures (Fig. 4) supports this interpretation, as it shows that when MTZ and H2Q molecules constituted the same solution there is a slight increase in the fractions with greater molecular size after fractionation with Sephadex G25. In addition, as the absorbance scan was performed at 200nm, the neoformed compounds eluting at this fraction were unsaturated and had a greater molecular mass than H2Q.
Voltammetric scanning (VS) of pure compound aqueous solutions (Fig. 5) provided a clear signal of MTZ with E1/2 value of −0.890V, which is therefore moved toward negative potentials in relation to the values presented in the bibliography6,7. Zuman and Rupp26 explain that these movements, which occur both in aqueous means and in solutions with different percentages of organic solvents, are due to pH increase and to the existence of an acid–base balance that precedes the transfer of the first e− of the R–NO2 group to form the nitro radical. This interpretation, which suggests a possible neoformation of electroactive species, might explain the results obtained in an aqueous means, which is a potential proton donor. On the other hand, CV scans also show that the presence of both MTZ and H2Q changes the electroactive compound concentration in potentials ranging between −0.500 and +0.500V, indicating that each mixture has a characteristic oxidant status, which helps anticipate that, as these mixtures are redox effectors, they generate significantly different electroactive behaviors in the systems where they are included20.
After performing DPV scans, when the electroactive behavior in the H2Q solution and the H2Q and MTZ mixture in the presence of ovine cells and bacteria was grouped by potential ranges (Fig. 6), significant differences between treatments were observed at some ranges (p<0.05). Using the concept of oxidant status led to overcome the evident difficulty of interpreting the influence that those partial differences had over adherence, as it helped compare the redox situation systemically. The oxidant status, calculated using the ranges that differed significantly (p<0.05), indicates that this variable had a higher value in the systems supplemented with the H2Q and MTZ combination (Fig. 6, inserted figure). Moreover, as the expression that defines oxidant status (see Materials and Methods) confirms that if there is a given concentration in an electroactive compound A with an E1 potential, this same concentration in a compound B with an E2 potential (being E2<E1) will originate a significantly greater oxidant status. Therefore, it may be concluded that although the H2Q and MTZ combination changes the number of redox compounds compared with H2Q alone at various potentials, an increase in the redox status will occur due to a concentration increase in the lowest negative potentials within the studied range, that is, between −1.0 and −1.2V. Based on this analysis, an increase in low-potential compounds should be associated to a decrease observed in the adherence phenomenon. In other words, adherence is reduced when there is an increase in low-potential redox couples which, under normal circumstances in a vaginal system (potentials greater than −1.0V), should behave as reducers. This result coincides with the results indicated in the bibliography, which show that cell–cell adhesion is induced by oxidant agents20.
Since MTZ is an antibiotic having redox behavior commonly used in both veterinary and human medicine, this paper aims to demonstrate the importance of considering how the environment in which it must work can be modified and consequently affect the speciation of the molecule, finally influencing bacterial adhesion. Although the redox nature of this complex relationship is not explained in this work, the results show that the study of behavior and fluctuations of the redox compounds that compose the vaginal fluid, in relation to concentration and species20, must be hierarchized in order to better contribute to understanding the early stages of microbial colonization in animal body cavities, such as the vaginal environment. Because of the results we wonder: if a given strain considered potentially probiotic fails to form biofilm, should not we investigate the redox conditions present in the medium before discarding their effectiveness? And on the other hand: could the redox status be modified through the incorporation of effector molecules in order to prevent pathogenic bacterial colonization?
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestsThe authors declare that there is no conflict of interests
Financing: Proyecto “Efecto de cambios de pH en suspensiones de líquidos biológicos sobre el comportamiento de un electroatractor antraquinónico extracelular”. 1 VET 135 Res C.S. 979/2012. Secretaría de Ciencia y Técnica – Universidad Nacional de Rosario.
The authors appreciate the comments made by the reviewers of this article.