The degree of antagonism exercised by fungi on geohelminth development varies according to the morphological alterations caused by different fungal species. Saprophytic fungi may exert ovicidal or ovistatic effects. The aim of this study was to apply scanning electron microscopy (SEM) to observe the action of two soil saprophytic species of Chrysosporium (C. indicum and C. keratinophylum) on Toxocara canis eggs. The fungal strains to be tested were incubated for 28 days at 28°C in 2% water agar with a suspension of unembryonated T. canis eggs. A suspension of T. canis eggs in 2% water agar was used as control group. The assay was done in triplicate for each fungus and the control group. SEM observations were performed on the 4th, 7th, 14th, 21st, and 28th day after inoculation. The effect of the fungi on eggs was evaluated in accordance with the alterations observed on the surface and the changes in the normal characteristics of the eggs. Hyphae around the eggs, appresoria penetrating the shell and changes in the typical egg membrane were observed in this assay. Type 3 effect (alterations that occur both in the embryo and the shell, and hyphal penetration of the eggs) was the prevalent effect. SEM allowed us to observe clearly the morphological alterations in T. canis eggs due to the effect of C. indicum and C. keratinophylum. Both saprophytic species of Chrysosporium alter the egg structure and alterations increase as exposure increases.
El grado de antagonismo ejercido por los hongos sobre el desarrollo de los geohelmintos depende de la especie fúngica y las alteraciones morfológicas que causan. Los hongos saprófitos pueden tener efecto ovicida u ovistático sobre los huevos. El objetivo fue aplicar la microscopía electrónica de barrido (MEB) para observar la acción de 2 especies de Chrysosporium (C. indicum y C. keratinophylum) saprófitas de suelos, sobre huevos de Toxocara canis. Las especies a ensayar se sembraron en agar agua al 2% con una suspensión de huevos no embrionados de T. canis y se incubaron 28 días a 28°C. Como grupo control se utilizó una suspensión de huevos de T. canis en agar agua al 2%. El ensayo se realizó por triplicado para cada hongo y el grupo control. Las observaciones con MEB se realizaron a los 4, 7, 14, 21 y 28 días de incubación. La acción de los hongos se evaluó según las alteraciones en la superficie y los cambios en las características normales de los huevos. En este ensayo se observaron: hifas rodeando los huevos, appresorios penetrando la cubierta y cambios en la membrana característica del huevo, prevaleciendo el efecto tipo 3 (alteraciones que se producen tanto en el embrión como en la cubierta y penetración de hifas al interior de los huevos). La aplicación de la MEB permitió observar claramente que las 2 especies de Chrysosporium saprófitas de suelos, afectan el normal desarrollo de los huevos de T. canis, alteran su estructura y las alteraciones aumentan con el tiempo de exposición.
Toxocariasis is the clinical presentation of human infection by Toxocara spp., a roundworm that lives in the small intestines of domestic dogs and cats. The infection occurs by the accidental ingestion of embryonated Toxocara eggs present in contaminated soil or on dirty hands6,14. Toxocariasis is present worldwide and is a consequence of the human habit of keeping dogs and cats for company, which favors the persistence of the parasite in the environment and its transmission13,22. Despite its extensive geographical distribution, infection is more frequent in tropical and subtropical regions, especially in populations with poor sanitary conditions20. Human T. canis infection is a public health concern in the Americas, Europe and in all developing countries. However, a full appreciation of the global burden of this disease may be greatly underestimated18.
Toxocara spp. eggs, as the eggs of other geohelminths, have a high degree of resistance to adverse environmental conditions and diverse chemicals, since they are protected by a thick, complex shell. This shell is made up of three membranes or layers – the outer vitelline layer, a middle chitinous layer, and the inner lipidic layer – that makes eggs resistant to chemicals and temperature changes, allowing them to survive outside the host for long periods22. Consequently, soil contamination with infective eggs is a worldwide health issue, and that is why the interest in finding biological control agents to reduce this contamination has increased in the past decades4.
Fungal parasitism on nematode eggs is a natural biological phenomenon that can be used for biological control of geohelminth eggs in the environment, since they are the most resistant stage in the life cycle of nematodes4,5. The penetration process of the hypha through the egg shell has not been completely elucidated yet. In 1995, Bonants et al.8 were the first to mention that the fungi colonization mechanism may be mechanical and/or enzymatic. Special penetration organs (“appresoria”) formed from the hypha help the fungus apply pressure on the egg shell (mechanical effect). Other investigations suggest the involvement of exoenzymes such as proteases and chitinases breaking up egg shells (enzymatic mechanism)4.
The degree of antagonism exercised by fungi on geohelminth development varies according to the morphological alterations caused by the different fungal species. Thus, a saprophytic fungus may exert ovicidal or ovistatic effects, where the ovistatic ability is shown by the delay in embryo development or inhibition with no morphological damage to the egg shell12. Therefore, several researchers have assayed the in vitro effect of different fungi on geohelminth eggs. Knowledge about the effect of the genus Chrysosporium is scarce, since there is no register of studies on the subject except for the study carried out by Ciarmela et al.10, who characterized Chrysosporium merdarium species as having very high ovicidal activity on Toxocara canis eggs, along with other soil saprophytic fungi.
The purpose of this study was to apply scanning electron microscopy (SEM) to observe the action of two soil saprophytic species of Chrysosporium, Chrysosporium indicum and Chrysosporium keratinophylum, on T. canis eggs.
Materials and methodsFungal strainsStrains IMR-MF-816 C. indicum and IMR-MF-40 C. keratinophylum deposited in the culture collection at the Mycology Department, Instituto de Medicina Regional, Universidad Nacional del Nordeste, Argentina, were assayed. Both strains were obtained from soils of parks of Corrientes city, Argentina23.
C. indicum and C. keratinophylum were selected for being the most commonly isolated strains from soils of parks in the area where the assay was conducted23 and, also because of the high ovicidal activity described for the C. merdarium species10.
Source of T. canis eggsAdult female worms of T. canis were obtained after deworming naturally-infected puppies. Eggs were extracted from the uterus of the female nematode, treated with 0.1% (v/v) NaClO and washed repeatedly with sterile distilled water. Eggs were resuspended in sterile distilled water at a final concentration of 1×103eggs/ml. Microscopic observation revealed that most eggs were unembryonated17.
Interaction assaysInteraction assays were conducted according to the technique described by Basualdo et al.3, with the following modifications: from a culture of C. indicum and C. keratinophylum in potato dextrose agar, a piece of agar (4mm in diameter) of each fungal strain was placed onto Petri dishes containing 2% water agar and they were incubated at 28°C for 4 days to obtain a considerable fungus growth. Subsequently, a suspension of approximately 1×103 per ml immature or non-embryonated T. canis eggs was added to each dish7,17. Once the dishes were inoculated with the egg suspension, they were incubated for 28 days at 28°C. A suspension of T. canis eggs in 2% water agar was used as control group. The assay was done in triplicate for each fungus and the control group.
Scanning electron microscopy (SEM)After inoculating the dishes with the T. canis egg suspension, observations with SEM were conducted on the 4th, 7th, 14th, 21st and 28th day under a Joel 5800 LV (Tokyo, Japan) scanning electron microscope at Servicio de Microscopia Electrónica (Universidad Nacional del Nordeste, Argentina).
Fixation, dehydration, critical point drying, setup, metallization and observation steps were carried out following the technique outlined by Sarmiento et al.24 with the following modifications. Fixation of the material was done for 48h in a 2% v/v freshly prepared formaldehyde solution. After 48h, dehydration was carried out through consecutive passages in ethyl alcohol in increasing concentrations (10%, 30%, 50%, and 70% v/v), and the material was left for 15min in each alcohol concentration.
At the Microscopy Service, the material was dehydrated again in situ, through passages into 70%, 85%, and 100% acetone. Then, critical point drying with CO2 was done, followed by setup of the dry material over a metal plate which was subjected to gold plating for 3min, prior to observation. Observations were conducted at different magnifications (220×–2200×).
Evaluation of the fungal effect on eggsThe effect of the fungi on eggs was evaluated in accordance with the alterations observed on the surface and the changes in the normal characteristics of 100 eggs, according to Lysek and Sterba19 and classified into: Type 1 effect, or lithic effect with no morphological damage to the shell or hyphal penetration through it; Type 2 effect, or lithic effect with morphological alteration in the embryo and shell but no penetration of the shell; and Type 3 effect, or lithic effect with morphological alteration of the embryo, penetration and internal colonization.
Statistical analysisThe statistical significance of the values obtained was evaluated using the Student's t-test. A probable value of p<0.01 was considered significant.
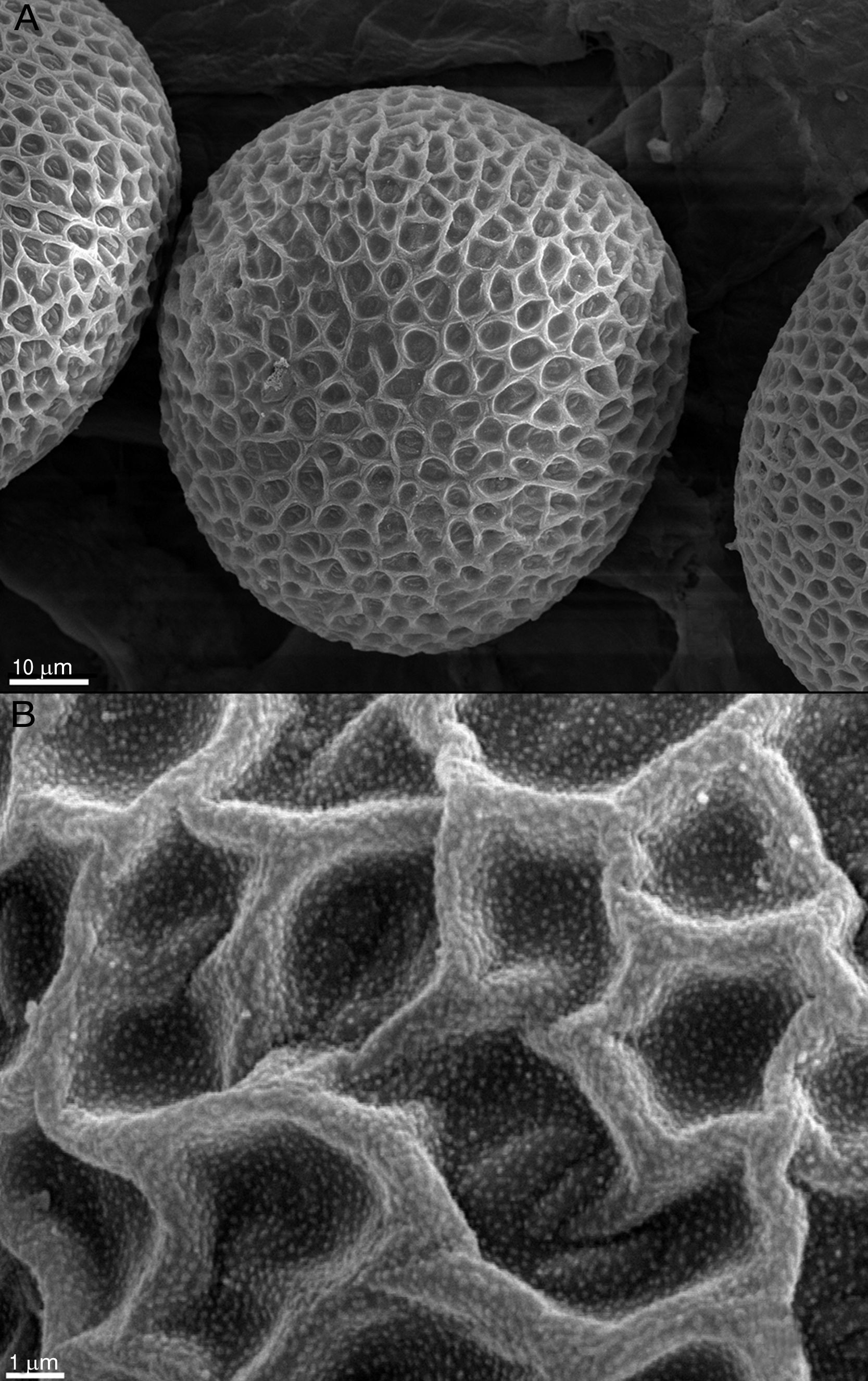
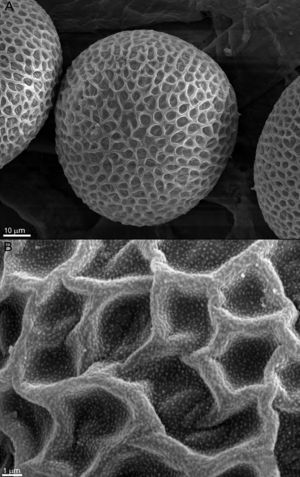
ResultsThe effect of fungi on the eggs was evaluated according to the alterations observed in the surface and the changes in the normal characteristics of the eggs. Figure 1A and B shows normally developed T. canis eggs of the control group after 14 days of incubation, with no morphological alterations and intact shells, exhibiting their typical surface.
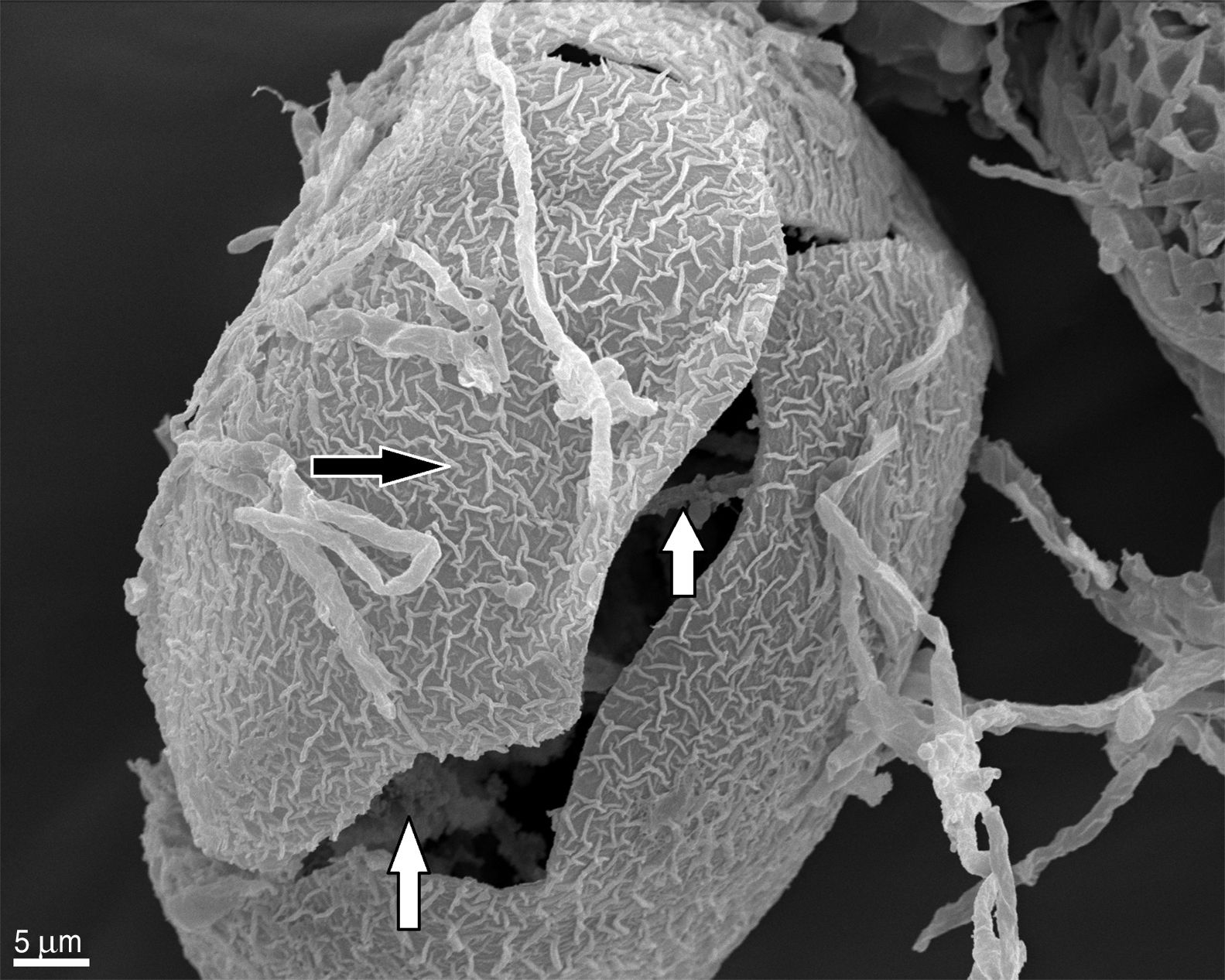
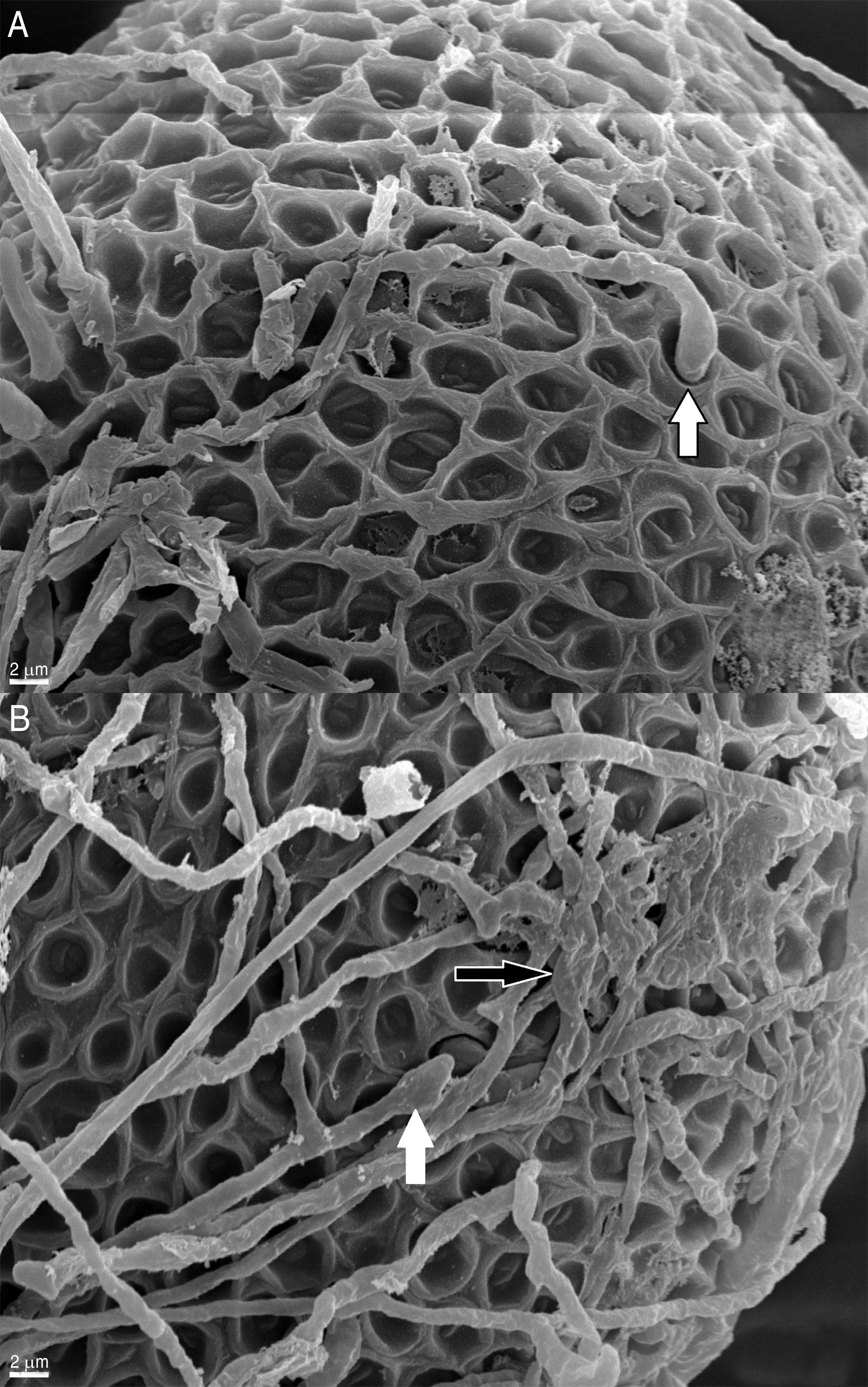
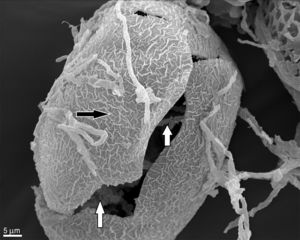
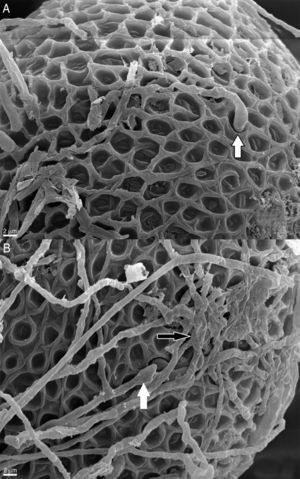
In contrast to the control group, T. canis eggs submitted to the interaction assays with both Chrysosporium were observed submerged into a hyphal network as from the 7th day of incubation and penetration (“appresoria”) organs that finally managed to penetrate the shell appeared. Figures 2 and 3B show hyphae surrounding the egg. Appresoria penetrating the shell can be observed in Figure 3A and B. hyphae around the eggs make the shell softer and thinner. Changes in the typical egg membrane can be observed in Figures 2 and 4.
The highest percentage of affected eggs was observed between days 7 and 14. After 7 days of incubation, 46% and 69% of the eggs were altered by C. indicum and C. keratinophylum, respectively. On day 14, this percentage had increased 68.7% with C. indicum and 74% C. keratinophylum. From day 14 onwards, no major changes were detected.
Non-significant differences were obtained between both Chrysosporium species.
Several structural changes of the egg shell occur when fungal hyphae contact the egg, consequently affecting the embryo. This observation of hyphae inside the egg can be possible only if, when assembled for the SEM, the shell breaks and allows to see what happens inside (Fig. 2), otherwise these observations are not possible by this technique.
According to Lysek and Sterba19, the effect of the fungi classified as Type 3 prevails in our study. This effect includes hypha penetration into the eggs and alterations occurring both in the shell and the embryo.
DiscussionIn order to contribute to the biological control of geohelminths, many studies in Latin America have assessed the effect of different fungi on T. canis eggs in Argentina3,10,11,16,17 and Brazil1,2,9,12,15. In Europe, the contribution of Mazurkiewicz-Zapalowicz et al.21 regarding the effects of several species of soil saprophytic fungi on T. canis deserves to be mentioned.
Chrysosporium is a filamentous keratinophilic fungus, commonly isolated from soils, vegetal material, manure and birds. It lives among the remains of hair and feathers on the soil. Besides being a common contaminant, it is occasionally isolated from human infections. This genus adapts to hot weather areas, is constant and dominant throughout northern Argentina and is cosmopolitan as regards its distribution23.
The use of SEM allowed us to observe clearly the interaction fungi-eggs and how C. indicum and C. keratinophylum affect the normal development and alter the structure of T. canis eggs. We also noted that these alterations increased depending on the length of exposure. A similar action was reported for only one Chrysosporium species by Ciarmela et al.10 in their studies on C. merdarium. These authors report a high in vitro ovicidal activity of C. merdarium and describe that the fungus growth and the egg alteration was visible after 21 days of incubation. Unlike our results, we already observed an active interaction as from the 7th day. At this time, a hyphal network surrounding the eggs with appressorium formation and the thinning and smoothness of the shell maintained throughout the days of exposure were observed with both Chrysosporium. As it was documented for C. merdarium, we observed that T. canis eggs had smooth shells since the 14th day post-incubation10.
It is worth considering that the eggs used in this study to conduct the in vitro test were immature or unembryonated. Lysek and Sterba19 mentioned other investigations in which Paecilomyces lilacinus (current name Purpureocillium lilacinum) colonizes Globodera pallida eggs more readily when they are in the early stages of development. Depending on their maturity, these authors reported that eggs have different degrees of resistance to being invaded. For most fungi, when eggs are in a more advanced stage or there is larval development inside them, the ovicidal activity and the ability to colonize eggs are reduced19.
Due to their ovicidal nature, P. lilacinum and Pochonia chlamydosporia are the most studied fungi in vitro. Only P. lilacinum has been isolated from soils in northeastern Argentina23, but its in vitro activity has been shown widely enough, and therefore was not considered for this study. P. lilacinus has been studied by Basualdo et al.3, who proved that T. canis eggs colonized by this fungus do not fully develop and the fungus produces special penetration organs, causing mechanical damage to the egg. Likewise, it is important to observe that both species of Chrysosporium tested in this study have formed hyphal networks around the eggs with attack-specialized mycelium (appresoria), which favored the penetration of this ovicidal fungus through resistant egg-shells inside the eggs, with the same effect.
Carvalho et al.9 also studied the interaction of P. lilacinum and P. chlamydosporia on T. canis eggs. These researchers believe that both fungi can destroy the eggs under laboratory conditions, and claim that the longer the contact between fungus and egg, the more efficient the ovicidal activity will be. In our study, SEM observations allowed us to reach similar conclusions on the effect of C. indicum and C. keratinophylum, with a high activity of these fungi from 7th day, being able to alter and penetrate the eggs from the 14th day.
Despite their slow growth, Ciarmela et al.10 show, their mechanically and enzymatically-driven ovicidal activity for the species C. merdarium, since, by the 14th day post-incubation, eggs with a smooth shell were observed, similarly to what we have reported in our assay (Fig. 2). Despite their high antagonic activity, Ciarmela considers it a control agent only for limited use because it may affect human beings10; however, the presence of these Chrysosporium species in soils can play a very important role as natural biological control agents.
This is the first assay conducted in northeast Argentina. We can conclude that both C. indicum and C. keratinophylum have, in vitro, a high capacity to destroy T. canis eggs, which can be well observed and described under SEM. We should deepen our studies to complete the knowledge about the mechanisms used by these fungi to, fully or partially, destroy T. canis eggs, as well as, to learn about the influence of the type of soil, humidity, temperature, and presence of other organisms on the in vivo action of these fungi on geohelminth eggs.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare no conflict of interest.
The authors wish to thank to: Secretaría de Ciencia y Técnica, Universidad Nacional del Nordeste, for their financial support; Biochemist María Mercedes Sarmiento, for isolating fungal strains and to Ms. Liliana Alegre for assisting in the preparation of materials. We also wish to acknowledge Laura Cipolla for her translation of the manuscript.