The aim of this study was to evaluate the microbiological quality of paprika produced in Catamarca, Argentina. Microbiological analyses were carried out for the enumeration of total aerobic mesophilic bacteria, coliforms, yeasts and molds, and the detection of Salmonella in samples obtained from different local producers during three consecutive years. The mycobiota was identified paying special attention to the mycotoxigenic molds. Standard plate counts of aerobic mesophilic bacteria ranged from 2.7×105 to 3.7×107CFU/g. Coliform counts ranged from <10 to 8.1×104CFU/g. Salmonella was not detected in any of the samples tested. Fungal counts (including yeasts and molds) ranged between 2×102 and 1.9×105CFU/g. These results showed a high level of microbial contamination, exceeding in several samples the maximum limits set in international food regulations. The study of the mycobiota demonstrated that Aspergillus was the predominant genus and Aspergillus niger (potential producer of ochratoxin A) the most frequently isolated species, followed by Aspergillus flavus (potential producer of aflatoxins). Other species of potential toxigenic fungi such as Aspergillus ochraceus, Aspergillus westerdijkiae, Penicillium chrysogenum, Penicillium crustosum, Penicillium commune, Penicillium expansum and Alternaria tenuissima species group were encountered as part of the mycobiota of the paprika samples indicating a risk of mycotoxin contamination. A. westerdijkiae was isolated for the first time in Argentina.
El pimentón es considerado una de las especias más proclives a contaminarse con diversos tipos de microorganismos, incluyendo patógenos como Salmonella y hongos capaces de producir micotoxinas. Existen muy pocos datos acerca de la contaminación microbiana del pimentón producido en nuestro país. El objetivo del presente trabajo fue evaluar la calidad microbiológica del pimentón (Capsicum annum L.) producido en la provincia de Catamarca, una de las principales zonas productoras del norte argentino. Se realizó el recuento de bacterias aerobias mesófilas, coliformes totales y mohos y levaduras, y la búsqueda de Salmonella en muestras obtenidas de diferentes establecimientos productores locales durante 3 años consecutivos. Se identificaron todas las cepas fúngicas (1.622 aislamientos) a nivel de género y se determinaron las especies pertenecientes a los géneros potencialmente toxinógenos. Los recuentos totales de bacterias aerobias mesófilas variaron entre 2,7×105 y 3,7×107UFC/g. Los coliformes totales estuvieron en el rango de<10 a 8,1×104UFC/g. Salmonella no fue detectada en ninguna de las muestras analizadas. Los resultados obtenidos muestran un alto nivel de contaminación, que excede en varias de las muestras los límites máximos establecidos en las regulaciones alimentarias internacionales. El estudio de la micobiota demostró que Aspergillus fue el género predominante. Otros géneros encontrados fueron Cladosporium, Rhizopus, Alternaria y Penicillium. Aspergillus niger (potencial productor de ocratoxina A) fue la especie aislada con mayor frecuencia, seguida de Aspergillus flavus (potencial productor de aflatoxinas). También se encontraron otras especies toxinógenas, lo que indica un riesgo potencial de contaminación con micotoxinas. Aspergillus westerdijkiae fue aislado por primera vez en Argentina.
Spices and herbs have been used for centuries for the aroma and flavor characteristics they convey to foods. The increasing popularity of highly spiced cuisines as well as a desire for flavorful foods which are low in sodium and fat have resulted in a continuing interest in the use of spices and herbs in food products29. Paprika (in Spanish referred to as “pimentón”) is a powdered spice with a deep orange-red color and a characteristic non-pungent flavor resulting from the dried and ground fruits of certain varieties of pepper (Capsicum annuum L. belonging to the family Solanaceae). Microbiological studies carried out with species, including paprika, have indicated high microbial loads which could pose a problem for food manufacturers 4,7,9,25,29,31,33,39. These commodities normally carry a great number of bacteria and molds, often of soil origin, and could be a major source of microbial contamination in foods. Current practices of harvesting, handling and production often cause additional contamination and microbial growth. Many spices are grown and harvested in poor sanitary conditions, which increase the risk of contamination even with pathogens such as Salmonella. A significant outbreak of human salmonellosis due to paprika and paprika-powdered potato chips was described and well documented in Germany in 199526. Owing to production conditions and poor storage practices, products derived from Capsicum are also susceptible to fungal contamination. Spoilage caused by fungi decreases the quality of the products and also imply a risk for health due to potential contamination with different mycotoxins. Toxigenic molds such as Aspergillus spp., Penicillium spp. and Fusarium spp. have been detected by several researchers in the mycobiota of Capsicum powder as well as natural contamination with aflatoxins and ochratoxin A16,19,23,28,42,43,45.
In Argentina, paprika production is a regional industry of increasing importance in some northern provinces (Catamarca, Salta and Tucumán). This study aimed to determine the microbiological quality of paprika from this region, paying a special attention to pathogenic microorganisms such as Salmonella and mycotoxigenic fungi.
Materials and methodsSamplesFifteen samples of paprika (Capsicum annuum L.) from Santa María Department, Catamarca Province, Argentina, belonging to 2010, 2011 and 2012 consecutive harvests were analyzed. These samples were obtained from different local producers. For each sample, 250g of paprika were collected in sterile containers; all samples were kept at 5°C until analysis.
Water activityWater activity was measured with a water activity meter (Aqualab, Decagon Devices CX3 02734) with an accuracy of ±0.002. Measurements were performed in duplicate.
Aerobic plate count (APC)One gram of paprika was homogenized in 9ml of 0.1% peptone water in an Erlenmeyer flask. Subsequent decimal dilutions were prepared in sterile peptone water. One ml of each dilution was plated by the pour plate method with agar plate count, in duplicate. The plates were incubated at 35°C for 48h5.
Total coliform countOne ml of subsequent decimal dilutions up to 1:1000 (previously prepared for the APC) was transferred to Petri dishes in duplicate. Ten ml of Violet Red Bile Agar (VRBA) was poured, swirled to mix and let to solidify. Then, it was overlaid with 10ml of melted medium. The plates were incubated at 35°C for 24h. Purple-red colonies (0.5mm or larger in diameter) surrounded by a zone of precipitated bile acids were counted. To confirm that the colonies were coliforms, 10 representative colonies were picked and each of them were transferred to a tube of Brilliant Green Lactose Bile Broth. The tubes were incubated at 35°C and were examined at 24 and 48h for gas production5.
Salmonella spp.Salmonella was investigated according to the reference method for paprika5. For the pre-enrichment, 25g of paprika were added to 225ml of Trypticase Soy Broth (TSB) and incubated at 35°C for 24h. The enrichment step was performed on selenite cystine broth and tetrathionate broth, and were incubated at 35°C for 24h. Isolations were examined on Bismuth Sulfite Agar (BSA) and Xylose Lysine Desoxycholate Agar (XLD), after incubation at 35°C for 48 and 24h, respectively. Suspected colonies of Salmonella pp. were tested on Triple Sugar Iron (TSI) and Lysine Iron (LIA) agar, incubated at 35°C for 24h. Colonies exhibiting typical reactions on TSI and LIA were purified and further characterized by traditional assays: urease, oxidase, phenylalanine descarboxylase, Voges-Proskauer, indole and citrate.
Mold and yeast countsMold and yeast counts were performed on dichloran 18% glycerol (DG18) agar, a medium that has lower water activity (aw=0.955) and favors xerophilic fungi development36. Plates were incubated at 25°C for 5 days.
Mold identificationPreliminary characterization at genera level of the isolated strains was performed according to Pitt and Hocking36. The strains belonging to genera producers of mycotoxins were identified to species level.
Isolates of Aspergillus and Penicillium were plated on Malt Extract Agar (MEA) and were incubated for 7 days at 25°C to obtain well-sporulated cultures. The spores of each strain were collected and placed in 1ml of a sterile aqueous solution with 0.05% Tween 80 and 0.2% agar. Aspergillus strains were cultured in MEA and 25% glycerol nitrate agar (G25N) and incubated at 25°C, and in Czapek Yeast Extract Agar (CYA) incubated at 25°C and 37°C. All plates were incubated in darkness for a standard time of 7 days for anamorphic species and 14 days for teleomorphic species. Taxonomic identification was done according to Klich24 and Pitt and Hocking36. Penicillium strains were cultured in MEA and Czapek Agar (CZ) and incubated at 25°C, and in CYA at 5, 25 and 37°C for 7 days. Identification was done according to Pitt35, Samson et al.40 and Samson and Frisvad41.
Alternaria strains were cultured in Potato Dextrose Agar (PDA) and incubated at 25°C for 5–7 days. Strains were subcultured on tap water agar 18% (TWA) and incubated at 25°C for 7–14 days under lights with a 12h-photoperiod. Taxonomic identification was performed according to Simmons and Roberts47 and Simmons46.
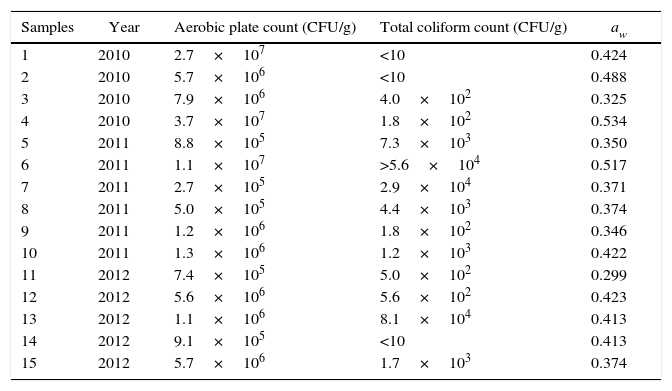
Results and discussionTable 1 shows the results of the aerobic plate count, total coliforms and water activity of the samples analyzed in the present study. Water activity of the samples showed levels between 0.299 and 0.534. Fernández-Trujillo and Escarabajal11 reported and recommended a range between 0.5 and 0.7 during storage of paprika from Murcia, with an optimal level of aw=0.5 to prevent product caking.
Total aerobic plate and total coliform counts (CFU/g), and aw levels of paprika samples from Santa Maria Department from Catamarca, Argentina
| Samples | Year | Aerobic plate count (CFU/g) | Total coliform count (CFU/g) | aw |
|---|---|---|---|---|
| 1 | 2010 | 2.7×107 | <10 | 0.424 |
| 2 | 2010 | 5.7×106 | <10 | 0.488 |
| 3 | 2010 | 7.9×106 | 4.0×102 | 0.325 |
| 4 | 2010 | 3.7×107 | 1.8×102 | 0.534 |
| 5 | 2011 | 8.8×105 | 7.3×103 | 0.350 |
| 6 | 2011 | 1.1×107 | >5.6×104 | 0.517 |
| 7 | 2011 | 2.7×105 | 2.9×104 | 0.371 |
| 8 | 2011 | 5.0×105 | 4.4×103 | 0.374 |
| 9 | 2011 | 1.2×106 | 1.8×102 | 0.346 |
| 10 | 2011 | 1.3×106 | 1.2×103 | 0.422 |
| 11 | 2012 | 7.4×105 | 5.0×102 | 0.299 |
| 12 | 2012 | 5.6×106 | 5.6×102 | 0.423 |
| 13 | 2012 | 1.1×106 | 8.1×104 | 0.413 |
| 14 | 2012 | 9.1×105 | <10 | 0.413 |
| 15 | 2012 | 5.7×106 | 1.7×103 | 0.374 |
The standard plate counts of aerobic mesophilic bacteria showed high levels of contamination in the samples, ranging from 2.7×105 to 3.7×107CFU/g. Several other studies also reported high microbial loads in herbs and species according to Mc Kee's review29. Surveys conducted in different countries determined that aerobic mesophilic bacterial plate counts ranged from several hundreds to several millions per gram. High counts were observed for all types of spices, being paprika one of the most contaminated, often with values greater than 7logCFU/g. The results of surveys conducted more recently revealed similarly high microbial loads in paprika4,7. Although microbiological criteria for spices have been recommended, few specific standards have been suggested in some countries6,25. Argentinian food regulations do not include microbiological specifications for paprika or other species. The International Commission on Microbiological Specifications for Foods (ICMSF)21 set up a maximum limit of 106CFU of total aerobic mesophilic bacteria/g of spice and 104CFU/g as the bacterial counts that distinguish good from marginal quality. A total aerobic mesophilic count of ≤104CFU/g is of acceptable quality and 104–106CFU/g is of marginal quality. Values above 106 are unacceptable. Applying these criteria, high rejection rates have been mentioned by several authors. Schwab et al.44 informed that 44% of the samples of paprika analyzed would not meet the ICMSF standard. Banerjee and Sakar7 reported that 51% of the samples of some spices marketed in India were in the unacceptable range (≥106CFU/g). A similar proportion of unacceptable samples was detected in the present study. On the other hand, results reported by Garbowska et al.14 report good hygienic conditions in the production process of spices and herbs available in the Polish market since 60% of the analyzed samples did not exceed 104CFU/g whereas the level regarded as unacceptable (≥106CFU/g) was not identified in any of the samples. However, most of the results published up to now confirm the poor hygienic quality of spices, including paprika, produced around the world 15,18,31,39,48. In the second edition of the ICMSF book on sampling for microbiological analysis22 the Commision accepted that, in retrospect, the above mentioned recommendations for spices were inappropriate because a considerable portion of these commodities in international commerce would not meet the suggested limits. Furthermore, failure to meet the limits might or might not be related to food quality or safety. The new recommendation was that spices be treated as raw agriculture commodities and, accordingly, the ultimate use of such products will be determinant. However, the original ICMSF specifications are still considered a guide to evaluate the microbiological quality of spices and herbs in international trade.
Gallardo Guerrero et al.13 studied the evolution of the microflora during dehydration of red pepper fruits during a period of 9 days at temperatures ranging between 30 and 40°C to obtain a high quality paprika protected by PDO (Product with Designation of Origin: “Pimentón de La Vera”). Initial counts of total mesophilic aerobic bacteria ranging between 8 and 11logCFU/g tended to decrease in the middle stages of processing and increased again at the end. These authors attribute such high microbial levels to soil pollution, the environment and feces of birds and other animals contaminating the red pepper fruits either during cultivation, their time in the dryer or handling operations. Similar sources of contamination, characteristic of handcrafted produced products, could be present in the region considered in the present work.
Total coliforms are used generally as indicators of hygienic quality. Coliform counts in the samples ranged from <10 to 8.1×104 (Table 1). A few samples exceeded the maximum count set by the ICMSF for this group of microorganisms (104CFU/g). These results agree with those of other authors7,44.
Salmonella spp. is a pathogen of greatest concern in spices due to its ability to persist in low aw environments and low dose for infection. In the present study Salmonella spp. was not detected in any of the samples tested. Other surveys also revealed that this pathogen was not present in spices7–9. However, Vij et al.49 reviewed spice recalls that took place in the United States from 1970 to 2004 involving 12 spice types contaminated with bacterial pathogens and reported that in all but one instance the recalled spices contained Salmonella spp.. Paprika was the spice most often involved in the recalls. Furthermore, different spices were identified as the contaminated food vehicle in several outbreaks of salmonellosis33. One of the most widespread outbreaks associated with contaminated paprika used to season potato chips ocurred in Germany in 199326. Some, if not all, of the paprika was imported from South America. There were an estimated 1000 cases of illness, mostly affecting children younger than 14 years of age. The estimated infectious dose in that outbreak was reported to be 4–45 salmonellae. The high fat content associated with the paprika-powdered chips may have protected Salmonella spp. from gastric acid in the stomach, resulting in the low infectious dose. It is important to note that many spices are frequently used in ready-to-eat foods (e.g. crackers and chips) or in cooked foods that are seasoned before consumption increasing the risk of foodborne disease. Although it could be apparent that Salmonella spp. contamination is rare or sporadic in spices, including paprika, this pathogen is of great concern and should be included in any sampling plan to monitor the microbiological quality of these products. Other pathogens such as Bacillus cereus and Clostridium perfringens seem to be rarely detected.
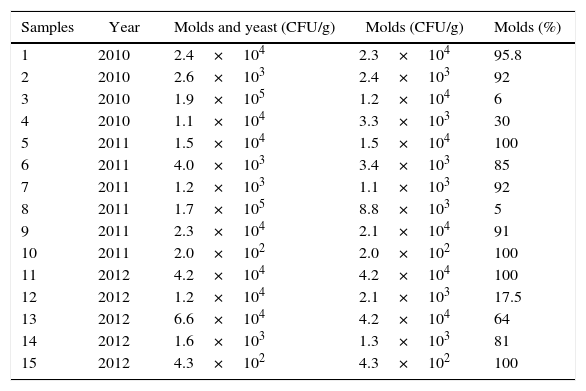
Table 2 shows the total molds and yeast counts and the percentage of molds detected in the samples. In most of the samples (10/15) molds account for more than 80% of the fungal load. However, yeasts were present in high numbers in some samples and they were absent in some others. Santos et al.42 reported that predominant contamination of Capsicum products produced in Spain was caused by yeasts. Baxter and Holzapfel8 analyzed selected spices and herbs in South Africa and also found that paprika contained a high number of viable yeasts. In the present study, fungal counts (including molds and yeasts) ranged between 2×102 and 1.9×105CFU/g. These results are consistent with other studies29. Santos et al.42 reported 2.3×104 mean total CFU/g of paprika in MEA and 3.8×102CFU/g of paprika in DG18. The low aw of this media, also used in the present work for fungal counts, mainly selects xerophilic species, being suitable for dried foods. The ICMSF21 set up a maximum limit of 104CFU of molds and yeasts/g of spices. In the present study six samples had acceptable counts, other six showed slightly higher levels while the other three reached the 105 level. A survey carried out in Australia in order to develop a database for unacceptable levels of fungal contamination of foods provided some information on species. Specifications for less than 1000 yeasts and molds/g were informed by most of the Australian food manufacturers. Some of them included a marginally acceptable category which allowed up to 2000fungi/g provided there was no organoleptic deterioration of the raw material. A few companies set the unacceptable level at 104 or 105CFU/g. The variation in acceptable levels by the different organizations is a reflection of the different end uses of the spices3.
Mold and yeast count (CFU/g) and percentage of molds detected in paprika samples from Santa Maria Department from Catamarca, Argentina
| Samples | Year | Molds and yeast (CFU/g) | Molds (CFU/g) | Molds (%) |
|---|---|---|---|---|
| 1 | 2010 | 2.4×104 | 2.3×104 | 95.8 |
| 2 | 2010 | 2.6×103 | 2.4×103 | 92 |
| 3 | 2010 | 1.9×105 | 1.2×104 | 6 |
| 4 | 2010 | 1.1×104 | 3.3×103 | 30 |
| 5 | 2011 | 1.5×104 | 1.5×104 | 100 |
| 6 | 2011 | 4.0×103 | 3.4×103 | 85 |
| 7 | 2011 | 1.2×103 | 1.1×103 | 92 |
| 8 | 2011 | 1.7×105 | 8.8×103 | 5 |
| 9 | 2011 | 2.3×104 | 2.1×104 | 91 |
| 10 | 2011 | 2.0×102 | 2.0×102 | 100 |
| 11 | 2012 | 4.2×104 | 4.2×104 | 100 |
| 12 | 2012 | 1.2×104 | 2.1×103 | 17.5 |
| 13 | 2012 | 6.6×104 | 4.2×104 | 64 |
| 14 | 2012 | 1.6×103 | 1.3×103 | 81 |
| 15 | 2012 | 4.3×102 | 4.3×102 | 100 |
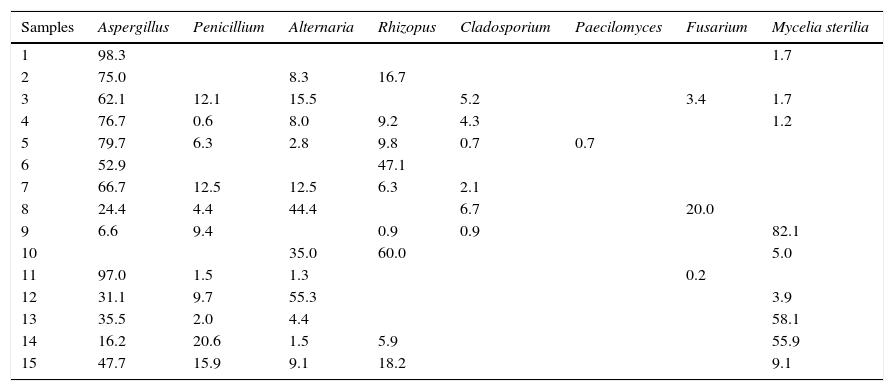
The study of molds in spices and herbs is of significance because the quality of the products has decreased as a consequence of fungal spoilage and there is also a health risk due to the potential production of mycotoxins. In the present work, the mycobiota of paprika was identified paying special attention to the mycotoxigenic molds. A total of 1622 isolates of molds were recovered from samples by the dilution plate method. All colonies were subcultured for taxonomic identification. Seven genus were identified and the percentage of each genus over the total number of isolates was calculated for each sample (Table 3). Aspergillus was prevalent since it was present in all but one sample and accounted for more than 50% of the mycobiota observed in most of the samples. Other genera widely distributed were Penicillium and Alternaria. Rhizopus was abundant in some of the samples, making it difficult to isolate the rest of the fungi. Cladosporium, Paecilomyces and Fusarium were less frequently isolated.
Percentage of fungal genera detected in each paprika sample analyzed from Santa Maria Department from Catamarca, Argentina
| Samples | Aspergillus | Penicillium | Alternaria | Rhizopus | Cladosporium | Paecilomyces | Fusarium | Mycelia sterilia |
|---|---|---|---|---|---|---|---|---|
| 1 | 98.3 | 1.7 | ||||||
| 2 | 75.0 | 8.3 | 16.7 | |||||
| 3 | 62.1 | 12.1 | 15.5 | 5.2 | 3.4 | 1.7 | ||
| 4 | 76.7 | 0.6 | 8.0 | 9.2 | 4.3 | 1.2 | ||
| 5 | 79.7 | 6.3 | 2.8 | 9.8 | 0.7 | 0.7 | ||
| 6 | 52.9 | 47.1 | ||||||
| 7 | 66.7 | 12.5 | 12.5 | 6.3 | 2.1 | |||
| 8 | 24.4 | 4.4 | 44.4 | 6.7 | 20.0 | |||
| 9 | 6.6 | 9.4 | 0.9 | 0.9 | 82.1 | |||
| 10 | 35.0 | 60.0 | 5.0 | |||||
| 11 | 97.0 | 1.5 | 1.3 | 0.2 | ||||
| 12 | 31.1 | 9.7 | 55.3 | 3.9 | ||||
| 13 | 35.5 | 2.0 | 4.4 | 58.1 | ||||
| 14 | 16.2 | 20.6 | 1.5 | 5.9 | 55.9 | |||
| 15 | 47.7 | 15.9 | 9.1 | 18.2 | 9.1 |
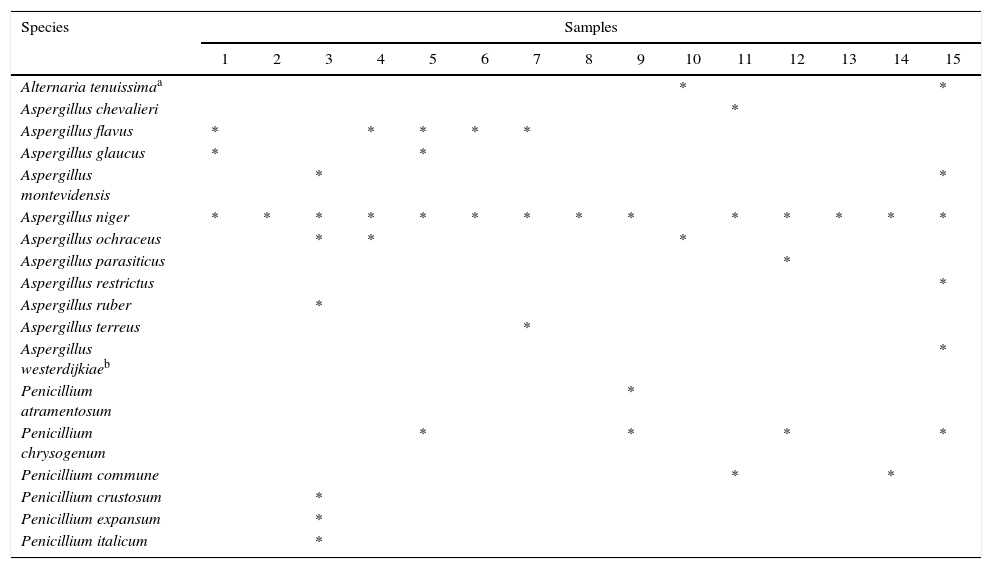
Strains belonging to mycotoxigenic genera Aspergillus, Penicillium and Alternaria were identified at species level. Table 4 shows the distribution of these fungal species among the samples analyzed. Due to the new single name nomenclature for fungi30, Hubka et al.20 provided a necessary nomenclatural revision and transferred all Eurotium species to Aspergillus. These changes were incorporated into the present study. Aspergillus montevidensis, Aspergillus chevalieri, Aspergillus glaucus and Aspergillus ruber, formerly considered to be Eurotium species, are xerophilic fungi very common in dry foods and were found in the mycobiota of Capsicum powder by other authors16,42. Furthermore, A. montevidensis is the current name of the well-known Eurotium amstelodami. The name change was due to the fact that E. amstelodami was an illegitimate name20,34.
Distribution of species belonging to mycotoxigenic genera (Alternaria, Aspergillus and Penicillium)
| Species | Samples | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
| Alternaria tenuissimaa | * | * | |||||||||||||
| Aspergillus chevalieri | * | ||||||||||||||
| Aspergillus flavus | * | * | * | * | * | ||||||||||
| Aspergillus glaucus | * | * | |||||||||||||
| Aspergillus montevidensis | * | * | |||||||||||||
| Aspergillus niger | * | * | * | * | * | * | * | * | * | * | * | * | * | * | |
| Aspergillus ochraceus | * | * | * | ||||||||||||
| Aspergillus parasiticus | * | ||||||||||||||
| Aspergillus restrictus | * | ||||||||||||||
| Aspergillus ruber | * | ||||||||||||||
| Aspergillus terreus | * | ||||||||||||||
| Aspergillus westerdijkiaeb | * | ||||||||||||||
| Penicillium atramentosum | * | ||||||||||||||
| Penicillium chrysogenum | * | * | * | * | |||||||||||
| Penicillium commune | * | * | |||||||||||||
| Penicillium crustosum | * | ||||||||||||||
| Penicillium expansum | * | ||||||||||||||
| Penicillium italicum | * | ||||||||||||||
Several toxigenic species of Aspergillus were detected, belonging to sections Flavi (Aspergillus flavus and Aspergillus parasiticus), Nigri (Aspergillus niger) and Circumdati (Aspergillus ochraceus and Aspergillus westerdijkiae). A. flavus, a potential producer of type B aflatoxins and cyclopiazonic acid, was quite frequent, being isolated from five samples, whereas A. parasiticus, generally a strong producer of type B and G aflatoxins, was present in only one sample. A. niger, reported as a potential producer of ochratoxin A (OTA), was the most frequently isolated toxigenic mold, being present in all but one of the samples. The predominance of Aspergillus section Nigri in the dried spice was not unexpected because members of this group can survive the drying process due to the relative resistance of black spores to sunlight and UV radiation38. Another OTA producer, Aspergillus carbonarius, very commonly found in grape products, was not detected.
Other species capable of producing OTA were A. ochraceus, isolated from three samples and A. westerdijkiae, present in one sample and isolated in Argentina for the first time.
Although ochratoxin A was first described from Aspergillus ochraceus, molecular studies indicate that A. westerdijkiae is the major ochratoxin A-producing species in Aspergillus Section Circumdati12. A. westerdijkiae is morphologically similar to A. ochraceus, though it is unable to grow at 37°C. Gil Serna et al.17 reported that A. westerdijkiae achieved the highest values of both growth and OTA production in a paprika-based medium in comparison with different matrix-based media, probably due to the sugar composition of Capsicum annuum fruits which might positively affect OTA production by this species.
The only Alternaria species identified, Alternaria tenuissima species group, is a potential producer of tenuazonic acid, alternariol, alternariol monomethyl ether, altenuene and altertoxins. However, it was present in only two samples. Some of the Penicillium species listed in Table 4 are able to produce toxic secondary metabolites such as cyclopiazonic acid (Penicillium commune), tremorgens (Penicillium crustosum) and patulin and citrinin (Penicillium expansum), but they were sporadically isolated.
Results of the present study are supported by numerous reports which consistently indicate that Aspergillus is the most commonly occurring genus in Capsicum powder as well as in other spices1,9,16,27,28,42,43,45. The same fungal genera and species isolated in the present work were isolated by Gherwaby et al.16 from chili in Saudi Arabia, being A. niger and A. flavus the most prevalent toxigenic molds. Santos et al.42 reported that Aspergillus and Eurotium were the predominant fungi in samples of paprika and chili produced in Spain. The most abundant species was A. niger. These authors also isolated A. flavus with relatively high frequency (60% of the paprika samples). Other Aspergillus species able to produce OTA (A. carbonarius, A. ochraceus and A. westerdijkiae) were also detected in the paprika samples but less frequently. Martín et al.28 analyzed the fungal contamination of smoked paprika and identified Aspergillus, Cladosporium, Penicillium and Fusarium as predominant genera. Among the toxigenic species isolated they mentioned A. niger (the most frequent), Fusarium verticillioides, Penicillium expansum and Penicillium citrinum, but they did not detect any aflatoxin-producing fungi.
Table 4 shows the co-occurrence of A. niger and A. flavus in several of the analyzed samples. This finding is in agreement with other studies which report a high co-occurrence of Aspergillus species able to produce aflatoxins and OTA16,43. Although the presence of toxigenic molds in foods does not necessary imply the presence of the toxins they can produce, it may be considered as an indicator of potential contamination with mycotoxins. Numerous surveys have confirmed the natural occurrence of aflatoxins and OTA in Capsicum products, including paprika, from different countries2,10,16,19,23,32,33,37,42, indicating a need to establish maximum levels for regulation.
ConclusionsFrom the results of the present study, it can be concluded that paprika produced in the northern region of Argentina presents a high microbial load as was expected according to the conditions of production and storage of this commodity. Sundrying of the fruits in contact with the soil is a critical step as well as the storage in environmental conditions conductive to microbial proliferation. Similar levels of microbial contamination of Capsicum products have been reported worldwide, indicating that a high proportion of the samples analyzed by several workers exceeded the limits established by food regulations. In order to improve the microbiological quality of paprika, good agricultural practices and good manufacturing practices should be applied throughout the supply chain. The presence of molds capable of producing mycotoxins in this product should be considered a potential hazard for public health.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Conflict of interestThe authors declare that they have no conflicts of interest.
We are grateful to Nilda Arapa for her technical assistance. S.M. Romero, A.G. Larumbre and G. Vaamonde are members of Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET-Argentina), InMiBo publication N° 215.