Leptospirosis is important in Uruguay due to the economic loss caused by the diseases of production animals, mainly bovines, and also due to frequent human infection. We decided to study anti-Leptospira antibodies in the sera of dairy workers, rice laborers, veterinarians, suburban slum dwellers and garbage recyclers. Our aims were to estimate the seroprevalence of infection by Leptospira spp. in these people at risk, the relative importance of the known risk factors associated with infection, and the impact of human infections in each setting. Groups at risk were identified and 35 visits to their locations were made, conducting field surveys and exchange talks for information and education. Simple epidemiological questionnaires were administered and sera samples were taken from 308 persons. The microagglutination Technique (MAT) and the IgM Indirect Immunofluorescence (IIF) assay were employed to detect antibodies. Environmental water samples, canine and equine sera were also examined. More than 45% of human sera were reactive and the studied groups were confirmed to be widely exposed to infection. Female sera were frequently reactive, though most illnesses occur in men, and the most severe cases in elderly males; the emergence and evolution of the disease may strongly depend on the host condition and functions. Animal contact and unsafe water usage were the main identified risk factors to be considered in prevention. Fifty per cent of the studied horses showed a positive MAT reaction. The underdiagnosis of the illness and its long-term symptoms require further study, as well as greater health and social attention efforts.
La leptospirosis es importante en Uruguay por las pérdidas económicas que ocasionan las enfermedades en los animales de producción, principalmente en los bovinos, y a causa de la frecuente infección humana. Decidimos estudiar anticuerpos anti-Leptospira en trabajadores de tambo y de plantaciones de arroz, así como en veterinarios, habitantes de asentamientos y recicladores de residuos. Buscamos evaluar la importancia de distintos factores de riesgo conocidos asociados con la infección por Leptospira spp., y estimar la frecuencia y el impacto de las infecciones humanas en la población expuesta. Se efectuaron 35 visitas a colectivos de riesgo, realizando relevamientos de campo e intercambios educativos, llenando formularios epidemiológicos sencillos y tomando muestras de sangre a 308 personas. Se investigaron anti-cuerpos séricos con técnicas de microaglutinación (MAT) e inmunofluorescencia indirecta para IgM. Se examinaron también, muestras de agua ambiental, y sueros caninos y equinos. Más del 45% de los sueros humanos fueron reactivos y se confirmó que los grupos estudiados estaban ampliamente expuestos a la infección. Los sueros de mujeres fueron frecuentemente reactivos, aunque la mayoría de las enfermedades ocurren en varones, y los casos más graves en varones añosos. La emergencia y la evolución de la afección pueden depender fuertemente del estado del huésped y su respuesta. El contacto con animales y el uso de agua insegura fueron los principales factores de riesgo identificados para considerar en la prevención. El 50% de los sueros equinos fueron reactivos por MAT. Es necesario incrementar los esfuerzos de atención social y sanitaria en estos sectores, superar el subdiagnóstico y estudiar la evolución y la sintomatología a largo plazo de los pacientes.
Leptospirosis is a worldwide zoonotic infection caused by spiral bacteria of the genus Leptospira2,22,25,30. DNA studies have allowed to describe 22 Leptospira genospecies; 15 of which have been characterized as fully or partially pathogenic for humans or animals and must be considered in diagnosis8,32. For practical purposes, they are classified into serogroups and serovars (that can be shared by several genospecies) according to the surface LPS antigenic composition.
In Uruguay this zoonosis is tightly linked to bovine infection. There are 12 million bovines and 3.3 million human inhabitants in the country distributed over a 176215km2 surface. Bovine production animals are frequently infected (seroprevalence is higher than 20%) with several species and serovars of Leptospira13,28,41,49.
Bovine leptospirosis causes reproductive bovine disorders, such as abortions, weak neonates, placental retention, infertility, agalactia or reduced milk production, infection of calves, among others. Important economic losses ensue, raising deep concern among meat and dairy farmers12,16,38,46.
Infected bovines that can excrete bacteria for up to 2 years, as well as other infected animals, can transmit Leptospira spp. to workers through the contact of urine, contaminated water or moist soil with abraded skin or the conjunctiva. Good animal vaccines are thus also useful resources for indirect control of the spread of this zoonosis to human groups at risk18,20,36,48.
Leptospirosis is an occupational illness. It has been described in the already mentioned cattle handlers, in rice workers, sugar cane laborers, sewage workers and other groups at risk, all of whom perform activities in highly humid environments that favor pathogen transmission.
Heavy rainfalls, floods, tight and careless contact with animals, their urine or infected organs are important risk factors for infection of humans in rural, suburban or urban settings3,11.
Human disease can be asymptomatic, can produce non-specific mild signs and symptoms that are similar to those of common viral illnesses, or may cause marked sickness leading sometimes to multiorgan dysfunction with renal or hepatic failure5,7,29,32,33,40,43,45.
Leptospirosis cases are regularly diagnosed in our laboratory through the microagglutination technique (MAT), the IgM Indirect Immunofluorescence (IgM IIF) assay, blood culture or PCR45.
From June 2015 to June 2017, 162 confirmed leptospirosis patients were diagnosed. Considering the diagnoses performed in other laboratories, and keeping in mind frequent underdiagnosing, non-notified cases, and usual subclinical infections10, more than 800 Leptospira infections may have occurred during that period in Uruguay. Most confirmed patients (71.4%) worked in rural areas (mainly young males) or in connection with production animals and derived products (60.2% and 11.2%, respectively). Three urban patients were waste collectors or recyclers. Only 10 (6.2%) were women and 9 (5.6%) were children <15 years old.
We estimate that human leptospirosis annual incidence is about 15/100000 inhabitants: approximately 500 human cases occur throughout the country, almost always with some recorded death each year.
Former studies have also estimated seroprevalence, revealing previous or recent contact with Leptospira spp. to be approximately 10% in the general population of blood donors, and near 50% in workers at risk45.
The hypothesis of this research program was that there are, however, some human groups linked by environmental or occupational conditions in which Leptospira infection and illness is not fully recognized due to social marginalization and fragmentation, or to difficult access to healthcare: population segments whose records are not included in epidemiological or health attention reports.
We decided to study anti-Leptospira antibodies in the sera of dairy workers, rice laborers, veterinarians, suburban slum dwellers and garbage recyclers6,9,15,44. Our aims were to estimate the seroprevalence of infection in people at risk, to identify the serum reactive serovars in MAT, the relative importance of known risk factors associated with infection, and the impact of infections on those human groups, in order to sensitize health and social authorities, thus favoring prevention measures that contribute to good health attention, labor stability and social inclusion.
MethodologySampling program and sample sizeIdentification of zones and human groups at risk was based on our laboratory records of diagnosed patients in recent years, and on published data regarding exposed human groups4,15,35,49. Information was additionally obtained from workers’ unions (waste classifiers, rice workers), rural setting owners, government Social Development and Health ministries, local official administrations, and non-governmental organizations.
Identified locations and human groups were visited, individual participation within the group being voluntary. In each location, the approach to the workers’ group was addressed with the help of the corresponding local health or community attention team.
Given the previously estimated seroprevalence of 50% in human groups at risk45, a sample size of 300 persons allowed us to study the global prevalence of infection in highly exposed people, within±5.5% of the estimated value with 95% confidence. Three hundred and eight persons were studied from April 2015 to April 2017, including rice workers in Cerro Largo, Treinta y Tres, Rocha and Lavalleja (through 12 visits); dairy farm workers in Florida, Colonia and Rio Negro (4 visits); slum dwellers dealing with informal waste recycling and animal raising in Montevideo and Canelones settings (3 locations, 9 visits); temporary workers at the recently organized urban waste recycling plants in Montevideo and collaborators of a non-governmental waste recycling organization (4 locations, 5 visits).
Five meetings were also organized for disseminating information and obtaining additional serum samples from field veterinarians, health personnel and rural workers in San José, Durazno, Salto, Tacuarembó and Treinta y Tres. The study comprised 300 persons exposed to known risk factors, and 8 non-exposed persons that were occasionally included when attending the exchange meetings and did not formally constitute a control group.
This work was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). A signed informed consent was obtained from each studied worker, as required by the School of Medicine Ethics Committee.
Personal survey questionnairesThese were administered to all studied workers for determining the presence of known risk factors for Leptospira infections: working conditions, potentially contaminated water collections or animals, among others. Each of them and also land owners, supervisors or health workers were simultaneously supplied with a brief information chart explaining the characteristics of the disease, known risk factors and preventive measures. Additional information about the study program was provided for obtaining their individual informed consent.
Sample collection for microbiological studies and diagnosis.Human samplesBlood was taken in sterile tubes containing accelerant gel for the laboratory study of specific antibodies in sera using the Microagglutination Technique (MAT) and the IgM-Indirect Immunofluorescence (IgM IIF) assay34.
The microagglutination technique for human serum antibodies investigation (IgM and IgG)MAT was performed in all serum samples according to the standard technique with a two-step procedure25,27,45. Each serum was initially diluted 1/25 in saline solution and mixed with equal volumes of each of the 18 live cultures of Leptospira serovars that yield reactive results very frequently in the region and are able to promote cross-reactions. The serogroups and serovars of the employed strains were: Australis Australis; Australis Bratislava; Autumnalis Autumnalis; Autumnalis Butembo; Ballum Castellonis; Canicola Canicola; Cynopteri Cynopteri; Grippotyphosa Grippotyphosa; Hebdomadis Hebdomadis; Icterohaemorrhagiae Icterohaemorrhagiae; Mini Mini; Pomona Pomona; Pomona Kennewicki; Pyrogenes Pyrogenes; Sejroe Hardjo; Sejroe Wolffii; Semaranga Patoc; Tarassovi Tarassovi.
In a second step, agglutinating serovars were tested against serial dilutions of the patient's serum. Titers equal or higher than 400 in a single serum sample against at least one serovar were considered to confirm a case of present leptospirosis infection.
Sera with lower titers (titer 50 for two or more serovars, titers 100 or 200 for at least one serovar) were recorded as reactive and interpreted as evidence of past or recent infection, revealing personal contact with Leptospira spp.
Indirect Inmunofluorescence (IgM IIF)Slide wells were coated with 20μl of a 1/100 dilution of a pool of four usually reacting serovars in the MAT assays: Australis Bratislava, Ballum Castellonis, Canicola Canicola and Icterohaemorrhagiae Icterohaemorrhagiae. After drying at room temperature for 24h, the antigen was fixed with 20μl acetone for 8–10min. Twenty μl of a 1/50 dilution of the problem sera were added to each well and the slides were incubated in a wet box at 37°C for 30min. Several washings with PBS 1× and distilled water were performed, and then the slides were allowed to dry at room temperature. Twenty μl of 1/15 anti-human IgM marked with fluorescein-isothiocyanate (SIGMA®) were then added to each well, and the slides were again incubated at 37°C for 30min in a wet box. After washing with PBS 1× and distilled water, the slides were air-dried, mounted with glycerol and a cover glass, and observed with an epifluorescence microscope (Nikon® ECLIPSE 80i, 495nm filter) using 20× and 40× lenses. This assay has a proven good specificity (100%)38.
Animal samplesBlood was also obtained from 50 dogs and 22 horses found in close relationship with workers in the rice fields, dairy farms and suburban slum settings. Animal samples were obtained in accordance with the ARRIVE guidelines (NC3Rs, UK). Anti-Leptospira antibodies were assessed by MAT in the same manner as for the human samples. Employed antigen strains were Ballum Castellonis, Autumnalis Butembo, Cynopteri Cynopteri, Canicola Canicola, Grippotyphosa Grippotyphosa, Icterohaemorrhagiae Icterohaemorrhagiae, Pomona Pomona and Pyrogenes Pyrogenes for dogs; Ballum Castellonis, Canicola Canicola, Icterohaemorrhagiae Icterohaemorrhagiae, Grippotyphosa Grippotyphosa, Pomona Pomona, Sejroe Hardjo, Sejroe HardjoBovis, Sejroe Wolffii and Tarassovi Tarassovi for horses21. Reactive sera (antibody titers≥100) revealed past or present infection of the animals.
Environmental samplesSamples were also taken from water bodies located near homes and workplaces; sampling points were levees, dikes, narrow canals, wells, animal drinking troughs. Ten samples (100ml each, approximately) were obtained from seven rice fields; 9 from 4 dairy farms and 6 from 3 slum settings. They were collected in sterile plastic cups, transported in 4–8°C refrigerated boxes to the laboratory and processed on arrival, 2–6h after sampling. Five hundred μl volumes of filtered sample through 0.2μm pore membrane were inoculated into Fletcher semisolid and Ellinghausen-McCullough-Johnson-Harris (EMJH) liquid culture media (Difco-BD®), incubated at 28°C and observed periodically in a dark field microscope to detect bacterial growth (Nikon Eclipse Ci-L®). Positive cultures were studied and identified as already described39.
Statistical analysisData analysis was performed using the SPSS (Statistical Package for the Social Sciences) and the Epi-Info 2000 software developed by the PAHO (Pan American Health Organization). Qualitative and quantitative variables were examined in accordance with their level of measurement. An initial univariate analysis was performed with the appropriate summary measures.
The bivariate analysis was based on the search of statistical associations to explain the dependent variables with adequate statistical tests according to the level of measurement of those variables and to the number of observations. Combined indicators were built for summarizing environmental or living situations (Table 1). The Chi-square test was used for excluding the independence between two variables when comparing results, with p<0.05. The Fisher's exact test was employed if sample sizes were small.
Categories of “socio-educational level” combined indicator
| High | Complete university studies. |
| Middle-High | Complete secondary education or incomplete university studies. With 2, 1 or 0 unsuitable situations referring to potable water, rodents or floors at home. |
| Incomplete secondary education, with no unsuitable situations referring to safe water, rodents or floors at home. | |
| Middle | Complete secondary education or incomplete university studies. 3 unsuitable situations referring to safe water, rodents or floors at home. |
| Incomplete secondary education. 1 or 2 unsuitable situations referring to safe water, rodents or floors at home. | |
| Complete primary school or incomplete secondary education, with no unsuitable situations referring to safe water, rodents or floors at home. | |
| Middle-Low | Incomplete secondary education. 3 unsuitable situations referring to safe water, rodents or floors at home. |
| Complete primary school with 1, 2 or 3 unsuitable situations referring to safe water, rodents or floors at home. | |
| Low | Incomplete primary school or no formal education, or slum dweller or waste recycling worker. |
The obtained potential associations were analyzed for detecting spurious relations, confounding variables or hidden links, employing stratified analysis. Finally, the binary logistic regression technique was employed for selecting the effect of participating variables that could actually explain seroreactivity.
Information and education for preventionThis activity was developed during the same visits that allowed the personal questionnaires and collection of samples. Its aim was to train workers on disease characteristics, hazards and prevention measures in their jobs, and also to sensitize and provide information to the health staff, veterinarians and managers of production facilities. The approach to the labor or dwelling group was previously arranged with local supervisors or social workers. An initial talk was organized for informing about leptospirosis, the risk factors for acquiring the infection, the signs and symptoms of the disease and the proper means of prevention. An information chart was provided and a valuable exchange ensued, based on doubts, questions, fears, and useful data produced by workers or supervisors about local cases, risk conditions and epidemiology. After reading and signing the informed consent sheet, the personal questionnaires were administered and the blood samples were taken.
Working places, sheds, barns, slums and surroundings were thoroughly visited and examined; labor conditions, living facilities, social and hygienic habits were observed and recorded; additional information was exchanged with workers or inhabitants; human-associated environmental water collections, dogs and horses were sampled.
The results of the serological tests were informed to the studied person or to his/her supervisor, providing interpretation or adding information when needed.
ResultsThe study involved 308 persons: 300 from 5 social groups at risk, and a small group (“others”, not exposed) that comprised a few health staff members, and some students or attendees to the exchange meetings. Eighty-eight female and 220 male persons were included (Table 2).
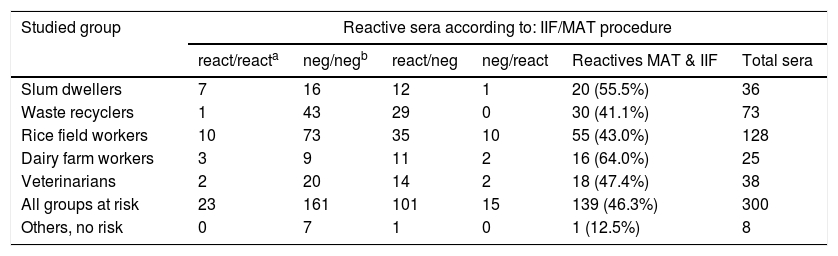
Reactive sera (as defined by IIF and/or MAT) found in each studied risk group
| Studied group | Reactive sera according to: IIF/MAT procedure | |||||
|---|---|---|---|---|---|---|
| react/reacta | neg/negb | react/neg | neg/react | Reactives MAT & IIF | Total sera | |
| Slum dwellers | 7 | 16 | 12 | 1 | 20 (55.5%) | 36 |
| Waste recyclers | 1 | 43 | 29 | 0 | 30 (41.1%) | 73 |
| Rice field workers | 10 | 73 | 35 | 10 | 55 (43.0%) | 128 |
| Dairy farm workers | 3 | 9 | 11 | 2 | 16 (64.0%) | 25 |
| Veterinarians | 2 | 20 | 14 | 2 | 18 (47.4%) | 38 |
| All groups at risk | 23 | 161 | 101 | 15 | 139 (46.3%) | 300 |
| Others, no risk | 0 | 7 | 1 | 0 | 1 (12.5%) | 8 |
One hundred and thirty nine of 300 sera from persons at risk (46.3%: range 41.1–64.0 in the different groups) were found to react with specific antigens in IIF and/or MAT procedure (Table 2), thus revealing probable contact with Leptospira. Seroprevalence was 12.5% for the others. Dairy farm workers revealed a higher frequency of serum reactivity than that of rice field workers or waste recyclers (p<0.05).
Reactivity was more frequent when assessed by IIF (124/300: 41.33%) than when studied by MAT (38/300: 12.66%) (p<0.05). IgM IIF reactivity was especially frequent in the sera of waste recyclers (30 of all reactive 30) and IIF negative/MAT reactive sera were often found in the rice field workers.
Most frequently reactive serogroups were Icterohaemorrhagiae in dairy farm workers; Canicola in slum inhabitants; Canicola, Autumnalis, Mini and Icterohaemorrhagiae in rice field workers; Pomona, Autumnalis and Icterohaemorrhagiae among veterinarians. Only one MAT reactive serum was found in waste recyclers.
In general, the Canicola Canicola reference strain reacted with 15 sera, Icterohaemorrhagiae Icterohaemorrhagiae and Autumnalis Autumnalis with 12, Mini Mini with 11 and Pomona Kennewicki with 4.
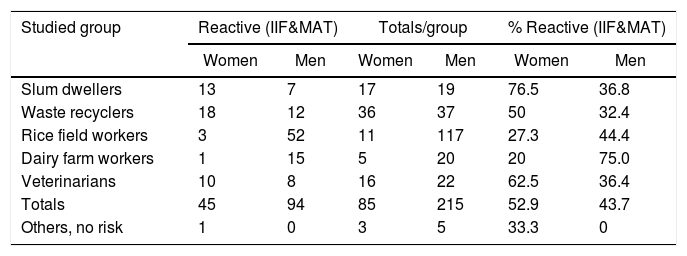
Sera from women were more often reactive than those of men, but no significant differences were observed between overall frequencies. However, when stratifying the analysis by groups, some associations were found: women exhibited higher serum reactivity than men among slum dwellers, and the opposite occurred with dairy workers (p<0.05 in both cases) (Table 3).
Reactive sera (as defined by IIF and/or MAT) in male and female persons
| Studied group | Reactive (IIF&MAT) | Totals/group | % Reactive (IIF&MAT) | |||
|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Women | Men | |
| Slum dwellers | 13 | 7 | 17 | 19 | 76.5 | 36.8 |
| Waste recyclers | 18 | 12 | 36 | 37 | 50 | 32.4 |
| Rice field workers | 3 | 52 | 11 | 117 | 27.3 | 44.4 |
| Dairy farm workers | 1 | 15 | 5 | 20 | 20 | 75.0 |
| Veterinarians | 10 | 8 | 16 | 22 | 62.5 | 36.4 |
| Totals | 45 | 94 | 85 | 215 | 52.9 | 43.7 |
| Others, no risk | 1 | 0 | 3 | 5 | 33.3 | 0 |
The age of the studied persons ranged from 13 to 73 years old (mean=38.3±13.3 standard deviation). Mean ages of men (38.6) and women (37.4) were not significantly different when examined with the “t” test for independent samples, assuming equal variance values.
However, the mean age was different in the different risk groups; slum inhabitants were the youngest (mean=31.6: 34.6 for women; 29.1 for men), followed by veterinarians (mean=36.0: 32.6 for women and 38.7 for men), and waste recyclers (mean=37.4: 38.2 for women and 36.4 for men). Rice field workers (mean=40.2: 41.2 for women and 40.0 for men) and dairy farm laborers (mean=44.0: 50 for women and 42.7 for men) had the highest mean ages, both statistically different from those of slum dwellers. One-way ANOVA analysis: p<0.01, with Duncan and Scheffé multiple comparison tests.
Most analyzed persons and most reactive sera belonged to the young age groups (Table 4).
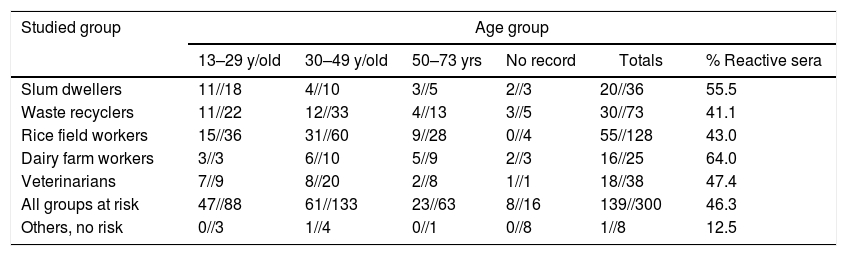
Reactive sera (MAT&IIF)//Analyzed sera in each age group
| Studied group | Age group | |||||
|---|---|---|---|---|---|---|
| 13–29 y/old | 30–49 y/old | 50–73 yrs | No record | Totals | % Reactive sera | |
| Slum dwellers | 11//18 | 4//10 | 3//5 | 2//3 | 20//36 | 55.5 |
| Waste recyclers | 11//22 | 12//33 | 4//13 | 3//5 | 30//73 | 41.1 |
| Rice field workers | 15//36 | 31//60 | 9//28 | 0//4 | 55//128 | 43.0 |
| Dairy farm workers | 3//3 | 6//10 | 5//9 | 2//3 | 16//25 | 64.0 |
| Veterinarians | 7//9 | 8//20 | 2//8 | 1//1 | 18//38 | 47.4 |
| All groups at risk | 47//88 | 61//133 | 23//63 | 8//16 | 139//300 | 46.3 |
| Others, no risk | 0//3 | 1//4 | 0//1 | 0//8 | 1//8 | 12.5 |
The mean age of 139 persons at risk with reactive sera (X=36.4) was slightly lower than the age of those yielding negative results (X=39.8). The “t” test for independent samples=2.11; p<0.05.
When considering symptoms and signs of illness experienced in previous weeks, only conjunctival hyperemia and abdominal manifestations (pain, diarrhea, vomits, hepatic illness) were definitely more frequent in the persons showing reactive sera than in the persons yielding negative serum results (p<0.05).
Our survey detected seven highly reactive sera (titers≥400) of workers that had been previously sick, or were currently ill, or later developed clinical leptospirosis. They were identified in two locations: one rice field establishment (5 persons) and one dairy farm (2 patients). These patients were all middle-aged male workers in contact with bovine and other animals. Their symptoms included general non-specific manifestations such as fever, headache, asthenia, joint and muscle pain, and added evidence of digestive system or kidney involvement in four cases. They all had accessible health attention services, but laboratory diagnosis was not readily achieved.
Fifteen per cent of 300 potentially exposed persons had not received formal education, or had not completed primary school; 67.2% had finished primary school or had not completed secondary education; 5.5% had completed secondary education or initiated university studies; 14.7% had completed university studies. More than half of the patients (55.7%) could be classified as having low (33.3%) or middle/low (22.4) socio-educational levels; 21.3% middle level; and almost one fourth of them middle/high (8.3%) or high (14.7) levels. The frequencies of found reactive sera were not distinctly different among the diverse socio-educational levels (Chi2=3.51; df=4; p>0.05). Associations were neither found between levels of other combined indicators and frequency of reactivity.
All veterinarians and dairy farm workers had contact with various animals, mainly bovine cattle. Slum inhabitants and waste recyclers had the same level of contact, but the involved animals were principally dogs, horses and rodents. Rice field workers had contact with a wide variety of animals, including those already mentioned and also sheep, pigs and others.
All waste recyclers and rice field workers with reactive sera had contact with animals; however, 9 and 19 respectively of those with negative sera did not, suggesting that animal contact may be linked to reactivity (p<0.05).
Considering all 300 surveyed persons at risk, 49.3% of those that had animals around their homes were serum-reactive; however, reactivity was 34.3% for sera of persons not living in close contact with animals (p<0.05).
Rodents were present in the vast majority of the working or living surroundings of those 300 persons. Sera were reactive in 49% of persons usually observing rodents in either setting, and 30,2% in persons that did not (p<0.05). Additionally, 48.2% of those declaring the close presence of rodents at work had reactive sera, while 30.4% sera of persons not working close to rodents yielded reactive results (p<0.05).
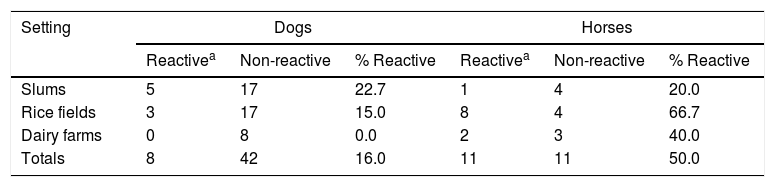
With regard to the animals that were actually studied, sera of horses were frequently reactive in MAT tests, in a higher proportion than dog samples (Table 5, p<0.05), especially in rice production settings. Canicola was the most frequently reactive serogroup in the MAT of dogs; Icterohaemorrhagiae in the MAT of horses.
Most slum dwellers and waste recyclers (both showing reactive or non-reactive sera) used safe water for drinking and washing. The opposite was true for dairy farm workers. Veterinarians and rice field workers used either type of water, but in the latter group, workers with reactive sera more frequently employed water from unsafe sources (50 of 55) than workers showing non-reactive results (47 of 72) (p<0.05). Globally, 38.3% of all persons using safe piped water yielded reactive sera results; figures were 50.7% for those employing unsafe water from tanks, wells, or field water collections. Chi2=4.14 for 1 df; p<0.05.
Almost all workers used safe shoes or boots, but only 76% of them used gloves; differences in serum reactivity were not significant between glove users and non-users. Other protection devices (face masks, aprons) were used in varied proportion by the different risk groups.
Frequency of sera reactivity was not different for the persons recorded as exposed or not exposed to potentially contaminated water collections, neither in general nor in each studied group. Considering the separate groups, most slum inhabitants were exposed (92%): more than the members of the other groups at risk, who were also often exposed (47%) (p<0.05).
Sera of persons living in areas exposed to floods were more frequently reactive than those persons having safer housing.
With regard to the factors probably associated with serum reactivity (dependent variable), a final logistic regression analysis was performed.
The results showed that the variables fitting the model of true associations with serum reactivity were: 1 – Presence of animals or contact with them at work, at home or their environment: OR 4.96 (1.29–19.07 with 95% confidence); 2 – Presence of rodents or contact with them at home, at work or their environment: OR 2.79 (1.32–5.91); 3 – Unsafe water usage for washing and cleaning at work: OR 1.82 (1.10–3.01); 4 – Home exposure to flooding: OR 1.54 (1.01–2.055). The probability of having a reactive serum increased with decreasing age: sera of young persons were more probably reactive. The antilogarithm of the Beta coefficient of age was 0.98 (0.96–0.998 interval limits with 95% confidence).
Six Leptospira spp. isolates were obtained from water samples in this working period. Five of them were identified as Leptospira biflexa non-pathogenic strains; one Leptospira meyeri isolate was also recovered from a slum water sample35.
Field observation, information and education for prevention were successfully performed in most visits to the groups at risk. A warm welcome was generally given, collaboration was provided, and talk about leptospirosis, its epidemiology and risk factors, doubts and fears of exposed people, signs and symptoms of sick patients and related subjects was useful for both workers and the research team. Personal and group exchanges revealed that in recent months or years several persons, especially in rural settings, had suffered illnesses that were not recognized or treated but had symptomatology and epidemiological conditions compatible with leptospirosis. The outcome of some of those cases was fatal. Health attention was usually available, despite the long distances from the attention services to the rural settings, but leptospirosis was not easily diagnosed or managed by health personnel, as has been already reported45. Other testimonies revealed that asthenia, headache, muscle and joint pain in confirmed cases often lasted for several weeks or months, leading to labor impairment and economic problems for the families of the affected patients.
DiscussionThe results shown suggest that humans pertaining to all the studied groups at risk were widely exposed to infection by Leptospira spp. The percentage of samples revealing serum antibodies to Leptospira spp. was higher than 40% in all those examined groups. As expected from previous studies, seroprevalence was lower among other persons, not exposed to known risk factors of infection, whose samples were occasionally obtained, not for formally organizing a control group. Similar results have been reported in regional and international surveys14,37.
More reactive sera were found by IIF than by MAT. This result cannot be explained as caused by low IIF specificity, for this method was shown to have no false positive results34. It may be due to known low sensitivity of MAT when studying recent infections25, or to usage of locally non-prevalent living strains as antigenic reagents. Actual circulating strains are being studied35,49 and must be included in the MAT panel for improving its performance. Early detection of new infections is easier by IgM IIF than by MAT. Recent infections seemed especially frequent in waste recyclers (30 by IIF of reactive 30, 29 of them non-reactive by MAT). In IgM IIF negative sera, MAT reactivity (exploring both IgG and IgM) was found in 10 rice field workers and others, and can be interpreted as revealing past or older contacts, with persistent IgG titers.
Frequently reactive serogroups in the performed MAT tests for our work should not be considered recognized infecting variants. Reactive serovars in MAT assays do not reliably show the infecting variant of Leptospira spp.25,47. However, after broad cross-reactivity in the acute phase of illness, relative serogroup specificity usually follows in convalescent samples. Frequent Canicola titers in slum dwellers agree with those of the close and large population of dogs living in such environment.
Project activities allowed us to recognize two clusters of patients that had not been jointly considered for implementing preventive measures; however, no human blood cultures were performed for this research work. In routine diagnostic tests conducted in our laboratory, three isolates were obtained throughout 2015–2017, all of which were from rural workers. They were identified as Leptospira interrogans serogroup Canicola, Leptospira kirschneri serogroup Pomona serovar Mozdok and Leptospira borgpetersenii serogroup Ballum35,49. Further blood cultures are required for gathering detailed knowledge about human-infecting Leptospira species and serovars in Uruguay.
In our serum survey, female human reactive sera were generally more frequent than male ones, but differences were not significant, except for slum inhabitants. Higher male seroprevalence in dairy workers probably reflects the fact that women are not usually allowed to perform direct milking tasks.
Globally, our data suggest that contact and infection of women with Leptospira spp. often occurs within human groups at risk; these figures are not consistent with the low proportion of female cases of laboratory confirmed leptospirosis disease (≥400 titer) in Uruguay and in the world. Male sex preference of clinical leptospirosis is a known phenomenon usually attributed to gender-specific occupational and peridomiciliary risk activities of women10. However, according to our results it would be necessary to analyze if gender-linked factors are involved in the development of clinical illness. Host response functions are involved in the pathogenesis of this disease50; they may behave differently in men or women, as well as in young or elderly male patients.
Alternatively, the low detected incidence of clinical leptospirosis in women, despite frequent infection, may be the result of an underdiagnosis of the disease or limited demand of attention by the female patients. This explanation is not presently supported by existing evidence; however, it deserves further examination.
Infecting contacts were detected more often in the young members of all the risk groups, and a similar age distribution usually occurs in confirmed cases. Children seldom show overt illness, and were not sampled in the studied groups. Leptospirosis is less frequent in old age groups, as was the detected serum reactivity, but severe cases occur more frequently in elderly workers10,35,45.
Persistent complaints can follow severe disease and result in working disability and reduced money income for involved families, as we could verify in the rural settings. Information about these long- term consequences of leptospirosis is not abundant in the medical literature; however, some detailed reports can be found revealing prolonged and marked fatigue, myalgia, malaise, headache, and a weak physical condition4,19. A national prospective study is needed of all notified cases, examining signs and symptoms of patients for long periods of time after diagnosis, and describing the personal and social consequences of extended illnesses.
Our results indicate that contact of humans with animals is an important risk factor for developing infection with Leptospira spp. in our country, at least in some defined social groups. The bovine component of the leptospirosis reservoir has been already studied, locally and worldwide, and its importance in Uruguay has been recently reported1,4,12,16,26,38,45,46,49. Reactivity of equine sera studied here was high (50%). This result agrees with regional findings, but requires a more complete survey; additionally, equine disease and the role of horses in Leptospira spp. transmission must be further studied31,39. Frequent infection and illness of horses raise concern among breeders and owners. Canicola and Icterohaemorrhagiae have been already described as prevalent reactive serogroups in the sera of infected dogs and horses, respectively39. Contact with rodents may also be an important risk factor for humans and other animals, as suggested by our recorded data and expressed in international literature9,17,23. Frequency of rodent infection still needs to be locally examined; however, published information includes reports revealing that the rodent population is not always part of the reservoir of infection1.
Most members of the studied human groups were exposed to several known risk factors. Direct or indirect exposure to animals, and usage of unsafe water were the most relevant conditions associated with the presence of serum anti-Leptospira antibodies in the examined population at risk. However, all prevalent risk factors have to be considered for designing effective prevention programs. Frequent contact with production animals (mainly bovines) and with horses and dogs employed as working aids are closely related to prevalent national economic activities, and seems to be the main reason explaining the relatively high incidence of leptospirosis in the rural workers of Uruguay. Poor environmental and social conditions, along with canine, equine and rodent contact, can be important risk factors in some suburban human groups and also in rural settings.
It is still necessary to conduct in our country further systematic studies on the presence and transmission of these infections in the human groups at risk, their living, working environment and animals, focusing on those poorly examined and infrequently considered. This is not easy because it requires dealing with population groups and zones of difficult access, variable composition, important mobility and usual marginalization from social and health attention. More information is particularly needed about the presence and transmission of leptospirosis in women and children that are rarely recognized as infected for reasons that should be especially explored24,27,42. Host organic reaction to infection in persons of different ages and gender must be carefully studied, looking for the reasons of observed differences in the incidence and severity of the disease.
Improved information and training of health personnel is still needed for early recognition, quick diagnosis and proper management of the illness. Considering directly obtained and published information that raises concern, prolonged attention to the evolution of confirmed cases shall be organized. Gathering thorough knowledge about health, social and economic long-term consequences of human leptospirosis will allow to improve care and rehabilitation of the affected persons and their families.
Conflict of interestThe authors declare that they have no conflicts of interest.
FundingThis work was supported by CSIC University Research Committee through its Social Inclusion Program, project no. 92, 2014.
The authors are grateful to Alicia Guerra and Teresa Monteverde from Health Division, Intendencia Departamental de Montevideo – IDM – and to Directors of Social Welfare Division, IDM; to Norberto Borba and Laura Fernández from Health Division, Intendencia Departamental de Cerro Largo; to Alicia Sosa and Mónica Olinisky from RAP, metropolitan public Primary Health Net; to Walter Migliónico from PIT-CNT, national workers association, Marcelo Amaya from SUTAA, rice workers association and Jorge Ramada from UCRUS, urban recyclers; to Laureana De Brun, from Microbiology Department, Veterinary Faculty, UdelaR; to members of San Vicente and Servando Gómez suburban dwellers organizations; to Virginia Gadea from SOCAT, Social Orientation Service, Ministry of Social Development; to Virbac Santa Elena laboratories; to María Sanguinetti from ACA, rice farmers association, and to members of all social, health and professional organizations that helped approaching to social groups at risk of Leptospira infection.
Special thanks to Miguel Meny, for valuable aid in data analysis and statistics.