Bovine leukemia virus (BLV) is an important cattle pathogen that causes major economic losses worldwide, especially in dairy farms. The use of animal models provides valuable insight into the pathogenesis of viral infections. Experimental infections of sheep have been conducted using blood from BLV-infected cattle, infectious BLV molecular clones or tumor-derived cells. The Fetal Lamb Kidney cell line, persistently infected with BLV (FLK-BLV), is one of the most commonly used long-term culture available for the permanent production of virus. FLK-BLV cells or the viral particles obtained from the cell-free culture supernatant could be used as a source of provirus or virus to experimentally infect sheep. In this report, we aimed to determine the minimum amount of FLK-BLV cells or cell-free supernatant containing BLV needed to produce infection in sheep. We also evaluated the amount of antibodies obtained from a naturally-infected cow required to neutralize this infection. We observed that both sheep experimentally inoculated with 5000 FLK-BLV cells became infected, as well as one of the sheep receiving 500 FLK-BLV cells. None of the animals inoculated with 50 FLK-BLV cells showed evidence of infection. The cell-free FLK-BLV supernatant proved to be infective in sheep up to a 1:1000 dilution. Specific BLV antibodies showed neutralizing activity as none of the sheep became infected. Conversely, the animals receiving a BLV-negative serum showed signs of BLV infection. These results contribute to the optimization of a sheep bioassay which could be useful to further characterize BLV infection.
El virus de la leucosis bovina (bovine leukemia virus [BLV]) es un importante agente patógeno del ganado que causa importantes pérdidas económicas en todo el mundo, especialmente en los rodeos lecheros. El uso de modelos animales proporciona información valiosa sobre la patogénesis de las infecciones virales. Se realizaron infecciones experimentales en ovejas usando sangre de bovinos infectados con BLV, clones moleculares de BLV infecciosos o células derivadas de tumores. La línea celular Fetal Lamb Kidney, persistentemente infectada con el BLV (FLK-BLV), es uno de los cultivos a largo plazo más utilizados para la producción permanente de virus. Las células FLK-BLV o las partículas virales obtenidas del sobrenadante del cultivo libre de células podrían usarse como fuente de provirus o de virus para infectar experimentalmente ovejas. En este trabajo, nuestro objetivo fue determinar la cantidad mínima de células FLK-BLV o de sobrenadante libre de células que contiene BLV necesaria para producir infección en ovejas. También evaluamos la cantidad de anticuerpos bovinos anti-BLV necesaria para neutralizar la infección. Observamos que las dos ovejas inoculadas experimentalmente con 5000 células FLK-BLV se infectaron, y que una de las dos ovejas que recibieron 500 células FLK-BLV se infectó. Ninguno de los animales inoculados con 50 células FLK-BLV mostró evidencia de infección. El sobrenadante FLK-BLV libre de células demostró ser infectivo en ovejas hasta la dilución 1:1000. Los anticuerpos BLV específicos mostraron actividad neutralizante, ya que ninguna de las ovejas se infectó. Por el contrario, los animales que recibieron un suero BLV negativo mostraron signos de infección por BLV. Estos resultados contribuyen a la optimización de un bioensayo en ovejas útil para caracterizar la infección por BLV.
Bovine leukemia virus (BLV) is a retrovirus that belongs to the Deltaretrovirus genus and the causative agent of enzootic bovine leukosis (EBL) in cattle and water buffaloes, their natural hosts2. The infection is distributed worldwide and the individual prevalence levels of infection vary among and within countries. In the European Union, measures of control and eradication were successful in most Western European countries1,22,25. Something similar occurred in New Zealand and Australia, which are BLV-free since 2008 and 2013 respectively12. In the Americas, there are different levels of BLV prevalence; in the case of Argentina, more than 30% of cattle and over 80% of dairy farms are infected32 with an individual endemicity higher than 80% in areas of intense production19. BLV infection is a serious concern since it causes major economic losses in the dairy productive system and in international trade9,30,32.
The virus can infect different cells of the immune system but has a preferential tropism for B lymphocytes. Therefore, it is a blood borne pathogen as Human T-lymphotropic virus 1 and 2 (HTLV-I and HTLV-II)2,31, pathogens from the same viral family with genetic and antigenic similarities. Active viral expression occurs during the first stages of the infection, after which the virus remains in a silent state in the infected host, permanently integrated into the cell genome as provirus DNA2,13. This produces a persistent immune response in the host without evidence of active viral circulation.
BLV has an intense transmission dynamics. All practices carried out without blood transmission control, such as blood extraction, vaccination, castration, dehorning, injection of medications, rectal palpation, tattooing, etc., are routes of potential infection, and animals with higher levels of provirus in blood are those that represent a higher risk for transmission8,21.
Most of the infected animals are asymptomatic carriers since they do not exhibit clinical changes and their leukocyte count remains normal. Between 20 and 30% of infected animals develop persistent lymphocytosis (PL)5,6, which is characterized by a permanent and stable increase in the number of circulating peripheral CD4+IgM+B cells. These animals do not show clinical symptoms, but an elevated white blood cell count can be observed at the hematological level37. Of all the infected animals, only 1–5% of them develop fatal lymphoma. The animals develop tumors of the lymphatic system by proliferation of B-cells, mostly affecting adult cattle (1–8 years old)7.
Cattle and water buffaloes are the natural hosts of BLV, but there are some species that can be experimentally infected with different clinical, hematological and immunological manifestations. The use of animal models is very important as they allow to study the different stages of viral infection along with the progression of the disease, as well as the characterization of samples for their infectivity, protective potential, among others. In rabbits, the infection is in most cases evidenced by the manifestation of circulating specific antibodies and the presence of the BLV genome. In some rabbits, a decreased T-cell responsiveness to phytolectin stimulation and signs of clinical disease, such as conjunctivitis, rhinitis, severe weight loss and unexpected death, can be also observed28,39. In rats and swine, the experimental infection only provokes the presence of anti-BLV antibodies as the virus does not produce pathogenic effects3,20. In chickens, anti-BLV antibodies can be found in all experimentally inoculated animals23, but only a small proportion of them develop leukemia4. Goats develop persistent anti-BLV antibodies and are very resistant to tumor development, although after a long period of time (8 years), death by lymphoma can be observed in some cases20,26.
Among the previously described animal models, sheep particularly exhibit interesting biological features for evaluating BLV pathogenesis and also for diagnostic purposes. In fact, conversely to other animal models, most of the sheep infected with BLV develop tumors typical of the disease. Moreover, sheep usually display lymphosarcoma symptoms faster (2.5 years after BLV infection) than the natural hosts20,26. Moreover, most of the experimentally-inoculated sheep develop persistent B-cell lymphocytosis after 1.5 years11. BLV experimental infection of sheep has been successfully performed using blood from BLV-infected cattle, in vitro produced BLV infectious molecular clones or cells derived from tumors of infected cattle20,24,27,29. Although some cell cultures derived from different animal species are permissive to BLV, infections are difficult to perform as they produce limited quantities of virus and large amounts of cellular debris14,33. The Fetal Lamb Kidney cell line persistently infected with BLV(FLK-BLV), established by Van der Maaten and Miller33, is one of the most commonly used long-term culture available for the permanent production of virus. FLK-BLV cells or the viral particles obtained from the cell-free culture supernatant (cell-free BLV) could be used as a source of virus to experimentally infect sheep and other species39. Previous reports have focused the use of the sheep model on the study of BLV pathogenesis, the process of leukemogenesis, potential treatments to eliminate tumors, molecular genetics and epigenetic modulation of viral expression, also the association with HTLV16,17. In this context, no information was found in the literature regarding the minimum infectious dose required to establish the infection in sheep using cell-free FLK supernatant or FLK-BLV viable cells.
The aim of the present study was to determine the effect of known amounts of FLK-BLV cells or cell-free supernatant obtained from FLK-BLV cultures in sheep, and to evaluate the amount of serum antibodies derived from a naturally-infected cow required to neutralize BLV infection.
Materials and methodsFLK-BLV cell cultureThe FLK-BLV cell line was generously provided by Dr. Luc Willems from the University of Liège, Belgium. The FLK-BLV cell line grows as a monolayer and has 4–7 copies of integrated proviral DNA and constantly secretes viral particles into the supernatant without any cytopathogenic effect, which allows to obtain large quantities of virus with a small amount of cellular debris. The FLK-BLV cells were cultured in RPMI 1640 medium (Corning, NY, USA) supplemented with 5% of inactivated fetal bovine serum (FBS, Internegocios, Buenos Aires, Argentina) until the formation of a confluent monolayer. The medium was then replaced with RPMI medium supplemented with 1% FBS to reduce the amount of debris, and cultured for 48h. The cell-free supernatant containing infectious BLV (cell-free BLV) was harvested, clarified by centrifugation at 3.000rpm for 15min and stored at −65±10°C until use. Cells were harvested by trypsinization, washed 3 times with PBS and resuspended in RPMI medium. Viable cells were counted by the trypan blue exclusion method. BLV virus (FLK-BLV supernatant) and infected cells (FLK-BLV cells) were used as inoculums for infection in lambs.
Experimental sheepAll experiments with animals were performed according to the guide on the care and use of laboratory animals of the National Institutes of Health10. The procedures followed for handling of animals, extraction and handling of samples were those of the guidelines described in the Manual of the Institutional Committee for Care and Use of Experimental Animals of the National Institute of Agricultural Technology (CICUAE-INTA). Twenty-eight 3–6 month-old BLV-free lambs (Pampinta breed) were used. Lambs were kept together in a natural grazing system until the end of the study, when they were euthanized.
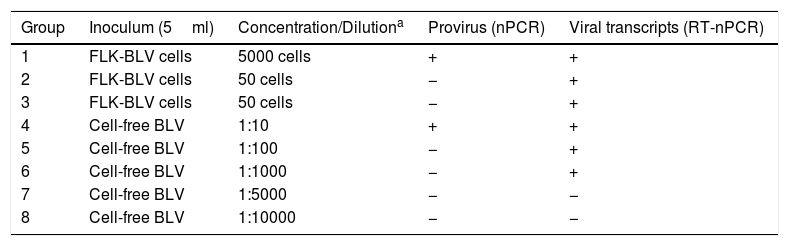
Experimental inoculations of sheepAssay A: BLV-FLK cells and cell-free BLV reducing amountsTo determine the minimum amount of BLV-FLK cells or cell-free BLV required to infect sheep, 16 lambs were divided into 8 groups (n=2 per group) and experimentally inoculated with different doses of FLK-BLV cells and cell-free BLV (Table 1). These inocula were obtained by serial dilutions in RPMI medium (Corning). All inocula were analyzed by nested PCR (nPCR) and retrotranscriptase-nested PCR (RT-nPCR) that amplifies a fragment of the BLV TAX gene (described in Nucleic acid extraction and amplification section) to detect the provirus and the virus, respectively38.
Inocula used in assay A: BLV-FLK cells and cell-free BLV reducing amounts (minimum infectious dose assay)
| Group | Inoculum (5ml) | Concentration/Dilutiona | Provirus (nPCR) | Viral transcripts (RT-nPCR) |
|---|---|---|---|---|
| 1 | FLK-BLV cells | 5000 cells | + | + |
| 2 | FLK-BLV cells | 50 cells | − | + |
| 3 | FLK-BLV cells | 50 cells | − | + |
| 4 | Cell-free BLV | 1:10 | + | + |
| 5 | Cell-free BLV | 1:100 | − | + |
| 6 | Cell-free BLV | 1:1000 | − | + |
| 7 | Cell-free BLV | 1:5000 | − | − |
| 8 | Cell-free BLV | 1:10000 | − | − |
Animals were subcutaneously inoculated in the armpit with 5ml of each inoculum. Whole blood samples with EDTA anticoagulant were taken by venous puncture of the jugular the day of the experimental infection, and 30 and 60 days post-infection (dpi). Plasma and buffy coat were obtained by centrifugation at 2500rpm for 15min and stored at −20°C until analyzed. All inocula were analyzed by nPCR and RT-nPCR.
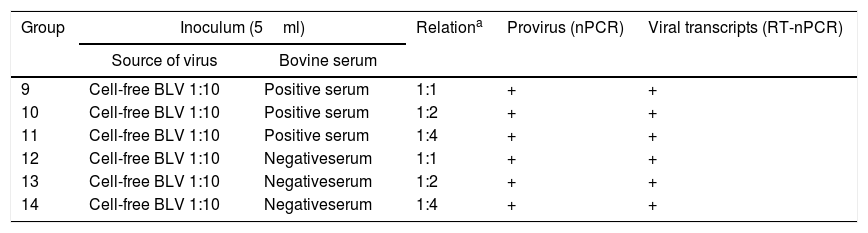
Assay B: neutralization assay with antibodies against BLVIn order to evaluate the neutralization of BLV infection with antibodies present in serum of naturally infected cattle, 6 lambs were divided into 3 groups (n=2 per group) and inoculated with cell-free BLV diluted 1:10, previously incubated with different amounts of a BLV-positive serum (Groups 9–11, Table 2). The BLV-positive serum had a titer of antibodies of 1/128 determined by ELISA as described in “Detection of BLV antibodies” section. As a positive control, 6 lambs were divided into 3 groups (n=2 per group) and inoculated under the same conditions but with cell-free BLV diluted 1:10, previously incubated with BLV-negative serum (Groups 12–14, Table 2). Animals were subcutaneously inoculated in the armpit with 5ml of each inoculum. Whole blood samples with EDTA anticoagulant were taken by venous puncture of the jugular the day of the experimental infection, and 30 and 60 days post-infection (dpi). Plasma and buffy coat were obtained as explained above and stored at −20°C until analyzed. All inocula were analyzed by nPCR and RT-nPCR.
Inocula used in assay B: neutralization assay with antibodies against BLV
| Group | Inoculum (5ml) | Relationa | Provirus (nPCR) | Viral transcripts (RT-nPCR) | |
|---|---|---|---|---|---|
| Source of virus | Bovine serum | ||||
| 9 | Cell-free BLV 1:10 | Positive serum | 1:1 | + | + |
| 10 | Cell-free BLV 1:10 | Positive serum | 1:2 | + | + |
| 11 | Cell-free BLV 1:10 | Positive serum | 1:4 | + | + |
| 12 | Cell-free BLV 1:10 | Negativeserum | 1:1 | + | + |
| 13 | Cell-free BLV 1:10 | Negativeserum | 1:2 | + | + |
| 14 | Cell-free BLV 1:10 | Negativeserum | 1:4 | + | + |
For the detection of BLV antibodies, an in-house ELISA test against BLV was performed (ELISA LKF) according to Trono et al.32 Briefly, ELISA plates were coated with complete BLV antigens concentrated from FLK-BLV cells by centrifugation on a discontinuous sucrose gradient. The plasma samples previously diluted 1/40 were dispensed into wells in duplicates. After incubation and washing, anti-bovine IgG peroxidase conjugated was added to each well. The presence of a secondary antibody was revealed with 3,3′,5,5′-tetramethylbenzidine and H2O2. Reaction was stopped using H2SO4 and the absorbance was read at 450nm. Normalized results were obtained as a sample-to-positive ratio. A weak positive control serum was used to calculate the ratio; its reactivity was set to 100% and all tested samples were referred to it and a cut-off level of 25% was established32.
Nucleic acid extraction and amplificationTotal RNA from inocula and whole blood samples was extracted using the High Pure RNA Purification Kit (Roche, Penzberg, Germany), according to the manufacturer's instructions. Extracted RNA was treated with DNAses prior to reverse transcription to eliminate DNA contamination. Reverse transcription was carried out with 0.02μg/μl of random hexamers (PROMEGA, Fitchburg, WI, USA) and 2.4U/μl of M-MLV (PROMEGA). The cycling conditions of the RT-PCR were: 1 cycle at 42°C for 60min and 1 cycle at 95°C for 5min. Total genomic DNA was extracted using the High Pure PCR Template Preparation Kit (Roche) according to the manufacturer's instructions.
Partial amplification of the tax gene by nPCR was performed according to Wu et al.38, using cDNA and DNA samples as template. Primers TAX7781F (5′-CAGACACCAGGGGAGCCATA-3′) and 8083R (5′-CTGCTAGCAACCAATTCGGA-3′) were used in the first round, and primers TAX7802F (5′-AGCCATACGTTATCTCTCCA-3′) and TAX8062R (5′-CAGGTTAGCGTAGGGTCATG-3′) in the second round. Positive and negative controls were included in each run. RNA samples without reverse transcription were analyzed to exclude the presence of genomic DNA in RNA samples. The expected size of the amplified product, 279bp, was confirmed by fluorescent nucleic acid gel staining (GelRed, Biotium, CA, USA). The estimated detection limit of the reaction is about one infected cell in 10000 non-infected cells.
ResultsProviral DNA and viral transcripts in the inoculation materialBLV provirus was detected by nPCR in the cell-derived inoculum containing 5000 FLK-BLV cells (Group 1, Table 1) and in the cell-free BLV diluted 1:10 (Group 4, Table 1). Viral BLV RNA was detected in the FLK-BLV cell preparations and in the dilutions 1:10 to 1:1000 of cell-free BLV (Groups 1–6, Table 1). Proviral DNA and viral BLV RNA were detected in all inocula of the neutralization assay (Groups 9–14, Table 2).
Sheep infectivityThe presence of BLV-specific antibodies in the plasma of the inoculated animals, along with the detection of circulating BLV proviral DNA, were considered indicators of infection in lambs.
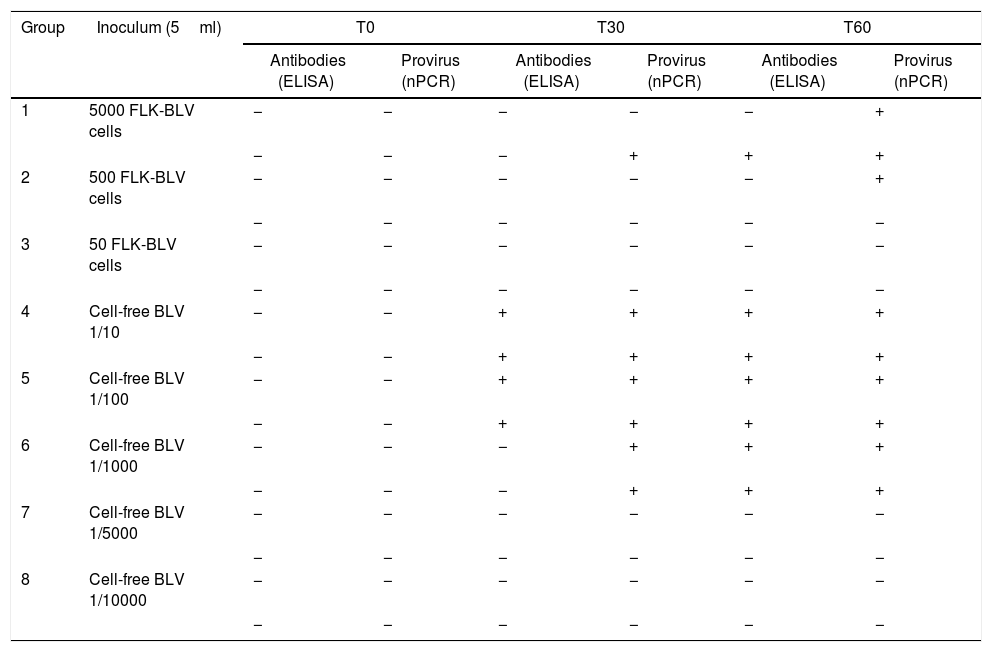
Both lambs inoculated experimentally with 5000 FLK-BLV cells showed evidence of infection (2/2), with detection of provirus and antibodies at T60 post-infection. With 500 FLK-BLV cells, only one of two sheep (1/2) became infected, and none of the sheep showed evidence of infection (0/2) with 50 FLK-BLV cells at the end of the trial. The cell-free BLV was infective down to 1:1000 dilution (2/2) (Table 3).
Detection of antibodies and proviral DNA at different times post-infection in lambs used in assay A (BLV-FLK cells and cell-free BLV reducing amounts)
| Group | Inoculum (5ml) | T0 | T30 | T60 | |||
|---|---|---|---|---|---|---|---|
| Antibodies (ELISA) | Provirus (nPCR) | Antibodies (ELISA) | Provirus (nPCR) | Antibodies (ELISA) | Provirus (nPCR) | ||
| 1 | 5000 FLK-BLV cells | − | − | − | − | − | + |
| − | − | − | + | + | + | ||
| 2 | 500 FLK-BLV cells | − | − | − | − | − | + |
| − | − | − | − | − | − | ||
| 3 | 50 FLK-BLV cells | − | − | − | − | − | − |
| − | − | − | − | − | − | ||
| 4 | Cell-free BLV 1/10 | − | − | + | + | + | + |
| − | − | + | + | + | + | ||
| 5 | Cell-free BLV 1/100 | − | − | + | + | + | + |
| − | − | + | + | + | + | ||
| 6 | Cell-free BLV 1/1000 | − | − | − | + | + | + |
| − | − | − | + | + | + | ||
| 7 | Cell-free BLV 1/5000 | − | − | − | − | − | − |
| − | − | − | − | − | − | ||
| 8 | Cell-free BLV 1/10000 | − | − | − | − | − | − |
| − | − | − | − | − | − | ||
(−) Negative, (+) positive.
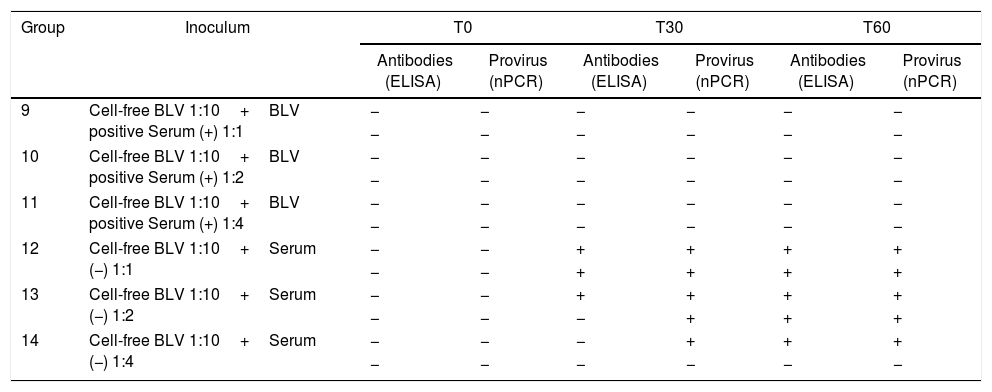
In the neutralization assay (Table 4), BLV positive serum showed a neutralization activity when it was previously incubated with cell-free BLV, as no sheep showed evidence of infection. As positive controls, the lambs inoculated with cell-free BLV when previously incubated with negative serum in relation showed evidence of infection 1:1 (2/2), 1:2 (2/2) and in 1:4 (1/2), with delayed detection.
Detection of antibodies and proviral DNA at different times post-infection in lambs used in assay B (neutralization assay with antibodies against BLV)
| Group | Inoculum | T0 | T30 | T60 | |||
|---|---|---|---|---|---|---|---|
| Antibodies (ELISA) | Provirus (nPCR) | Antibodies (ELISA) | Provirus (nPCR) | Antibodies (ELISA) | Provirus (nPCR) | ||
| 9 | Cell-free BLV 1:10+BLV positive Serum (+) 1:1 | − | − | − | − | − | − |
| − | − | − | − | − | − | ||
| 10 | Cell-free BLV 1:10+BLV positive Serum (+) 1:2 | − | − | − | − | − | − |
| − | − | − | − | − | − | ||
| 11 | Cell-free BLV 1:10+BLV positive Serum (+) 1:4 | − | − | − | − | − | − |
| − | − | − | − | − | − | ||
| 12 | Cell-free BLV 1:10+Serum (−) 1:1 | − | − | + | + | + | + |
| − | − | + | + | + | + | ||
| 13 | Cell-free BLV 1:10+Serum (−) 1:2 | − | − | + | + | + | + |
| − | − | − | + | + | + | ||
| 14 | Cell-free BLV 1:10+Serum (−) 1:4 | − | − | − | + | + | + |
| − | − | − | − | − | − | ||
(−) Negative, (+) positive.
FLK-BLV cells are a persistently infected cell line, with the integrated proviral BLV genome, which secretes viral particles into the supernatant with minimum cell debris. Previous studies have shown that this cell line is a good choice for experimental infection assays. However, the earlier reported articles showed poor details about the amounts and/or dilutions used for inoculation, regarding starting materials, viable cells or cell-free supernatants19,26. Thus, in the present study, cell-free BLV and FLK-BLV cells were used as source of virus and provirus, respectively, in different amounts.
Previous results from our laboratory have shown that the cell culture supernatant contains the maximum concentration of BLV particles 48h after the monolayer has reached 100% confluence. Afterwards, the concentration decreases as the particles begin to degrade. A semiquantification using serial dilutions of different FLK-BLV cell supernatants up to the dilution 1:1000 sowed to be positive by RT-PCR (data not shown). Consequently, although the exact number of viral particles used in the inocula could not be estimated since in vitro viral titration assays are not available, the protocol for collecting FLK-BLV supernatant used in this study has been previously optimized by our group to obtain the maximum amount of viral particles.
The presence of BLV-specific antibodies in the plasma of inoculated lambs, along with the detection of BLV proviral DNA, indicates that our experimental approaches based on FLK-BLV cells or cell-free BLV were successful in establishing the infection with the virus in lambs. As expected, the dose response effect observed showed a reduction in infectivity for both initial materials at higher dilutions. The presence of proviral DNA in the absence of antibodies in the 500 FLK cell inoculum suggests that as template. In this sense, a syncytium assay was used in the past for the detection of neutralizing antibodies using the CC81 cell line as indicator culture and the FLK cell line as effector culture, both in the presence of bovine or sheep serum that provokes syncytium inhibition in the CC81 culture in the case of neutralization activity15. This approach was not reproducible in our hands; nevertheless, we took great care in assaying multiple variables (data not shown).
Without an in vitro viral titration system and an in vitro assay for the detection of the neutralizing activity, and with the need to find a way to proceed in future experiments, we decided to advance with the previously reported in vivo assays using sheep. Even if the bovine is the natural host of BLV infection, this species is not the choice for characterization assays such as viral titration or neutralizing antibody detection. Neither is the choice for reproducing the disease, as the progression of infection is slow and only a small percentage of the infected animals develop fatal lymphoma after long subclinical periods. Experimental transmissions have been reported in many species with different clinical, hematological and immunological manifestations. In this respect, the sheep model has proven to be very useful in terms of obtaining information regarding immunity, viral persistence and pathogenesis. The asymptomatic period in sheep is much shorter than in cattle, with 100% of the experimentally-infected sheep developing persistent lymphocytosis and most of them progressing to the development of lymphosarcomas around 18 months post-infection. This model has been widely used not only for the analysis of natural samples but also to test the infectivity and pathogenesis of BLV molecular clones34–36. Furthermore, considering the close proximity of BLV with HTLV I and HTLV II, the sheep model was used for experimental treatments16,17.
The results presented in this work contribute to the optimization of a sheep bioassay used to further characterize the BLV infection. Particularly, the aim of this work was to determine the minimum number of FLK-BLV cells or cell-free BLV necessary to induce infection in sheep and to determine the capacity of antibodies of naturally infected bovines to neutralize the experimental infection. This approach can be useful as it can be used in a wide range of experiments such as testing infectivity and/or the protective potential of different samples, as well as for the study of viral pathogenesis. In fact, we are currently using this approach for the analysis of the BLV-inactivating potential of heat treatments of milk and colostrum18.
The minimum infectious dose is the minimum estimated number of organisms required to produce infection in animals exposed by a given route. In the assay done in this work, we were able to determine the minimum infectious dose of provirus and virus using both FLK-BLV cells and cell-free BLV. In both cases, as the inocula were more diluted, the time of appearance of evidence of infection was longer, and the first indication of this was the detection of proviral BLV genome by nPCR and later the detection of specific antibodies against BLV by ELISA.
The results of the neutralization assay indicate that the serum of a BLV-free animal did not interfere with the infection process. The exception was the last inoculum with the highest amount of serum that suggests unspecific serum toxicity. We conclude that the assay should not be performed at 1:4 or higher proportions, as in the case of a BLV-infected animal, it would not be possible to state if the result is a consequence of the presence of specific antibodies or of the unspecific effect of the serum itself. When BLV-positive serum (titer 1/128) was added, all the proportions were capable of protecting the lambs against the virus.
This work allowed us to progress toward the setup of feasible assays to detect infectivity and neutralizing antibodies in addition to the previously reported syncytium assay which has shown to be not easy to perform, insidious to read and even difficult to reproduce. The sheep bioassay is important for future experiments and projects in our research group that needs to characterize and state parameters derived from natural and/or experimental infections. Prior to this work, we performed the experimental inoculations using the complete product of the trypsinization of a T25 flask containing a confluent monolayer of FLK-BLV cells (cells and supernatant); now, we know the limit of infection of both cell-free BLV and viable FLK-BLV cells. Together with the positive detection of neutralizing activity, we are ready to progress toward experiments that can make use of this helpful tool.
Conflict of interestThe authors declare that they have no conflicts of interest.
This work was financially supported by Asociación Cooperadora de INTA Rafaela, project FONCYT PICT No. 2013-0929, project INTA PNSA-1115054 and extrapresupuestary funds from specialized technical services of the Laboratorio de Virus Adventicios (INTA) administered by Fundacion Argeninta. Porta N, Alvarez I, Ruiz V and Trono K were also supported by Consejo Nacional de Investigaciones Cientificas y Tecnicas (CONICET).