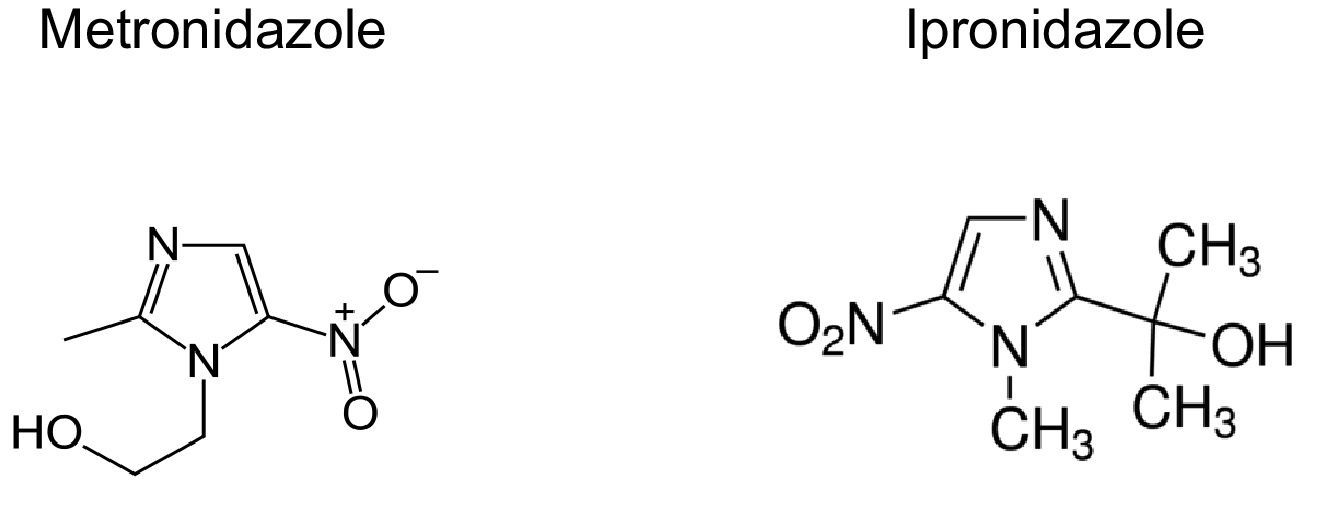Latent tuberculosis has been associated with the persistence of dormant Mycobacterium tuberculosis in the organism of infected individuals, who are reservoirs of the bacilli and the source for spreading the disease in the community. New active anti-TB drugs exerting their metabolic action at different stages and on latent/dormant bacilli are urgently required to avoid endogenous reactivations and to be part of treatments of multi- and extensively-drug resistant tuberculosis (M/XDR-TB). It was previously reported that azole drugs are active against M. tuberculosis. For that reason, the aims of this study were to determine the in vitro activity of azole drugs, imidazole (clotrimazole, CLO and econazole, ECO) and nitroimidazole (metronidazole, MZ and ipronidazole, IPZ), against a collection of MDR M. tuberculosis clinical isolates; and to analyze their potential use in both the LTB and the active forms of M/XDR-TB treatments.
MethodsA total of 55 MDR M. tuberculosis isolates and H37Rv were included. MZ and IPZ activity against M. tuberculosis isolates were tested using anaerobic culture conditions. The activity of ECO and CLO was measured by the minimal inhibitory concentration (MIC) using a microdilution colorimetric method.
ResultsMZ and IPZ showed bacteriostatic activity against M. tuberculosis strains. MIC50 and MIC90 to ECO was 4.0μg/ml, while MIC50 to CLO was 4.0μg/ml and MIC90 was 8.0μg/ml respectively.
ConclusionAll azole compounds tested in the study showed inhibitory activity against MDR M. tuberculosis clinical isolates.
La tuberculosis (TB) latente ha sido asociada a la persistencia de Mycobacterium tuberculosis durmientes en el organismo de las personas infectadas, las cuales constituyen un reservorio del bacilo y una fuente de diseminación de la enfermedad en la comunidad. Urge la necesidad de contar con nuevos fármacos antituberculosos con acción sobre el bacilo en estado latente/durmiente, a fin de evitar reactivaciones endógenas y para ser incluidas en el tratamiento de la TB multirresistente y extensivamente resistente (M/XDR-TB). Se ha reportado que los azoles son activos contra M. tuberculosis. Por esta razón, los objetivos del presente estudio fueron determinar la actividad in vitro sobre aislamientos clínicos de M/XDR-TB de distintos azoles, incluyendo los imidazoles econazol (ECO) y clotrimazol (CLO) y los 5-nitro-imidazoles ipronidazol (IPZ) y metronidazol (MZ), así como analizar su potencial uso contra las formas latente y activa de esta enfermedad.
MétodosFueron incluidos 55 aislamientos clínicos de M. tuberculosis MDR y la cepa de referencia H37Rv. Se evaluó la actividad del MZ y el IPZ sobre los aislamientos en condiciones de cultivo anaeróbico, mientras que la actividad del ECO y el CLO fue estimada determinando la concentración inhibitoria mínima (CIM) mediante el método colorimétrico de microdilución en placa.
ResultadosEl MZ y el IPZ presentaron actividad bacteriostática frente a las cepas de M. tuberculosis. La CIM50 y CIM90 del ECO fue de 4μg/ml, mientras que el CLO presentó una CIM50 de 4μg/ml y una CIM90 de 8μg/ml.
ConclusiónTodos los compuestos azólicos evaluados presentaron actividad inhibitoria frente a aislamientos clínicos de M. tuberculosis.
Latent tuberculosis (LTB) has been associated with the persistence of dormant Mycobacterium tuberculosis in the organism of infected individuals, who are reservoirs of the bacilli and the source for spreading the disease in the community10.
The Word Health Organization (WHO) estimates that one-third of the world population is infected with M. tuberculosis and around 10% might develop the disease during their life31,32. In 2013, it was estimated that almost 480 000 new multidrug-resistant tuberculosis (MDR-TB, simultaneously resistant to isoniazid, INH, and rifampicin, RIF) cases occurred worldwide. The extensively drug-resistant TB (XDR-TB) caused by MDR microorganisms with resistance also to an injectable agent (amikacin, kanamycin or capreomycin) and to a fluoroquinolone, has been reported by 100 countries and in accordance with the WHO, around 9.0% of people with MDR-TB have XDR-TB9.
From year 2000 to 2012, Argentina reported more than 80 XDR-TB cases with 2.2% of incidence rate among new MDR-TB cases15.
XDR-TB is considered an almost incurable disease and a limited number of active antibiotics that could be used are available. The contacts of M/XDR cases could also be infected by these resistant mycobacteria, developing and spreading the disease in the community. Therefore, new active anti-TB drugs exerting their metabolic action at different stages and on latent/dormant bacilli are urgently required to avoid endogenous reactivations and to be part of treatments for M/XDR-TB.
Azole drugs, such as imidazole, econazole (ECO) and clotrimazole (CLO) are widely used antifungal agents, which are also active against some gram positive bacteria. There are many data on their safety, side effects, pharmacokinetics and pharmacodynamics4,32. Furthermore, it has been previously reported that azoles are also active against in vitro M. tuberculosis and in the mouse model2,17,18,24.
The distribution of Cytochrome P450 (CYP) mono-oxygenases in fast-growing and slow-growing mycobacteria evidence that these enzymes are highly conserved in the genus Mycobacterium unlike other bacteria having no CYP, such as Escherichia coli. Azole drugs coordinate to the heme iron of CYPs of the microorganism; therefore, they are potent CYP inhibitors. M. tuberculosis contains 20 different CYPs11,12,19,23. There is no doubt about the activity of some azoles against mycobacteria such us PA824, metronidazole (MZ), delamanid, ECO, CLO and many new molecules of chemical synthesis; however, their mechanism of action is not fully elucidated20,24. It has been postulated that the main mechanism of resistance to azoles is the increased drug efflux mediated by the MmpS5-MmpL5 system in M. tuberculosis11,16,24.
Antimicrobial drugs that showed no significant activity against M. tuberculosis in aerobic culture condition may be active in the dormant state induced by a drop of the oxygen tension in the culture medium. This suggests the possibility that these drugs may have a place in the chemotherapy of MDR-TB and LTB alone and/or in combination with other drugs28.
Nitroimidazoles are synthetic chemotherapeutic agents that are active against fungi, parasites and anaerobic bacteria, and which have also demonstrated their bactericidal activity against dormant M. tuberculosis, since the targets of these drugs are expressed during the latent state1,3. It has been postulated that these drugs exert bactericidal activity by the reduction of its nitro group. Although 5-nitro and 2-nitroimidazoles are available, only the 5-nitro derivative is effective as antibacterial and antiparasitic agent14. MZ is the main drug of 5-nitroimidazoles and it is believed that it acts as an electron acceptor, inhibiting the released H2 and ATP production. The active structure of MZ remains unknown; however MZ reduction requires three electrons; therefore it is supposed that there is a free radical or another highly electrophilic molecule acting as electron acceptor. Reduced MZ derivatives act on DNA, producing an extensive break of this molecule as well as, it is believed, a possible inhibition of DNA repair mechanisms5. It has also been postulated that MZ is active against M. tuberculosis by affecting the synthesis of fatty acids in mycobacteria22. The redox potential of MZ is −420mV and the lowest potential in aerobic systems is −350mV, for this reason, MZ is only active under anaerobic conditions.
MZ acts on gram positive, gram negative and anaerobic bacteria. The antiparasitic effect includes amebas, trichomonas and giardias. In M. tuberculosis, MZ is considered a prodrug that is activated by mycobacterium enzymes, such as the oxidoreductase formed by Rv2454c and Rv2455c. This enzyme would be expressed around day 25th of incubation under hypoxic culture conditions12,13. Genes codifying for CYP450 are essential for the virulence and persistence of M. tuberculosis in the host. It has been previously reported that under hypoxic conditions, MZ has bactericidal activity against non-replicating M. tuberculosis25.
Some clinical trials evaluated MZ activity for pulmonary TB demonstrating that it produced early sputum smear and culture negative conversions but it was too neurotoxic and hepatotoxic to be part of a long term anti-TB treatment8.
Moreover, delamanid is a MZ derivative recently approved for MDR-TB treatment in combination with other anti-TB drugs (http://ec.europa.eu/health/documents/community-register/2015/20150424131446/anx_131446_es.pdf).
The aims of this study were to determine the in vitro activity of azole drugs, imidazole and nitroimidazole against a collection of MDR M. tuberculosis clinical isolates to compare the activity of MZ with IPZ and among imidazoles ECO and CLO, and to analyze their potential use in both LTB and the active forms of M/XDR-TB treatments.
In this study we tested MZ and IPZ as a prototype of 5-nitroimidazole compounds.
IPZ (2-isopropyl-1-methyl-5-nitroimidazole) is an antiprotozoal drug similar to MZ that had been used in veterinary medicine, such as in the treatment of histomoniasis, swine dysentery, trichomoniasis and giardiasis in turkeys and dogs26,30. In our knowledge there are no previous reports testing the in vitro activity of IPZ on M. tuberculosis strains.
On the other hand, ECO and CLO were used to test the imidazole activity among our MDR M. tuberculosis clinical isolates.
Materials and methodsA retrospective study including a collection of MDR M. tuberculosis strains isolated from patients diagnosed in the Reference Laboratory of Tuberculosis Control Program at Dr. Cetrangolo Hospital was conducted.
The Informed consent from each patient was obtained at the moment of the bacteriological TB diagnostic was made. No ethical approval was required for this study since it was a retrospective study that only included M. tuberculosis isolates and these experiences did not interfere with treatment decisions or medical behavior. However, approval was obtained from the Ethical Committee of Dr. Cetrangolo Hospital at the moment of the study design.
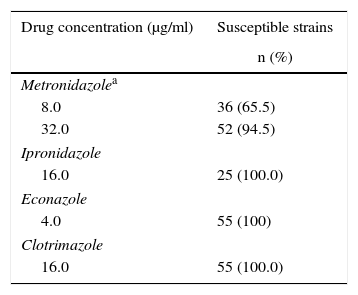
M. tuberculosis strains and clinical isolatesA total of 55 MDR M. tuberculosis strains were included in the study. This collection of MDR-TB strains were kept at −20°C and at the moment of the study they were unfrozen and sub-cultured in fresh Middlebrook 7H9 broth (M7H9) with OADC enrichment (Becton Dickinson, Buenos Aires, Argentina). M. tuberculosis H37Rv ATCC 27294 was used as fully drug-susceptible reference strain (Table 1).
Number and percentages of M. tuberculosis strains considered to be susceptible for each one of the drugs from the REMA and hypoxia experiments
| Drug concentration (μg/ml) | Susceptible strains |
|---|---|
| n (%) | |
| Metronidazolea | |
| 8.0 | 36 (65.5) |
| 32.0 | 52 (94.5) |
| Ipronidazole | |
| 16.0 | 25 (100.0) |
| Econazole | |
| 4.0 | 55 (100) |
| Clotrimazole | |
| 16.0 | 55 (100.0) |
To obtain anaerobic M. tuberculosis cultures (Can), a system adapted from Wayne and Hayes29. was used. Briefly, vacuum 5ml Vacutainer tubes (Becton Dickinson, Buenos Aires, Argentina) were filled, using syringe and needle (26 ½), with 3.0ml M7H9/OADC. Then, the tubes were inoculated with 30μl 1:100 dilution from a M. tuberculosis 1 McFarland suspension that was in the middle of the exponential phase of the growth curve and previously passed three times by syringe and needle (26 ½). These tubes were immediately incubated at 37°C during 35 days to achieve the hypoxic condition. Hypoxia is indicated by discoloration in the ‘control discoloration tube’, which was prepared as above mentioned but also adding 1.5mg/l of methylene blue (MB). MB is decolorized when the culture achieves the hypoxic condition27,29.
In vitro activity of MZ and IPZ in anaerobiosisMZ (Fluka, VETRANAL™, USA): once cultures of M. tuberculosis (H37Rv and MDR clinical isolates) reached hypoxia, 8.0μg/ml and 32.0μg/ml of MZ (Fluka, Germany) were added in different tubes to determine MZ activity. A free-drug tube was used as control of mycobacterial growth.
IPZ (Sigma–Aldrich, USA): once M. tuberculosis H37Rv achieved the hypoxic condition, 8.0μg/ml, 16.0μg/ml, 32.0μg/ml, 64.0μg/ml and 128.0μg/ml were added to determine their activity. MDR M. tuberculosis isolates were tested at 16.0μg/ml and 128.0μg/ml. A free-drug tube was used as control of mycobacterial growth.
In both cases (MZ/IPZ), the tubes were immediately re-incubated for 15 days at 37°C.
Determination of time to exit from the hypoxic/dormant stateAfter 15 incubation days, and in order to remove the MZ/IPZ from the drug-containing tubes, the whole content was transferred to a 15.0ml sterile empty plastic tube and was centrifuged at 3000rpm for 10min in a refrigerated centrifuge. The supernatant was removed and the pellet was washed with 3.0ml of PBS 1X, vortexing gently and centrifuging again. Afterwards, the pellet was resuspended in 3.0ml of PBS 1X. Then, 500.0μl of the washed culture were loaded into a BACTEC MGIT 960/OADC tube (Becton Dickinson, Buenos Aires, Argentina) to evidence the inhibitory effect of the drugs. The EpiCenter Software (Becton Dickinson, Buenos Aires, Argentina) connected to the MGIT system was used to detect the growth units, indicating the exit of dormancy and the restoration of the cellular metabolism in aerobic conditions6.
ECO and CLO activityDetermination of the minimal inhibitory concentration (MIC) to ECO and CLO was performed by the previously described resazurin (RES) microtiter assay using 96-well microplates. RES is a redox indicator that changes the color when bacteria consume O2. Briefly, the outside wells were filled with 200μl of sterile water and the inside wells with 100μl of M7H9/OADC medium. Afterwards, 100μl of ECO or CLO were added, the initial drug concentration being 32.0μg/ml; then two-fold serial dilutions were performed up to a lower concentration of 1.0μg/ml of the drug (range: 16.0–8.0–4.0–2.0–1.0–0.5μg/ml). After adding the drugs (except in the growth control well, GC), 100μl of a 1/25 dilution from 1 McFarland standard mycobacterial suspension, containing 106–108CFU/ml were added in each one of the wells loaded into the plates.
The plates were light-protected and incubated for 5 days at 37°C. Then, the GC was filled with 30μl of RES and incubated for one more day. If no color change was evidenced, a second GC well was developed and incubated for 24h (h) more and so on. When bacterial growth was observed, the remaining wells were filled with RES and re-incubated for 24h more for the final reading. The MIC was defined as the lowest drug concentration that completely inhibited the microorganism growth, which was evidenced by the absence of color change of the indicator21.
StatisticsVariable summary statistics were determined by the MedCalcv. 16.4.3 software (Mariakerke, Belgium). The t-test and the multiple line graphs to determine the MZ, IPZ, ECO and CLO activity was used to estimate the significance of the differences between drug activity against M. tuberculosis.
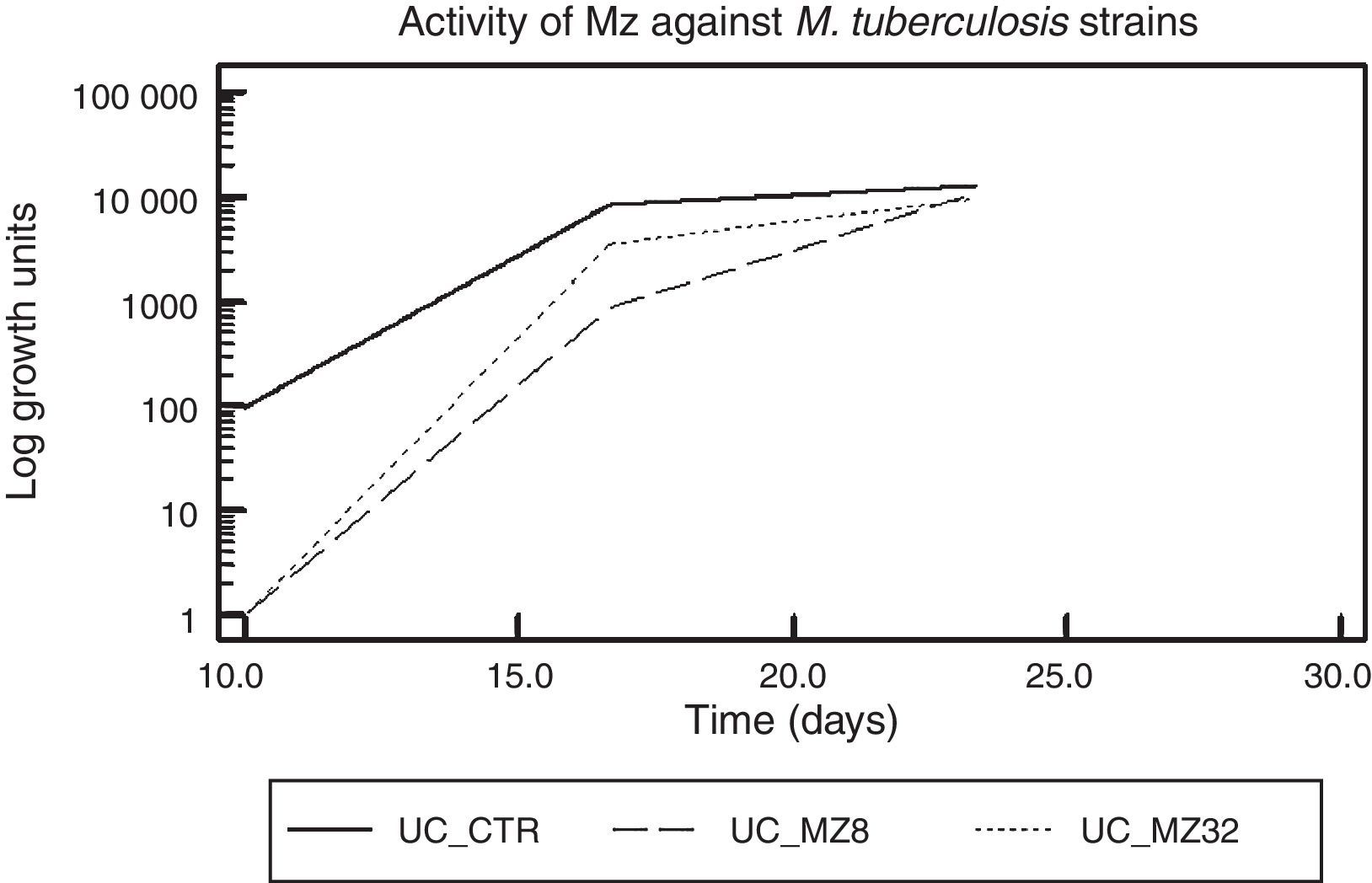
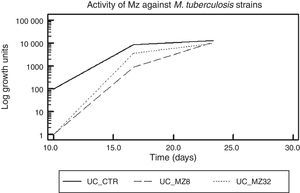
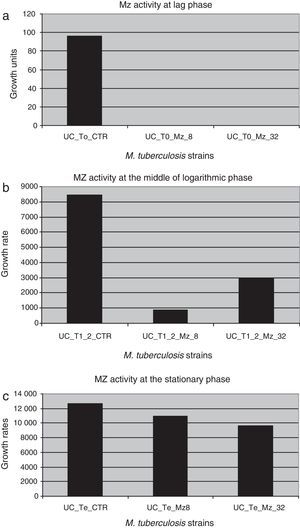
ResultsMetronidazol and IpronidazolMZ was tested as onH37Rv and on a total of 55 MDR M. tuberculosis strains. A total of 52 (94.5%) M. tuberculosis strains showed valid and interpretable results. Figure 1 shows the global activity of MZ against M. tuberculosis strains, with a difference of approximately 2 logarithmic units (2Ulog, p<0.0001) between the drug-free tube and MZ (8.0 and 32.0μg/ml)-containing tubes when the mycobacteria reached the exponential growth phase. This difference diminishes throughout the growth curve, and at the mid of the log phase a difference of 1.0Ulog between the control tube and the MZ (8.0μg/ml)-containing tube was observed (p<0.0001) while the difference between control tube and MZ (32.0μg/ml) was 0.4Ulogs (p<0.0001); furthermore, no significant difference was observed between both MZ tested concentrations (p>0.01). There was no difference between the Ulog control tube and the MZ-containing tubes (Ulog 0.06 and 0.12 for MZ 8.0 and MZ 32.0μg/ml respectively, p>0.01) at the moment in which the mycobacterium growths reached the stationary phase.
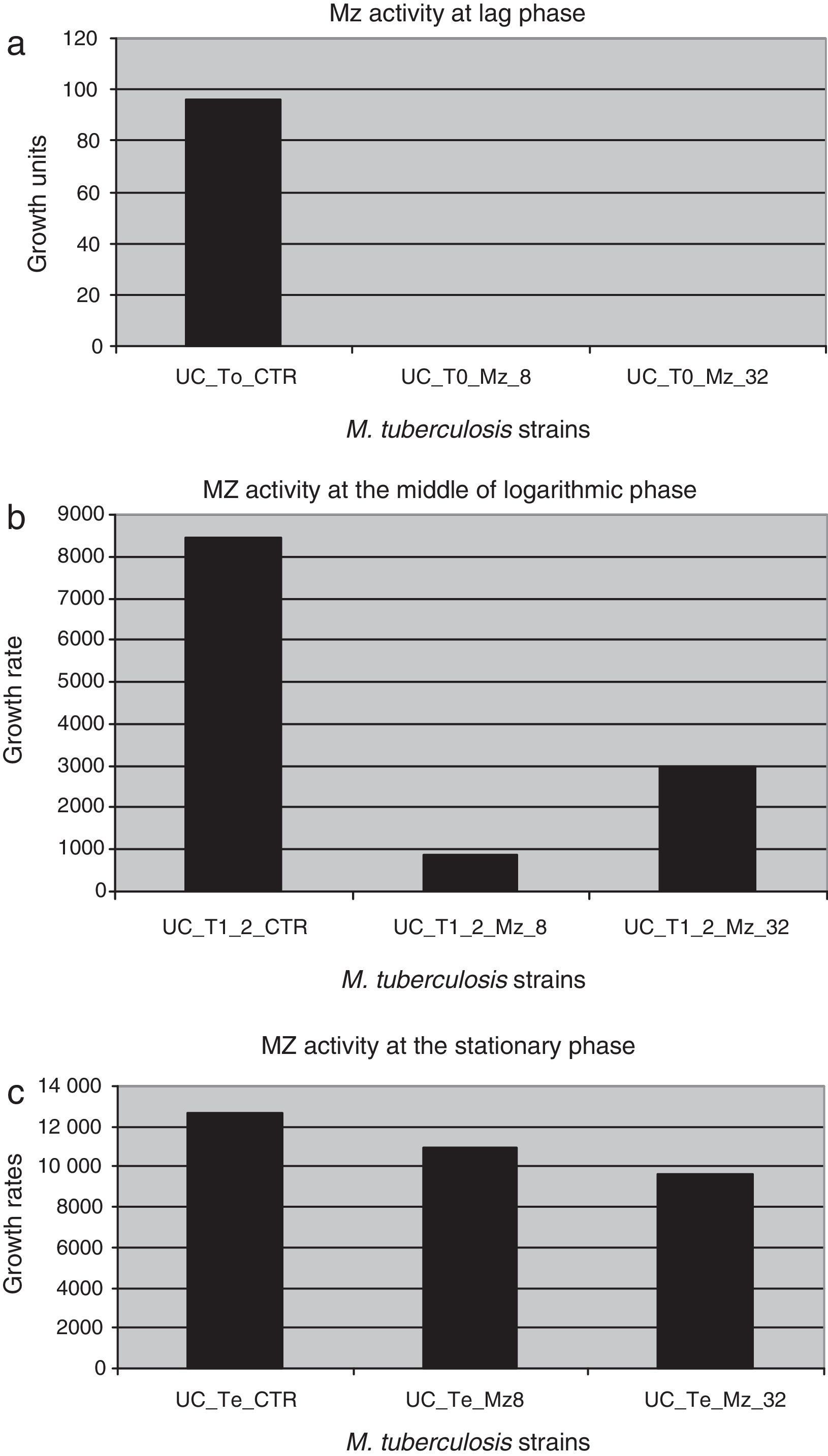
Figure 2 shows the difference in each phase of the curve for each MZ concentration.
Metronidazole activity against dormant M. tuberculosis strains in different phases of the growth curve. (a) UC_To_CTR/UC_To_Mz8/UC_To_Mz32, growth units to reach the logarithmic phase of the strains without metronidazole/8.0μg/ml of metronidazole/32.0μg/ml of metronidazole. (b) UC_T1_2_CTR/UC_T1_2_Mz8/C_T1_2_Mz32, growth units at the mid of the logarithmic phase of the strains without metronidazole/8.0μg/ml/32.0μg/ml of metronidazole. (c) UC_Te_CTR/UC_Te_Mz8/UC_Te_Mz32, growth units at the stationary phase of the strains without metronidazole/8.0μg/ml/32.0μg/ml of metronidazole.
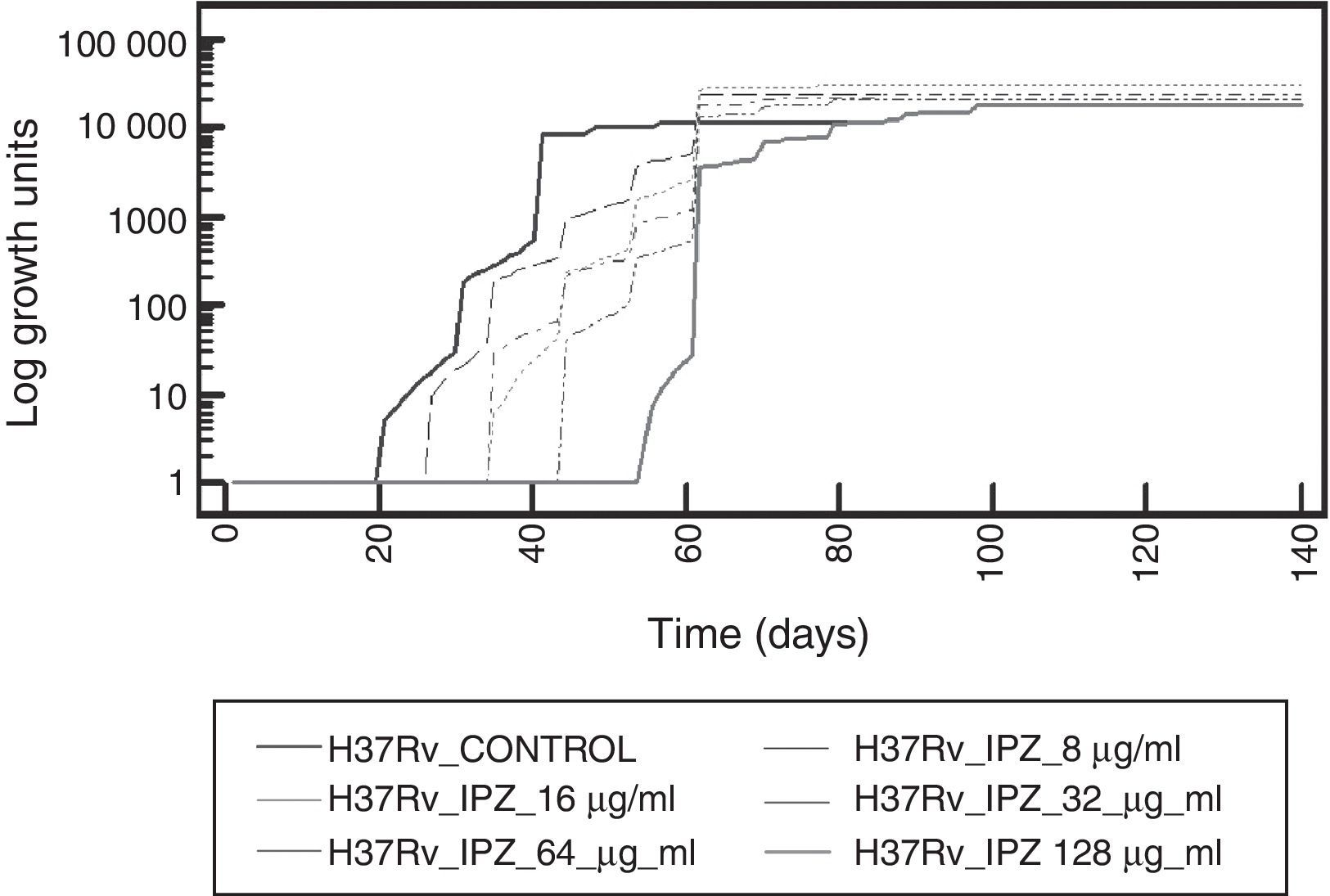
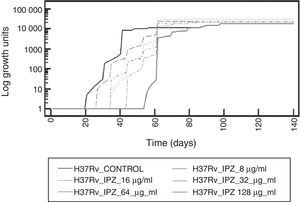
To determine IPZ activity, this drug was first tested on M. tuberculosis H37Rv strain. An increased inhibitory effect was observed as long as the drug concentration was also increasing. Figure 3 shows the effect at different concentrations of IPZ on dormant M. tuberculosis H37Rv.
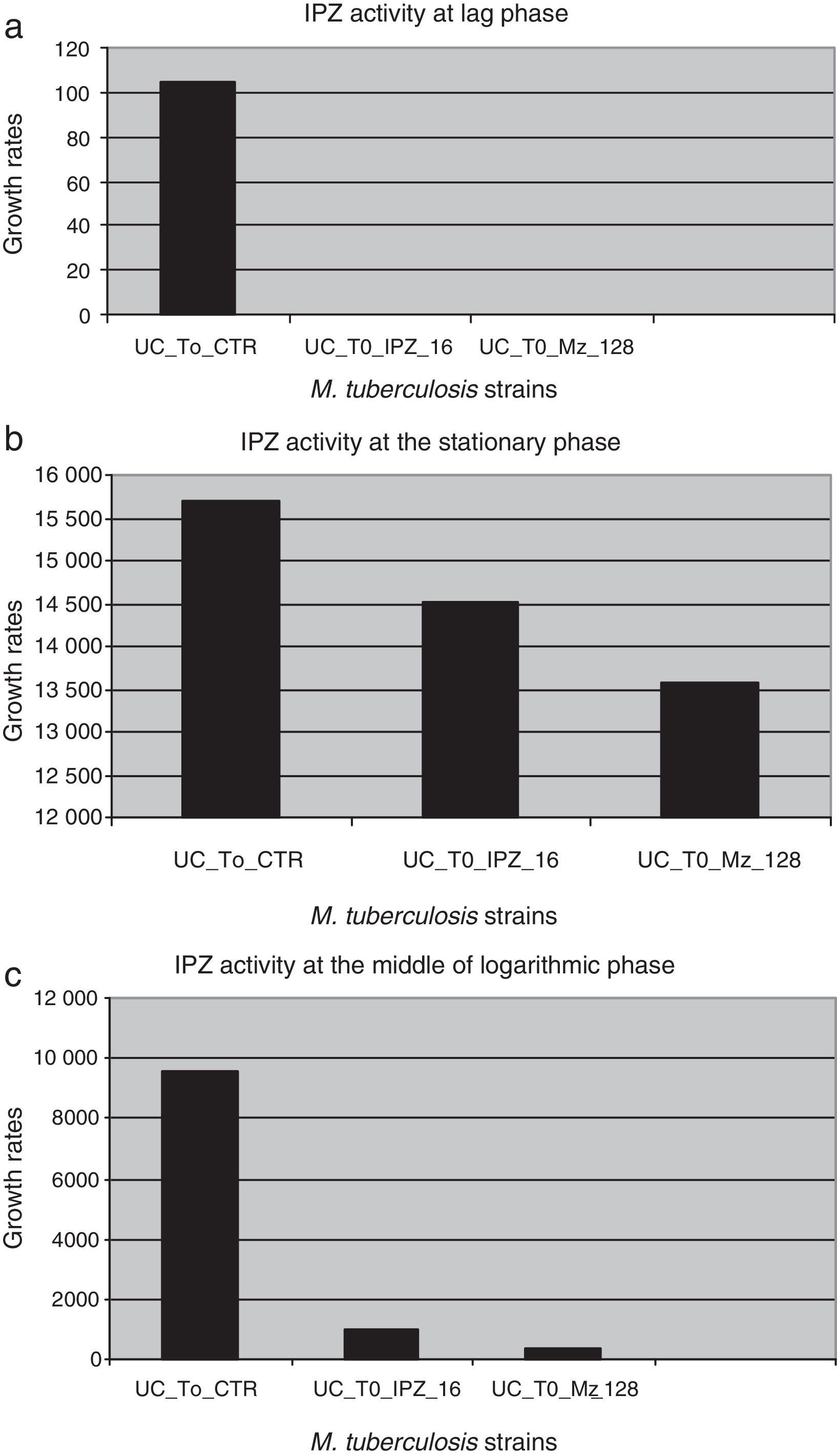
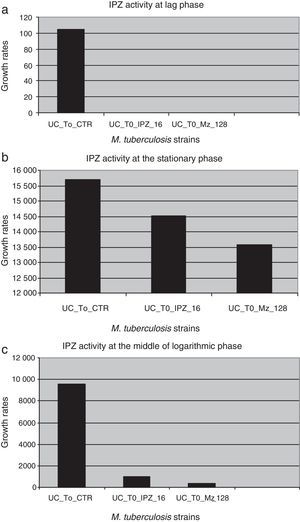
IPZ activity at 16.0μg/ml and 128.0μg/ml was also tested on 25 MDR M. tuberculosis isolates. Figure 4 shows the difference in each phase of the curve for each one of the IPZ concentrations tested on the M. tuberculosis isolates. As Figure 4 shows, greater activity was observed using IPZ 128.0μg/ml than 16.0μg/ml (p<0.001) at the mid of the log phase, while in the stationary phase, no difference was observed between both concentrations tested (p>0.01). Moreover, the 25 MDR M. tuberculosis clinical isolates were inhibited by both 5-nitro-imidazole drugs.
Ipronidazole activity against dormant MDR M. tuberculosis clinical strains in different phases of the growth curve. (a) UC_To_CTR/UC_To_IPZ16/UC_To_IPZ128, growth units to reach the logarithmic phase of the strains without ipronidazole/16.0μg/ml of ipronidazole/128.0μg/ml of ipronidazole. (b) UC_T1_2_CTR/UC_T1_2_IPZ 16/C_T1_2_IPZ128, growth units at the mid of the logarithmic phase of the strains without ipronidazole/16.0μg/ml/128.0μg/ml of ipronidazole. (c) UC_Te_CTR/UC_Te_IPZ16/UC_Te_IPZ128: growth units at the stationary phase of the strains without ipronidazole/16.0μg/ml/128.0μg/ml of ipronidazole.
ECO and CLO showed inhibitory activity against H37Rv and MDR M. tuberculosis clinical isolates. MIC results were as follows: MIC: 0.5μg/ml (ECO, n: 8, 14.5%; CLO, n: 6, 10.9%), MIC: 2.0μg/ml (ECO, n: 6, 10.9%; CLO, n: 9, 16.4%), MIC: 4μg/ml (ECO, n: 41, 74.5%; CLO, n: 15, 27.3%), MIC: 8.0μg/ml (CLO, n: 17, 30.9%), MIC: 16.0μg/ml (CLO, n: 8, 14.5%). MIC50–90 to ECO was 4.0μg/ml, while MIC50 and MIC90 to CLO was 4.0μg/ml and 8.0μg/ml respectively.
DiscussionIn this study and under the system used, MZ showed a reversible inhibitory bacteriostatic activity against MDR M. tuberculosis strains, although bactericidal activity of MZ was previously reported by other authors28. Long-term (35-day-old) non-replicating M. tuberculosis was not sterilized after 15-day-exposure to MZ. The combination of azole drugs plus other anti-TB agents could increase the effect of this kind of drugs12. At the concentrations of MZ and IPZ tested, a delay was obtained in M. tuberculosis growth that was re-established in the stationary phase. This may suggest that the maintenance of constant drug serum levels attained by the dose scheme based on the half-life of the drug, might potentially produce a prolonged inhibitory effect, and could be an effective alternative in MDR-TB treatment or in the chemoprophylaxis of MDR-LTB. According to previous reports, an average of 8.0μg/ml (13.0±2.3μg/ml) is reached in humans using doses of 400mg of MZ7,13. In this study no inhibitory differences were observed using either 8.0μg/ml or 32.0μg/ml of MZ.
Furthermore, the fact that IPZ has also been tested on M. tuberculosis provides new preliminary data about the inhibitory effect of another compound of the 5-nitroimidazole family. In this study and under our tested conditions, unlike what was observed with MZ, IPZ inhibited M. tuberculosis growth and the greater the concentration of the drug tested, the greater the inhibitory effect. These observed differences could be related to the action mechanism of each drug and with their pharmacokinetic characteristics. As observed with MZ, the inhibitory effect of IPZ was also bacteriostatic. No previous reports exist about the use of IPZ in humans. It has been used as antiprotozoal agent in animals. IPZ is effective by oral administration in drinking water and feed and also by parenteral administration, i.e., subcutaneous, intramuscular, intravenous, intraperitoneal or intraruminal injection.
Although we used a Wayne adapted hypoxic system based on oxygen deprivation to test nitroimidazole compounds, a bacteriostatic and non-bactericidal effect of these kinds of drugs was observed on M. tuberculosis strains. It could suggest that a change in the conditions to achieve the hypoxic culture – like fatty acid deprivation in the liquid medium –, could produce a bactericidal effect of nitroimidazole compounds.
Regarding ECO and CLO, the activity against M. tuberculosis isolates was evidenced under aerobic conditions. Furthermore, it was possible to find the MIC value inhibiting most of the mycobacterial amount, suggesting their possible use in combination with other anti-TB drugs for MDR-TB treatment.
Both systems used in this study, allowed us to evaluate the azole activity against M. tuberculosis strains. MZ, IPZ, ECO and CLO showed activity against the H37Rv strain and on MDR-TB clinical isolates.
ECO as well as CLO were found to have strong antimycobacterial potential against M. tuberculosis under in vitro conditions.
This study shows that azole drugs exerted an inhibitory action among a collection of Argentinean MDR clinical isolates. Azole drugs bear significant therapeutic potential against M. tuberculosis.
Although the use of MZ for TB treatment has been discarded due to its adverse effects, both 5-nitroimidazoles tested in this study were used as a model of their class of drugs. Results obtained with both drugs suggest that similar kinds of drugs, with fewer side effects than MZ, could be effective in MDR-TB treatment and in the treatment of MDR-LTB caused by contact with an MDR-TB source.
Ethical disclosuresProtection of people and animalsThe authors state that no human or animal experiments have been performed for this research.
Confidentiality of the dataThe authors state that no patient data appears in this article.
Right to privacy and informed consentThe authors state that no patient data appears in this article.
FundingNo funds were obtained for this work.
Conflict of interestAll authors declare no conflicts of interest.
BRI and AAC are fellows from CONICET, Argentina. We thank Marcelo Mazza and Guillermo Alonso for their technical support.