Macrophomina phaseolina is a polyphagous phytopathogen, causing stalk rot on many commercially important species. Damages caused by this pathogen in soybean and maize crops in Argentina during drought and hot weather have increased due its ability to survive as sclerotia in soil and crop debris under non-till practices. In this work, we explored the in vitro production of plant cell wall-degrading enzymes [pectinases (polygalacturonase and polymethylgalacturonase); cellulases (endoglucanase); hemicellulases (endoxylanase) and the ligninolytic enzyme laccase] by several Argentinean isolates of M. phaseolina, and assessed the pathogenicity of these isolates as a preliminary step to establish the role of these enzymes in M. phaseolina–maize interaction. The isolates were grown in liquid synthetic medium supplemented with glucose, pectin, carboxymethylcellulose or xylan as carbon sources and/or enzyme inducers and glutamic acid as nitrogen source.
Pectinases were the first cell wall-degrading enzymes detected and the activities obtained (polygalacturonase activity was between 0.4 and 1.3U/ml and polymethylgalacturonase between 0.15 and 1.3U/ml) were higher than those of cellulases and xylanases, which appeared later and in a lesser magnitude. This sequence would promote initial tissue maceration followed by cell wall degradation. Laccase was detected in all the isolates evaluated (activity was between 36U/l and 63U/l). The aggressiveness of the isolates was tested in maize, sunflower and watermelon seeds, being high on all the plants assayed. This study reports for the first time the potential of different isolates of M. phaseolina to produce plant cell wall-degrading enzymes in submerged fermentation.
Macrophomina phaseolina es un fitopatógeno polífago, causante de podredumbre carbonosa. Los daños que genera en cultivos de soja y maíz bajo siembra directa en Argentina, en períodos secos y calurosos, se incrementaron por su habilidad para sobrevivir como esclerocios en suelos y restos de cosecha. El propósito del trabajo fue estudiar la producción in vitro de enzimas degradadoras de pared celular vegetal (pectinasas [poligalacturonasa y polimetilgalacturonasa]; celulasas [endoglucanasa]; hemicelulasas [endoxilanasa] y la enzima ligninolítica lacasa) de varios aislamientos argentinos de M. phaseolina y evaluar la patogenicidad de esos aislamientos, como paso preliminar para establecer el papel de estas enzimas en la interacción M. phaseolina-maíz. Se estudió la cinética de crecimiento del hongo y la de la producción de dichas enzimas en medios de cultivo líquidos sintéticos con ácido glutámico como fuente de nitrógeno y con pectina, carboximetilcelulosa (CMC) o xilano como fuentes de carbono. Las pectinasas fueron las primeras enzimas detectadas y los máximos títulos registrados (1,4 UE/ml [poligalacturonasa] y 1,2 UE/ml [polimetilgalacturonasa], respectivamente) superaron a los de celulasas y xilanasas, que aparecieron más tardíamente y en menor magnitud. Esta secuencia promovería la maceración inicial del tejido, seguida luego por la degradación de la pared celular vegetal. Se detectó actividad lacasa en todos los aislamientos (36 a 63U/l). La agresividad de todos los aislamientos resultó alta en los 3 hospedantes evaluados: semillas de maíz, de girasol y de melón. En este trabajo se investiga por primera vez el potencial de distintos aislamientos de M. phaseolina para producir enzimas degradadoras de pared celular vegetal en cultivo líquido.
Fungal plant pathogens secrete a wide range of enzymes such as pectinases, xylanases, cellulases and ligninases that are able to depolymerize each cell wall component38. These fungi are of interest for the search of new enzyme activities due to their potential application in bioconversion processes17. Hydrolytic enzymes play an important role in plant pathogenicity by facilitating tissue colonization of the host3,13.
Pectin, a heteropolysaccharide defined as galactosyluronic acid-rich polymers, is composed by α-1,4 linked galacturonate chains with high percentage of methyl esterification. Plant pathogenic organisms are capable of degrading pectin by the combined action of several enzymes which produce the breakdown of polygalacturonic acid through two enzymatic processes: lyases split the α-1,4 glycosidic bond between galacturonic acid residues by transelimination, while polygalacturonases catalyze a hydrolytic cleavage33. The action of pectinolytic enzymes, and in particular of endopolygalacturonase on cell walls, appears to be a prerequisite for cell wall degradation by other enzymes. Therefore, pectinases are the first enzymes secreted by most fungal pathogens when attacking plant cell walls, followed by hemicellulases and cellulases35. A positive correlation has been established between the production of pectinolytic enzymes, virulence and disease symptoms in several pathosystems16,31.
Cellulose is an unbranched glucose polymer composed of β-1,4-glucose units linked by a β-1,4-d-glycosidic bond. Many plant pathogenic organisms are capable of degrading cellulose by producing a cellulose complex which involves the synergistic action of three main enzymatic complexes, endoglucanase, exoglucanase that releases either glucose or cellobiose, and β-1,4-glucosidase that hydrolyzes cellobiose and cellodextrins to glucose25,27.
Xylan is the major constituent of hemicellulose. β-1,4-Xylans are heteropolysaccharides with a homopolymeric backbone chain of 1,4-linked β-d-xylopyranose units. O-acetyl, α-l-arabinofuranosyl, α-1,2-linked glucuronic, or 4-O-methylglucuronic acids are the most frequent substituents on the backbone. Xylan hydrolysis mainly requires the action of endo-β-1,4-xylanase and β-xylosidase. However, the presence of other accessory enzymes is needed to hydrolyze substituted xylans15.
The complex aromatic polymer lignin, a component of plant secondary cell walls, provides a barrier to fungal entry and to the diffusion of fungal toxins and enzymes into plant cells8. The lignin-degrading enzyme, laccase, is a copper-containing enzyme produced by some fungi that catalyzes the oxidation of phenolic substrates. Several phytopathogenic fungi are known to produce lacasse18,20. Laccase protects phytopathogenic fungi from the plant defense system activated in response to a microbial infection, by degrading toxic substances such as phytoalexins, tannins and other phenolic compounds24.
The charcoal rot fungus Macrophomina phaseolina (Tassi) Goid, an Ascomycete which belongs to the family Botryosphaeriaceae, is a soil-borne pathogen distributed worldwide. The pathogen has a wide host range, infecting more than 500 plant species5. The fungus attacks a broad spectrum of economically important crops such as maize, soybean, sorghum, sesame, cotton, beans, sunflower or cucurbits6,10. In maize it causes stalk rot during hot and dry weather during plant senescence37. The symptoms are similar to other fungal stalk rots and the characteristic sign is the production of black microsclerotia in vascular tissues and inside the rind of the stalk. Microsclerotia in crop debris and in soil are the primary inocula of the disease, surviving up to three years in soil6. No-till practices are widely adopted in Argentina, and crop debris remains on the soil surface until complete degradation, increasing the amount of the pathogen inoculum. M. phaseolina owns a large repertoire of hydrolytic enzymes able to degrade all major components of the plant cell wall and cuticle, including cellulose, hemicellulose, pectin, lignin, and cutin12. Moreover, cellulolytic activity of M. phaseolina was shown to be significantly higher than that of other fungal species (i.e., Aspergillus niger and Trichoderma reesei), demonstrating the pathogenic potential of this fungus15. More than twenty laccase genes, which might be involved not only in lignin degradation but also in appressorial melanization and pathogenicity19 have been identified in M. phaseolina12.
In the present work, we (i) explored the in vitro production of plant cell wall-degrading enzymes by several Argentinean isolates of M. phaseolina, a fungal pathogen associated with corn stalk rot disease, as a preliminary step to establish the role of these enzymes in M. phaseolina–maize interaction; (ii) assessed the pathogenicity of these isolates on watermelon (Citrullus lanatus), sunflower (Helianthus annuus) and maize (Zea mays), and evaluated the relationship (if any) with their ability to produce cell wall-degrading enzymes.
Materials and methodsMicroorganismsIsolates BAFC 3591, 3595, 3820, 3821, 3864 and 3865 (Colección de Cultivos de Hongos del Dpto. de Cs. Biológicas, FCEN, UBA) of the anamorphic species M. phaseolina were used in these experiments. These isolates were previously isolated from samples of corn stems showing typical symptoms of a natural infection by M. phaseolina. These samples were collected from three locations in Buenos Aires province: Luján (isolates 3591 and 3865), Rafael Obligado (isolates 3820 and 3821) and San Antonio de Areco (isolates 3595 and 3864). Microsclerotia of M. phaseolina were obtained from stem tissues and placed on potato dextrose agar (PDA) plates. Pure cultures were developed by removing growing hyphal tips from a single microsclerotium colony and culturing them on PDA at 28–30°C for 5–7 days in darkness23.
Basal culture mediumGlutamic acid, 9.0g; MgSO4·7H2O, 0.5g; H2KPO4, 0.5g; HK2PO4, 0.6g; CuSO4·5H2O, 0.4mg; MnCl2·4H2O, 0.09mg; H3BO3, 0.07mg; Na2MoO4·2H2O, 0.02mg; FeCl3, 1mg; ZnCl2, 3.5mg; thiamine hydrochloride, 0.1mg; distilled water up to 1 l. Alternatively different carbon sources were added. In order to test pectinase, cellulase or xylanase production, the basal medium was supplemented either with 10g of pectin from apple, 10g of carboxymethylcellulose (CMC) or 10g of oat spelt xylan, respectively. To evaluate laccase production, glucose 10g was used as carbon source, and CuSO4·5H2O as laccase inducer18 was added to the medium, final concentration 0.2mM. Final pH was adjusted to 3.5 with either NaOH or HCl 1N.
Culture conditionsOne hundred milliliters Erlenmeyer flasks with 25ml of medium were inoculated with one agar plug (0.25cm2), cut out from a colony grown on Bacto-agar 2%. Incubation was performed at 28±1°C under stationary conditions. Cultures were harvested every three days during three weeks, filtered through a filter paper using a Büchner funnel and dried overnight at 70°C. Dry weight of mycelia was then determined. The culture supernatants were used as enzyme sources.
Enzyme assaysPolymethylgalacturonase (PMG) or polygalacturonase (PG) (endo plus exo activities) were assayed by following the release of reducing groups from apple pectin or polygalacturonic acid in 50mM sodium acetate buffer, pH 4.8 at 37°C, in accordance with the Somogyi–Nelson method26. One unit of enzymatic activity was defined as the amount of enzyme releasing 1μmol of galacturonic acid per min. Laccase activity was measured with 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) in 0.1M sodium acetate buffer (pH 3.5) at 50°C. Oxidation of ABTS was determined by the increase in A420 (420=36/mMcm)4. Endo-β-1,4-glucanase activity was assayed at 50°C and endo-β-1,4-xylanase activity was determined at 37°C by measuring the reducing sugars released respectively from CMC or xylan in 50mM sodium acetate buffer at pH 4.8, in accordance with the Somogyi–Nelson method26. One unit of enzymatic activity was defined as the amount of enzyme releasing 1μmol of glucose or xylose per min. Enzymatic units (U) were used (μmol/min). Enzyme activity was expressed as EU/ml of culture filtrate. Results are the average of three triplicate experiments with a standard error lower than 5%.
Pathogenicity testThe M. phaseolina isolates were grown in potato-sucrose agar (PSA) in Petri dishes and incubated in darkness at 28°C for 7 days. When dishes were completely colonized by the fungus and covered by microsclerotia, seeds of watermelon, sunflower or maize, previously disinfected with 2% sodium hypochlorite for 2min and rinsed twice in sterile water, were placed on the plates. Each treatment of six seeds arranged in two plates was replicated three times. Evaluation was done after 5 days, using the following severity assessment key: 0=healthy seed; 1=discoloration on a portion of the seedling in contact with the mycelium; 2=seed teguments invaded by mycelium and microsclerotia but healthy seedling; 3=seed teguments free from the fungus but infected seedling; 4=seed tegument and infected seedling; 5=infected seed and not germinated21. The disease index was calculated by multiplying the number of seeds by the degree of disease severity. Values greater than 3 were considered susceptible. Non-parametric analysis of variance (Kruskal–Wallis) was performed using Infostat software7.
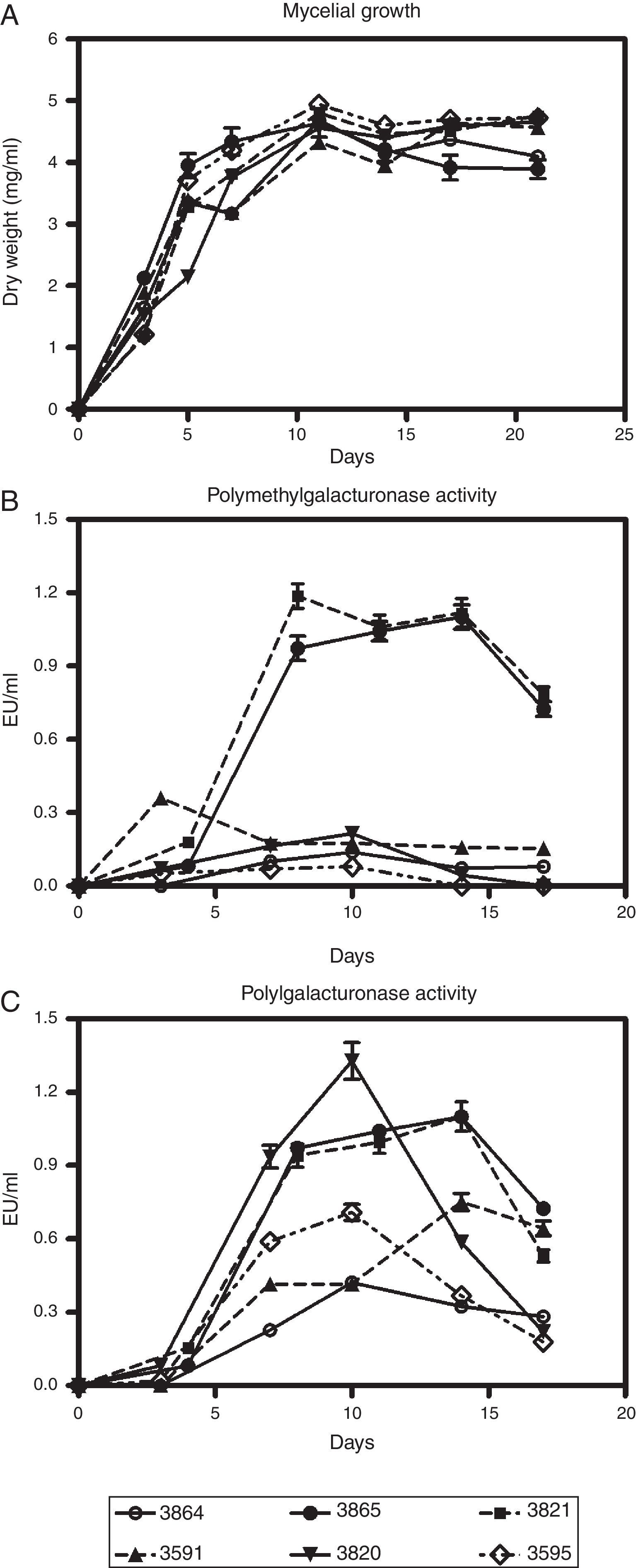
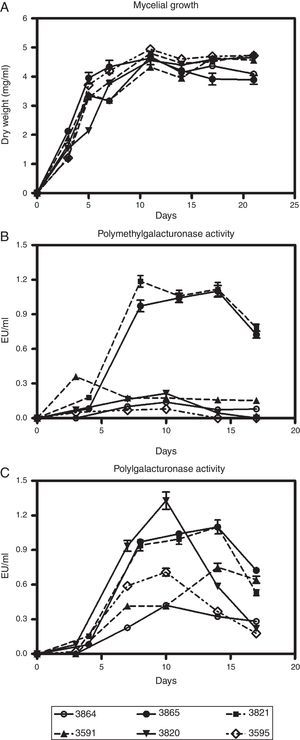
Results and discussionSix fungal isolates of M. phaseolina were grown in a synthetic medium with several carbon sources alternatively employed to assess the production of different cell wall-degrading enzymes. Figures 1A–C, describe growth and pectinolytic enzyme production in a medium with pectin as carbon source. All isolates were able to grow in this medium, and showed PMG and PG activities having the potential to degrade α-1,4 bonds in pectic substances hydrolytically. The disparity observed in enzyme production among the isolates cannot be attributed to fungal growth, since no major differences were found. Maximal growth values were around 4–4.8mg/ml of medium, and were usually registered after 11 days of cultivation (Fig. 1A). The peak of PMG activity, which appeared between days 7 and 10 of incubation, preceded, in general, the day of maximal growth (Fig. 1B). On the contrary, PG activity reached its highest level (between day 8 and 14 of culture) after or simultaneously with the growth peak (Fig. 1C), as previously reported in Botrytis cinerea22. Among the isolates assayed, M. phaseolina BAFC 3865 and BAFC 3821 rendered the highest levels of the two enzymes. Isolate BAFC 3820 showed high PG production but low PMG production (Figs. 1B and C). PG titers obtained from M. phaseolina isolates (between 0.4 and 1.3U/ml) were similar to those detected when other phytopathogenic fungi were grown in a medium with pectin. PG-production by Fusarium oxysporum f. sp. niveum reached a maximum of 0.4U/ml36, Colletotrichum lindemuthianum produced 0.24U/ml11, Colletotrichum trucatum rendered 1.08U/ml29, while in Fusarium graminearum higher levels of PG (5.4U/ml) than in other phytopathogenic fungi were measured17. PMG activities (between 0.15 and 1.3U/ml) were similar to those obtained for C. truncatum (1.2U/ml)29 and for F. graminearum (1.53U/ml)17.
Kinetics of growth (A), polymethylgalacturonase (B) and polygalacturonase production (C) by M. phaseolina isolates from different regions in Argentina, in minimum salt medium supplemented with pectin and glutamic acid as carbon and nitrogen sources, respectively. Values represent the mean of three replicates and SEM.
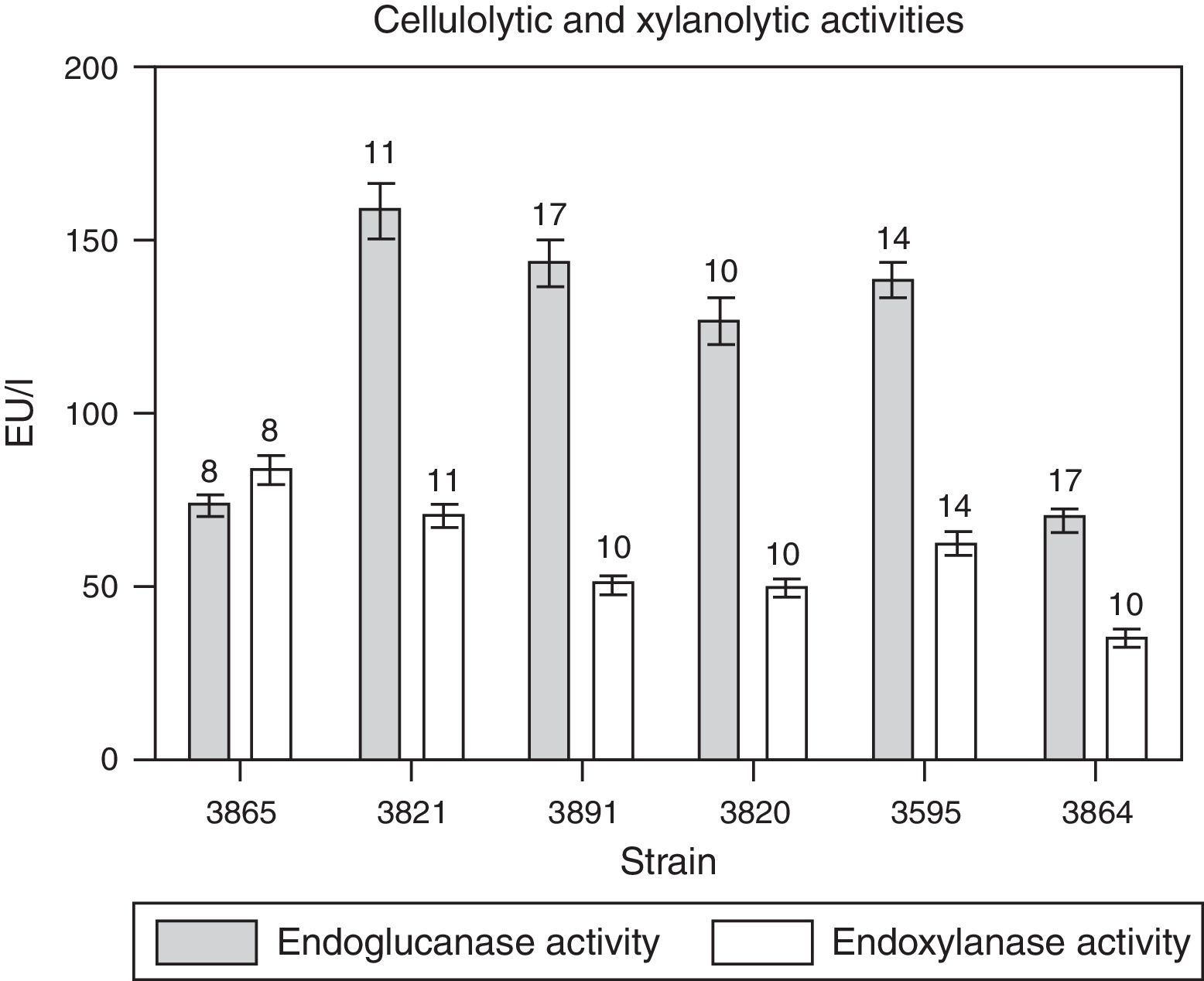
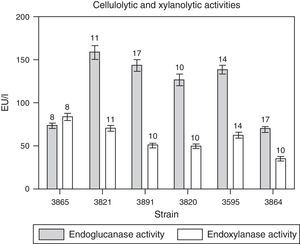
All the isolates tested were able to grow in the media with CMC or with xylan as carbon sources and produced cellulolytic and xylanolytic enzymes; however, their production was much lower than that of pectinases. Cellulolytic (endoglucanase) activity was generally detected after 11 days of cultivation while endoxylanase was observed between day eight and eleven (Fig. 2). Cellulolytic activity detected from M. phaseolina isolates was between 70U/l for isolate BAFC 3864 and 164U/l for isolate BAFC 3821, while maximum endoxylanase activity was registered in isolate BAFC 3865 (84U/l) and the minimum in M. phaseolina BAFC 3864 (35U/l) (Fig. 2).
Time courses for the production of endoglucanase and endoxylanase activities by M. phaseolina isolates from different regions in Argentina, in minimum salt medium supplemented with carboxymethylcellulose (for cellulases) or xylan (for xylanases) as carbon sources and glutamic acid as nitrogen source. The numbers over the bars indicate the day when the highest value was obtained. Values represent the mean of three replicates and SEM.
In this work, pectinases were the first extracellular enzymes detected related to the degradation of the main components of a plant cell wall, and the activity obtained was higher than that of cellulases and xylanases, which appeared later and in a lesser magnitude. Similar results were obtained by Kikot et al.17 when studying cell wall-degrading enzymes production by F. graminearum. The earlier production of galacturonases during M. phaseolina in vitro cultivation coincides with their postulated role in pathogenesis in other phytopathogenic fungi. Ahmad et al.1 reported that in several pathogens including M. phaseolina, pectinase was the chief enzyme while initiating the process of cell wall degradation as it showed the highest activity before cellulases. EndoPG and endoPMG facilitate the entry of the pathogen into the host tissues. In M. phaseolina, penetration generally occurs from an appresorium formed over anticlinal walls of epidermal cells or through natural openings. The fungal hyphae grow first intercellularly and then intracellularly through the xylem, producing numerous microsclerotia that plug the vessels39. The B. cinerea genome contains at least six endopolygalacturonase-encoding (Bcpg) genes, strains mutated in genes Bcpg1 and Bcpg2 were reduced in virulence14, which indicates that pectin degradation is necessary for successful infection. Furthermore, in the pathogenic fungus F. oxysporum, responsible for causing vascular wilt disease that is characterized by a severe degradation of vascular tissue, the amount of PG activity correlated highly with the development of the disease2.
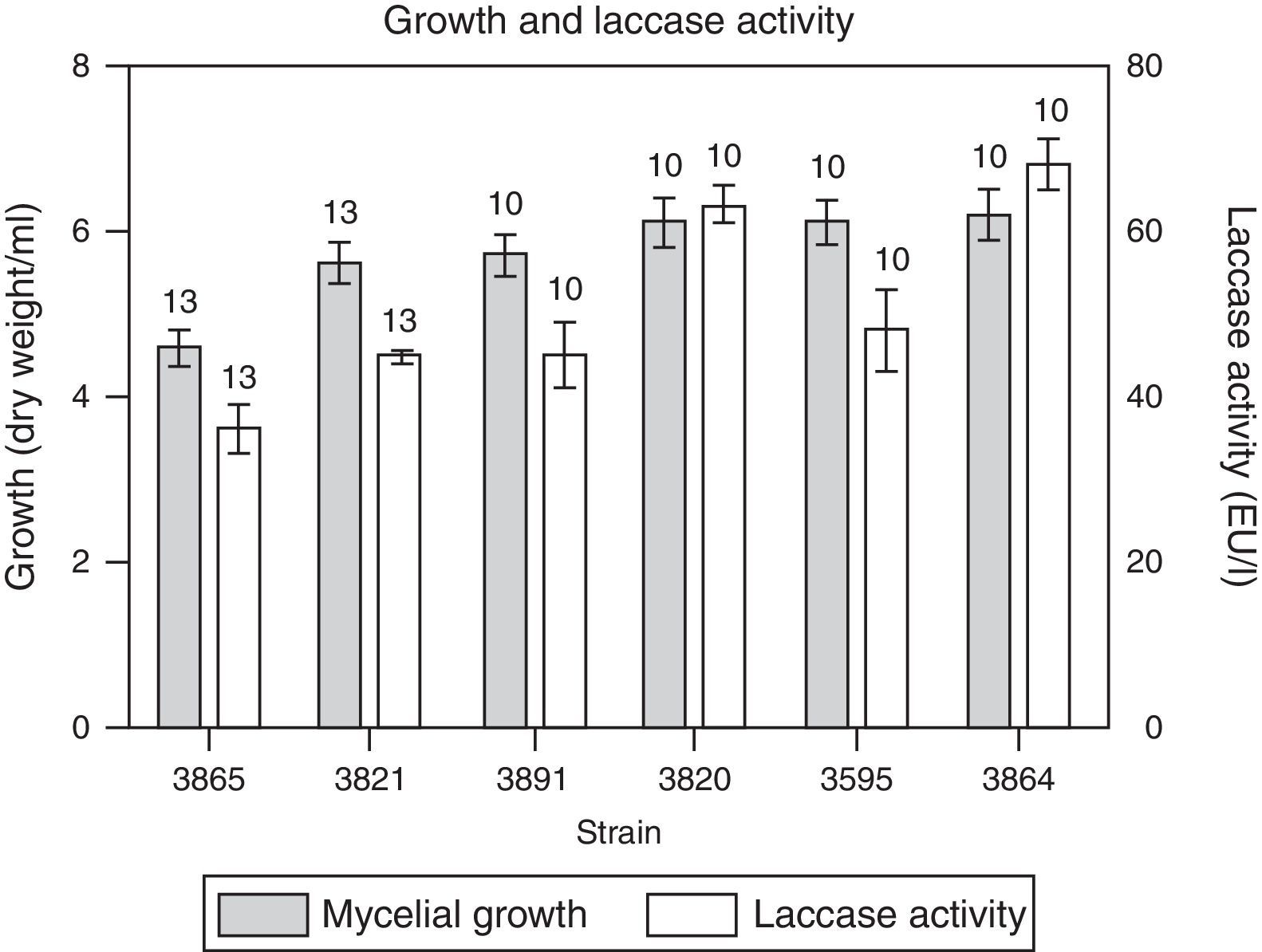
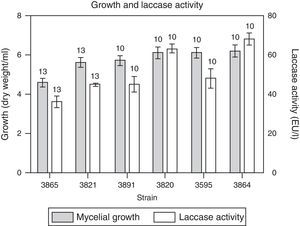
Laccase activity was confirmed in the six isolates evaluated, starting in every case before mycelial biomass peaked (i.e. it was present in the primary phase growth) (data not shown), and reached its maximum on day 10 coincident with the highest biomass recorded (Fig. 3). Biomass production in all the isolates evaluated varied between 5.2 and 6.2mg/ml medium. Laccase activity obtained for all isolates assayed was between 36U/l and 63U/l. Laccase production was comparable with that of C. truncatum18, when using glucose (41U/l) or pectin (44U/l) as carbon sources without the addition of CuSO4. However, laccase production by F. solani f. sp. glycines under submerged fermentation in a medium supplemented with soybean roots, attained higher values, even exceeding the titers obtained by white rot fungi such as Schizophyllum commune cultured under similar conditions20. This study reports for the first time the potential of plant cell wall-degrading enzyme production, assayed in submerged fermentation, by different isolates of M. phaseolina. Recently, Kaur et al.15 reported that one of the isolates of M. phaseolina was a potential source of several hydrolytic enzymes, such as cellulases, hemicellulase and amylase, with biotechnological applications28.
Growth and laccase enzyme production by M. phaseolina in minimum salt medium supplemented with glucose and glutamic acid as carbon and nitrogen sources, respectively, supplemented with CuSO4 0.2mM. The numbers over the bars indicate the day when the highest value was obtained. Values represent the mean of three replicates and SEM.
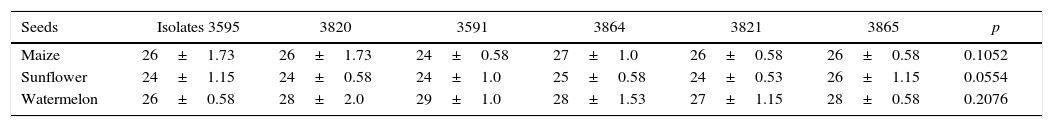
The degree of disease severity caused by all M. phaseolina isolates tested was high, exceeding degree 3 in the scale described by Manici et al.21 in every case. The six isolates evaluated invaded and infected all the seeds of the species assayed (Table 1), confirming the polyphagous condition of this phytopathogenic fungus34. The comparison of mean values among different plant seed reactions showed no significant differences (p≤0.05) in the disease index. Manici et al.21 depicted different degrees of pathogenicity in M. phaseolina isolates in a wide variety of crops, such as soybean, sunflower, sorghum, melon and sugar beet, among others, but none was pathogenic on maize. High pathogenicity was also observed by Gill-Langarica et al.9 in soybean crops. Rayatpanah and Dalili30 studied the pathogenicity of 24 isolates of Iranian M. phaseolina; the pathogenicity test demonstrated that no one was pathogenic on maize, while all of them showed pathogenic ability on soybean and sunflower. However, the Argentinean isolates evaluated in this work showed comparable pathogenicity even on maize. Reyes-Franco et al.32 investigated the pathogenicity of isolates of M. phaseolina from different countries, among them, Argentina. Results showed differences between isolates, being the most aggressive those from Mexico, Brazil and Colombia. Considering the results obtained in the present work, the aggressiveness of Argentinean isolates of M. phaseolina should be investigated more thoroughly.
Pathogenicity of isolates of Macrophomina phaseolina on maize, sunflower and watermelon seeds
| Seeds | Isolates 3595 | 3820 | 3591 | 3864 | 3821 | 3865 | p |
|---|---|---|---|---|---|---|---|
| Maize | 26±1.73 | 26±1.73 | 24±0.58 | 27±1.0 | 26±0.58 | 26±0.58 | 0.1052 |
| Sunflower | 24±1.15 | 24±0.58 | 24±1.0 | 25±0.58 | 24±0.53 | 26±1.15 | 0.0554 |
| Watermelon | 26±0.58 | 28±2.0 | 29±1.0 | 28±1.53 | 27±1.15 | 28±0.58 | 0.2076 |
Severity was recorded according to a 0 to 5 scale. Severity multiplied by the number of diseased seeds=pathogenicity. Values represent the mean of three replications of each species and SEM.
Although we could not establish a relationship between differences in cell wall-degrading enzyme production among isolates and pathogenicity, the in vitro production of plant cell wall-degrading enzymes by M. phaseolina could be related to their production and role in vivo: initial pectinolytic production followed by cellulolytic and ligninolytic secretion. This sequence indicates that pectic enzymes are needed to increase the accessibility of cell wall components for degradation by other enzymes, cell lysis and plant tissue maceration. Laccase would contribute not only with tissue disintegration in an advanced phenological stage but would also protect the pathogen against oxidative plant defenses and might be involved in appressorial melanization and pathogenicity.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare that they have no conflicts of interest.