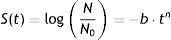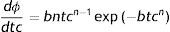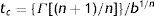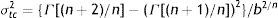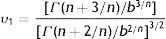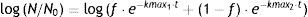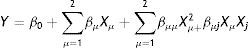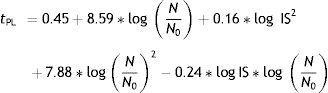The purpose of this study was to analyze the response of different initial contamination levels of Alicyclobacillus acidoterrestris ATCC 49025 spores in apple juice as affected by pulsed light treatment (PL, batch mode, xenon lamp, 3pulses/s, 0–71.6J/cm2). Biphasic and Weibull frequency distribution models were used to characterize the relationship between inoculum size and treatment time with the reductions achieved after PL exposure. Additionally, a second order polynomial model was computed to relate required PL processing time to inoculum size and requested log reductions. PL treatment caused up to 3.0–3.5 log reductions, depending on the initial inoculum size. Inactivation curves corresponding to PL-treated samples were adequately characterized by both Weibull and biphasic models (Radj2 94–96%), and revealed that lower initial inoculum sizes were associated with higher inactivation rates. According to the polynomial model, the predicted time for PL treatment increased exponentially with inoculum size.
El objetivo del presente trabajo fue evaluar la influencia de la concentración de esporas de Alicyclobacillus acidoterrestris ATCC 49025 en la respuesta de inactivación por acción de la luz pulsada (modo estanco, lámpara de xenón, 3pulsos/s, 0–71,6J/cm2) en jugo de manzana comercial. Para caracterizar la relación existente entre la concentración de esporas y el tiempo de tratamiento con las reducciones logarítmicas alcanzadas luego de la exposición a la luz pulsada (LP), se aplicaron 2 modelos: el de Weibull y el bifásico. Adicionalmente, se estimó la relación entre el tiempo de tratamiento con LP y la concentración inicial de inóculo en el jugo con las reducciones logarítmicas logradas mediante regresión múltiple y la metodología de superficie de respuesta (MSR). La inactivación por LP provocó entre 3 y 3,5 reducciones logarítmicas, según la concentración inicial de esporas. Las curvas de inactivación fueron adecuadamente caracterizadas por los modelos matemáticos propuestos (Rajustado2=94–96%). El análisis por MSR permitió predecir un aumento exponencial del tiempo de tratamiento requerido conforme se incrementa el nivel de contaminación inicial.
Heat pasteurization is the most commonly used technique for fruit processing as it ensures microbial safety and shelf life of juices. Nevertheless, it is well-known that traditional thermal processes cause significant damage on the organoleptic, nutritional and physicochemical properties of fluids foods11. In addition, Alicyclobacillus acidoterrestris is a thermoacidophilic, non-pathogenic and spore-forming bacterium which is capable of surviving the pasteurization process1. Therefore, this bacteria has been frequently involved in spoilage incidents related to high-acid fruit and vegetable products34, as it can produce a taint compound, identified as guaiacol, which causes an offensive smelling “smoky”, “antiseptic” or “disinfectant”-like flavour18. Not being associated with secondary gas or acid production, this spoilage is hard to spot with the naked eye; but it might show an increase in turbidity and sediment formation35.
Emerging preservation processes are being explored and implemented to provide safe, fresher-tasting, nutritive foods without the use of heat or chemical preservatives22. In the last decades, a wide range of contemporary physical factors have been intensely investigated with the purpose of inactivating A. acidoterrestris spores, encompassing high pressure CO27, microwave17, high electric field-alternating current36, ultraviolet light3 (UV-C), pulsed light (PL)9,13, among others.
PL has been increasingly used since 1996 when the US Food and Drug Administration (FDA) approved its use to sterilize, sanitize or reduce microbial load in foods, food packaging materials, as well as surfaces, environments, plants, devices and media (water, air) involved in food processes2. The implementation of PL for microbial inactivation has gained interest because of the very short treatment time required to achieve a desired microbial decontamination21. This technology is based on the application of short intense pulses (100–400μs) of a broad spectrum between 100 and 1100nm with 54% of emitted energy in the UV range19,28. The germicidal action of PL has been attributed to three different mechanisms which may coexist: the photochemical effect, encompassing the UV fraction, which is responsible for the formation of thymine dimmers that impair cell replication19; the photothermal effect, caused by localized overheating of microbial cells during PL treatment15,40, and the photophysical effect, which induces structural damages in microbial cells due to the pulsing effect25. The relative importance of each mechanism would depend on the fluence imparted to the food and target microorganism19.
PL effectiveness has been attributed mainly to food transparency to the desired light wavelengths. Additionally, other parameters such as turbidity, particle size, suspended solids, presence of particulate materials, dose, composition of the emission spectrum, sample volume, the number and design of lamps have a direct relevance and affect the sample–light interaction21.
Contamination level of food materials has demonstrated to influence the effectiveness of a wide range of emerging technologies, such as pulsed electric fields27, ozone25, high hydrostatic pressure41, among others. Different responses in inactivation effectiveness were reported by increasing the initial inoculum level, depending on the applied factor and matrix. In particular, the effect of inoculum size in PL effectiveness has not been thoroughly studied. The literature reports controversial effects, either increasing20 or decreasing PL decontamination37 while decreasing initial inoculum size.
The aim of the present work was to study the influence of initial contamination level on A. acidoterrestris ATCC 49025 spores in apple juice treated by PL. Furthermore, the suitability of Weibull and biphasic models to quantitatively characterize PL inactivation kinetics for a range of different inoculum sizes was analyzed. Additionally, response surface methodology was employed to optimize PL processing time according to the initial inoculum size and the required spore log reductions. To determine the influence of inoculum size (IS) on the decontamination efficiency of PL processing, three different initial spore concentrations (103, 104 and 106CFU/ml) were inoculated in a 100mm Petri dish.
Materials and methodsSpore production and inoculum preparationExperiments were performed using A. acidoterrestris ATCC 49025 spores. The initial inoculum was prepared by transferring a loopful of a fresh stock culture maintained in Bacillus acidoterrestris medium (BAM) to an Erlenmeyer-flask containing 20ml of BAM Broth and subsequently incubated at 43±1°C for 24h. Spore production was performed by sowing the inoculum onto bottles containing A. acidoterrestris medium (AAM) and incubating 1 week at 43±1°C. Spores were removed by the procedure explained by Silva and Gibbs33, and maintained at −18±1°C until use. BAM used in this study was prepared according to Silva and Gibbs33.
Apple juice samplesCommercial clarified apple juice without any additives (TROPICANA® PepsiCo, Argentina) was used to analyze the influence of initial spore contamination on PL effectiveness. Uninoculated juice samples were analyzed for the presence of A. acidoterrestris spores in BAM agar following the procedure described in “Materials and Methods” section, and were considered control samples. Likewise, as sterility control, uninoculated juice samples were evaluated for the presence of total mesophilic aerobes and yeasts and mold counts, in Plate Count Agar at 37°C for 72h or Chloramphenicol Glucose Agar at 25°C for 5 days, respectively.
Measurements of physicochemical juice parametersFor these studies, uninoculated juice samples were used. The pH of the juice was determined by a pH meter (PerpHect pH/ISE, 310 model, Orion Research Inc., Beverly, USA), while the soluble solid concentration (°Bx) was determined by a refractometer (PR-101 Palette, ATAGO Co. LTD, Japan). Juice turbidity was measured by a turbidimeter (LaMotte, Maryland, USA) using a formazin pattern (100NTU).
The particle size of apple juice was determined in triplicate in the range from 0.6nm to 6μm by dynamic light scattering (DLS) at 20°C in a Zetasizer Nano-Zs (Malvern, Worcestershire, UK) provided with a He–Ne laser (633nm) and a digital correlator (Model ZEN3600). Measurements were carried out at a fixed scattering angle of 173°, with a measuring range according to the manufacturer. The intensity distribution obtained was converted to volume distribution, using the Mie theory26. A refractive index (RI) of 1.35 and an absorption parameter of 0.1 were used, according to the specifications provided by the manufacturer for colored samples26.
Absorbance of juice (diluted to 0.5%, v/v) was determined before PL treatment in 1cm-path quartz cuvettes at 254nm using a UV–vis spectrophotometer (Jasco V-630, Tokyo, Japan) in order to determine transparency of apple juice to the most germicidal fraction of UV light.
Pulsed light treatmentPL treatment was performed using a RS-3000B Steripulse-XL system (Xenon Corporation, Wilmington, MA, USA), which produces polychromatic radiation in the wavelength range from 200 to 1100nm. The PL device consisted of an RC-747 power/control module, a treatment chamber that housed a xenon flash lamp (nontoxic, mercury free) and an air cooling system attached to the lamp housing to avoid lamp overheating during operation16. It generated high intensity PL at a pulse rate of 3 pulses per second and a pulse width of 360μs. According to the specifications supplied by the manufacturer, each pulse delivered 1.27J/cm2 for an input of 3800V at 1.9cm below the quartz window surface of the lamp. The different PL doses were obtained by altering the number of applied pulses. Fluence measurements were taken by a pyroelectric head model ED500 (Gentec Electro-Optics, Québec, Canada) connected to a TDS 2014 model oscilloscope (Tektronix, Beaverton, USA), with an aperture cover of 20.3cm2. Experiments were performed in triplicate.
Having filled a 100-mm Petri dish with inoculated apple juice to a depth of 1mm (approximately 5ml), it was exposed to PL treatment for 60s (71.6J/cm2, 1.1×103J/ml, initial temperature=2±1°C) at a distance of 0.1m from the quartz window. The Petri dish was placed into a container, filled with ice flakes before PL treatment, to minimize temperature increase of the sample. Therefore, juice temperature immediately decreased to 2–3±1°C, reaching a final value of temperature (TPLf) of 12±1°C after PL processing. Temperature evolution of juices during PL treatment was monitored using a T-type thermocouple connected to a data logger Digi-Sense model 69202-30 (Barnant Company Division, Barrington, USA). Experiments were performed in triplicate.
Electrical energy per order (EEO) estimationEEO, an electrical figure of merit defined as the electric energy in kilowatt hours [kWh] required to reduce the microbial load by one order of magnitude in 1m3 of a contaminated sample, was calculated to determine the involved energy delivered to the treatment and thus, its efficiency. EEO values were estimated according to the equation proposed by Bolton et al.5 for batch operations.
Microbial enumerationAfter PL treatments and before plating, A. acidoterrestris spores were shock-heated for 10min at 80±1°C, in order to stimulate spore germination and to inactivate vegetative organisms10,30,38. Peptone water (0.1%, w/v) tenfold dilution aliquots were surface plated by duplicate onto BAM using a spiral plater (Autoplate 4000, Spiral Biotech, USA). When the treatment resulted in low counts (longer treatment times), up to 3-ml of fruit juice was directly poured into each Petri dish. Plates were incubated for 72h at 43±1°C. A counting grid was used for the enumeration of colonies in the case of spiral plating. Survival curves were generated from experimental data by plotting log N/N0 (where N is the number of CFU/ml at a given time and N0 the initial number of CFU/ml) vs. treatment time.
Mathematical modelingMicrobial inactivation data were fitted with the cumulative form of a Weibull type distribution of resistances29:
where S(t) is the fraction of survivors at a given time and b and n are the scale and the shape parameters, respectively. The b value in the Weibull distribution function represents the rate of inactivation of the cells, while n indicates the concavity of the survival curve (n>1 indicates a downward concavity and n<1, an upward concavity. A log linear shape is a special case when n=1). The values of b and n were then used to generate the resistance frequency curves using the following equation:where tc is a measure of the organism's resistance or sensitivity and dϕ/dtc is the Weibull distribution corresponding to tc. Other statistical parameters which better explains the observed frequencies (distribution mode, tcm; mean, t¯c; variance, σtc2; and coefficient of “skewness”, υ1) were calculated from the following equations29:where Γ is the gamma function. The distribution mode, tcm, represents the treatment time at which the majority of the population dies or is inactivated. The mean, tc corresponds to the inactivation time on average with its variance, σtc2. The “skewness” coefficient, υ1, represents the skew of the distribution.Inactivation curves were also fitted by the biphasic model proposed by Cerf8, which can be formulated as follows,
where f is the fraction of the initial population corresponding to the subpopulation more sensitive to the treatment, (1−f) is the fraction of the initial population corresponding to the subpopulation that is more resistant to the treatment and kmax1 and kmax2 are the specific inactivation rates of the two populations, respectively.Model performance was evaluated using the root mean square error (RMSE); the Akaike information criterion (AIC) and the Bayesian Schwarz criterion (BIC) according to the equations described by Ferrario et al.16. The RMSE, which measures the average deviation between the observed and the fitted values, was used to evaluate the performance of models. The other criteria were used to detect model overfitting. According to Akaike's and Bayesian's theories, the most accurate and parsimonious model yield the smallest AIC and BIC values31. Both criteria are closely related and can measure the efficiency of the parameterized model in terms of predicting the data; however, the BIC criterion is slightly more conservative because the penalty term is greater in BIC than in AIC.
Principal component analysisThe principal component analysis (PCA) was applied to illustrate the spatial relationship among the tested juices and the Weibull or biphasic model parameters. The Cophenetic Correlation Coefficient (CCC) was obtained as a measure of how faithfully the analysis preserves the original Euclidean distances among data points. A good PCA analysis corresponds to a CCC value close to 1.0. The Pearson correlation coefficient (significant level set at p<0.05) was calculated to find correlations between the biphasic model parameters and the IS value.
Multiple regression analysisThe response surface methodology was employed to optimize PL processing. An incomplete three-level two-factor design was adopted. It was assumed that a mathematical function, φ, exists for the response variable, Y (predicted PL treatment time), in terms of two independent process variables X1 (log of initial inoculum size) and X2 (required A. acidoterrestris log reductions, log N/N0). The three levels corresponding to each independent variable were coded as −1 (log IS=3, 0.0 required log reductions), 0 (log IS=4, 1.5 required log reductions) and +1 (log IS=6, 3.0 required log reductions).
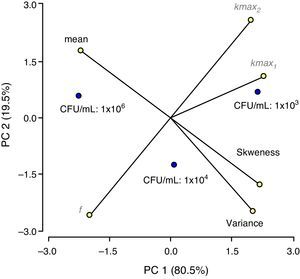
A second order polynomial on two variables was used:
where Y is the response (necessary PL treatment time); xμ,j are the coded independent variables, linearly related to Xμ; and β0, βμ, βμμ and βμj are the regression coefficients. In order to select the best equation, analysis of variance, test of lack of fit, partial F-test for individual parameters and analysis of residuals were performed following the backward selection procedure24. The computer-generated response surface and contour plot were obtained using the best regression equation. Statistical validation analyses were performed using InfoStat 2009 (InfoStat Group, FCA-UNC, Córdoba, Argentina) and Statgraphics Plus para Windows 5.1 (Statistical Graphics Corp., Washington, USA).Results and discussionPL inactivation curvesThe apple juice (pH 3.1±0.1, 13.1±0.1°Bx) was characterized by low particle size (1.00±0.01nm) and few suspended particles (turbidity: 1.18±0.06NTU). The low physicochemical parameters obtained are expected to foster PL efficacy, as previous studies have shown a negative correlation between the log reductions of relevant microorganisms exposed to PL processing (static device, 0–71.6J/cm2) and turbidity, particle size and absorbance at 254nm of freshly pressed strawberry, orange and apple juices14.
During the PL treatments, apple juice temperature increased linearly with time as a consequence of the absorption of light16; however according to the adopted process design (pre-cooled sample with initial temperature 2±1°C), the sample temperature was always below 20°C (data not shown).
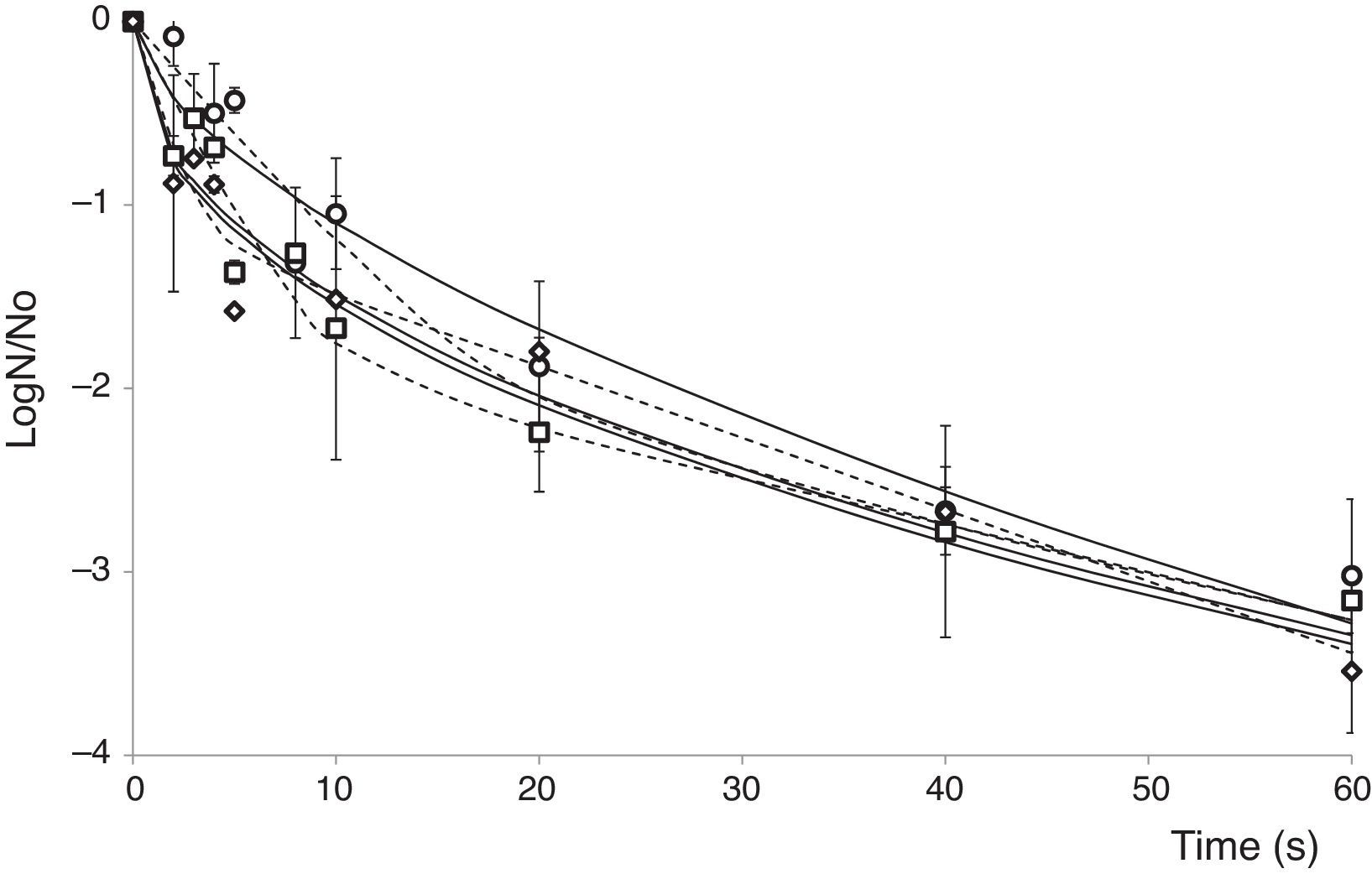
Survival curves of A. acidoterrestris spores in apple juice processed by PL at different doses and different IS (103–106CFU/ml) are shown in Figure 1. All inactivation curves were characterized by a more pronounced decrease during the first 10s of treatment (fluence≤12J/cm2), and then the number of survivors decreased slowly as the treatment time increased (Fig. 1). Inactivation curves exhibited a marked upward concavity, reaching after 60s (71.6J/cm2) of PL treatment between 3.0 and 3.5 log reductions depending on the initial IS value. The upward concavity can be traced back to a less inactivation effect with an increase in the PL dose. Nevertheless, no tail was observed within the dose range. These changes in the inactivation curve shape stem from either different sensitivities in the population or the presence of two subpopulations with different resistance to PL. The upward concavity as well as the more pronounced decrease during the first portion of the survival curve were previously observed16, when the inactivation kinetics of Escherichia coli ATCC 35218, Listeria innocua ATCC 33090 and Salmonella Enteritidis MA44 inoculated in commercial and natural apple (pH: 3.5; 9.5–12.7°Bx) and orange (pH: 3.9, 12.8°Bx) juices processed by PL (batch mode, 0–71.6J/cm2) was evaluated. In contrast to these results, the survival curves in orange and freshly squeezed apple juices exhibited a tail, which could be attributed to the higher absorption of samples in the UV region compared to commercial apple juice16 since the presence of suspended solids impairs the disinfection process.
Experimental survival curves (points) and fitted values derived from the Weibull (
) and biphasic (----) models for A. acidoterrestris ATCC 49025 spores in commercial apple juice treated with PL. (0–71.6J/cm2, temperature range: 2–12°C) at different inoculum (IS) values. CFU/ml: 1×103 (♦), 1×104 (■) and 1×106 (●), (I) standard deviation.Baysal et al.3 examined the efficacy of UV-C light processing (0.5J/cm2) at constant depth (0.15cm) on the inactivation of A. acidoterrestris DSM 3922 spores in white grape (¿absorptivity254nm: 5.8cm−1, 5.5NTU) and apple (¿absorptivity254nm: 12.0cm−1, 10.0NTU) juices. In accordance with our work, the survival curves exhibited upward concavity, and A. acidoterrestris spore reduction significantly depended on the UV dose applied and the type of fruit juice. Between 2.1 and 5.5 log reductions were achieved in apple and white grape juices, respectively. In addition, Chaine et al.9 examined the inactivation of A. acidoterrestris ATCC 49025T spores by PL in a continuous flow (1.5J/cm2) and reported almost 4.0 log reductions in 65°Bx sugar syrup. In contrast, these authors reported a linear inactivation response for A. acidoterrestris spores decay which may be attributed to the continuous flow mode implemented for the PL treatment.
Regarding the influence of IS on PL effectiveness, the reports of the literature are controversial. Gómez-López et al.20 studied Listeria monocytogenes LMG 13305 inactivation by PL (batch mode, 8.4cm distance from the lamp) exposure as a function of the superficial growth degree in nutrient agar. In agreement with the results obtained in this work, a higher inactivation was observed at lower L. monocytogenes initial counts, as 1.0 and 4.0 log reductions were achieved when the initial counts were 1×108 and 1×105CFU/ml, respectively. Conversely, Uesugui et al.37 investigated PL inactivation of L. innocua FSL C2-008 on stainless steel substrates at various levels of inoculum, reporting that microbial inactivation increased with inoculum size. They attributed these findings to cell distribution on the solid surface, in which hidden cells due to surface imperfections may survive; whereas cells that are directly exposed to the PL will be inactivated. Consequently, the increase in inoculum level, also increases the number of cells directly exposed to the PL provoking a higher level of reduction at high inoculum levels.
The EEO calculated for PL decontamination of A. acidoterrestris spores was in the range of 2.9×106–3.3×106kWh/m3/order. Unfortunately, there is little information regarding EEO estimation for reducing the microbial load in juices or other food matrixes processed by PL or UV-C light for comparison purposes. In previous studies, Ferrario et al.12 evaluated PL decontamination efficacy of E. coli ATCC 35218, S. Enteritidis MA44 and Saccharomyces cerevisiae KE 162 inoculated in commercial (pH: 3.5, 11.1°Bx) and freshly squeezed (pH: 3.5, 12.6°Bx) apple juices treated by single PL (0–71.6J/cm2, T<12°C) in batch mode operation. In agreement with the results shown in the present work, similar EEO values were obtained when applying single PL (6.2×106–3.8×107kWh/m3/order) treatment.
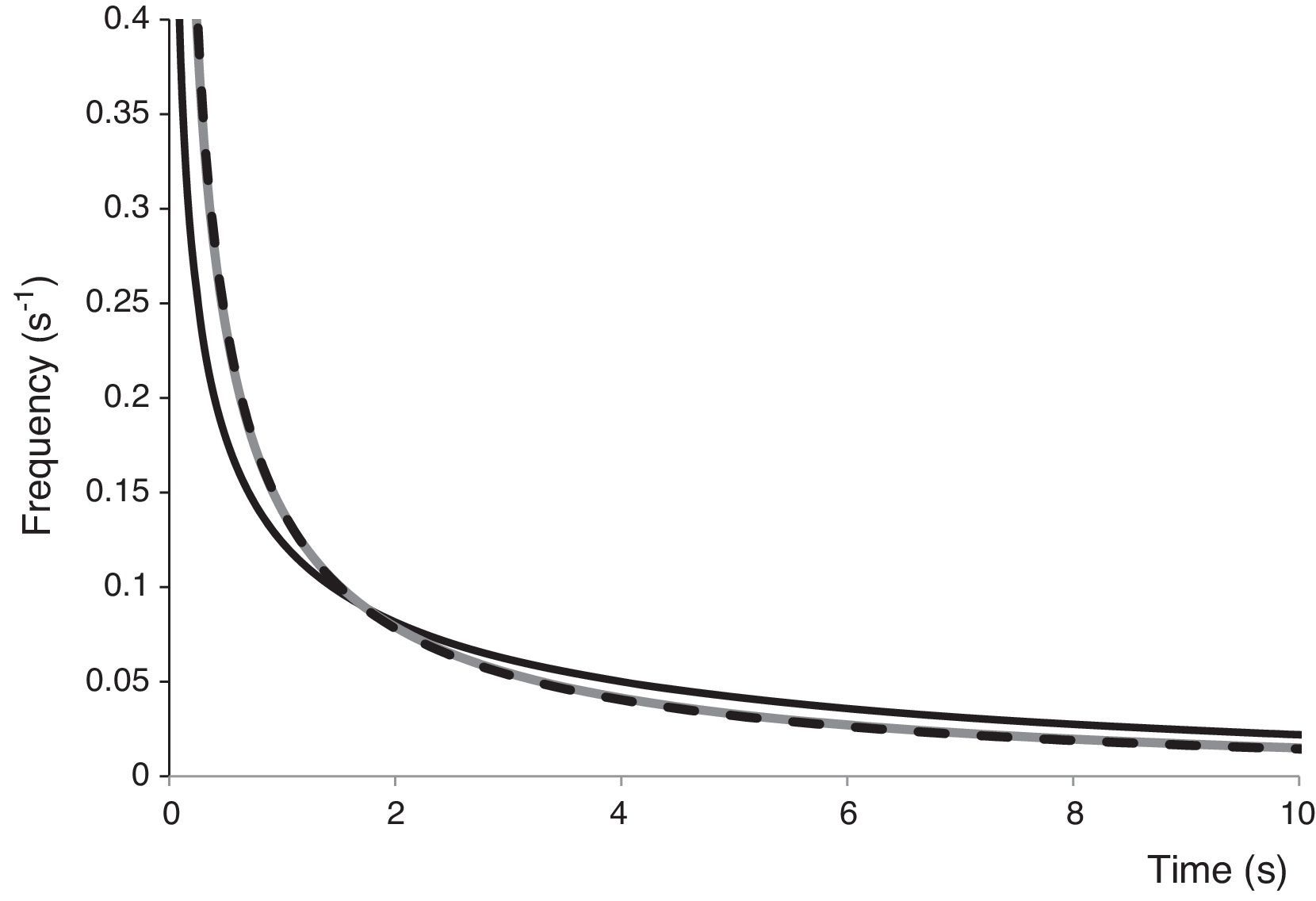
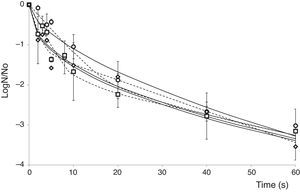
Mathematical modelingThe Weibull distribution of resistances model was appropriate for representing A. acidoterrestris spore inactivation by PL, exhibiting high Radj2 values, which indicate that 94.0–96.0% of the variation in the experimental data could be explained by the selected model (data not shown). All survival curves showed n values less than 1 (Table 1), as expected according to the notorious upward concavity. The b and n parameters were used to generate the frequency distributions of resistances (Fig. 2) and to calculate the associated statistics: mode, mean, variance and coefficient of skewness for obtaining a better explanation on the effect of PL on spore inactivation of microorganisms. All distributions of resistances lacked mode and were strongly skewed to the right, showing that the majority of cells in the population were sensitive to PL treatment at very low doses (Fig. 2). Frequency distributions corresponding to 1×103 and 1×104CFU/ml IS values completely overlapped; whereas, the distribution corresponding to 1×106CFU/ml IS value exhibited a slightly heavier tail and higher variance, due to the lower efficacy of PL decontamination at higher IS values. Higher mean values and lower skewness coefficients were obtained when IS increased (Table 1), indicating that the PL treatment became less effective. Similarly, previous studies12 characterized inactivation curves of E. coli ATCC 35218, L. innocua ATCC 33090 and S. Enteritidis MA44, inoculated in commercial and freshly squeezed apple juices, and treated by PL in batch mode (71.4J/cm2) by the Weibull model. Likewise, frequency distributions lacked mode and were strongly skewed to the right.
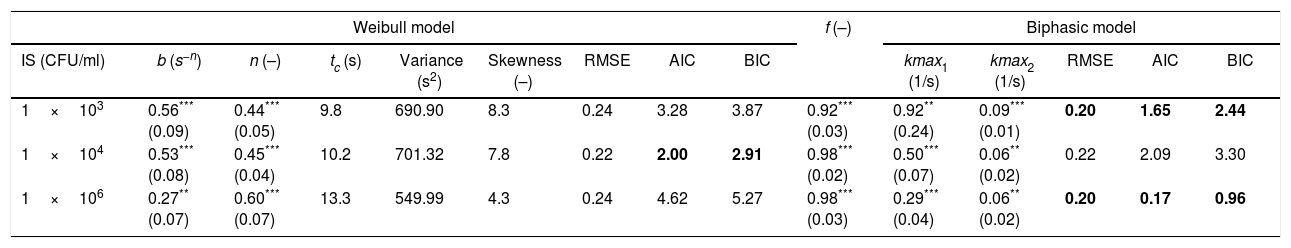
Estimated parameters of the Weibull and biphasic models, Weibull model-related statistics,a and corresponding values of the associated statistics for model performance, minimum RSME, AIC and BIC to characterize Alicyclobacillus acidoterrestris ATCC 49025 spore inactivation in apple juice treated with PL during 60s
| Weibull model | f (–) | Biphasic model | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IS (CFU/ml) | b (s−n) | n (–) | tc (s) | Variance (s2) | Skewness (–) | RMSE | AIC | BIC | kmax1 (1/s) | kmax2 (1/s) | RMSE | AIC | BIC | |
| 1×103 | 0.56*** (0.09) | 0.44*** (0.05) | 9.8 | 690.90 | 8.3 | 0.24 | 3.28 | 3.87 | 0.92*** (0.03) | 0.92** (0.24) | 0.09*** (0.01) | 0.20 | 1.65 | 2.44 |
| 1×104 | 0.53*** (0.08) | 0.45*** (0.04) | 10.2 | 701.32 | 7.8 | 0.22 | 2.00 | 2.91 | 0.98*** (0.02) | 0.50*** (0.07) | 0.06** (0.02) | 0.22 | 2.09 | 3.30 |
| 1×106 | 0.27** (0.07) | 0.60*** (0.07) | 13.3 | 549.99 | 4.3 | 0.24 | 4.62 | 5.27 | 0.98*** (0.03) | 0.29*** (0.04) | 0.06** (0.02) | 0.20 | 0.17 | 0.96 |
tc, distribution of the mean, variance and skewness. Boldface RSME, AIC or BIC value is the best value in the row for model comparison.
Several studies reported that the Weibull model could quantitatively describe microbial inactivation by PL in both liquid12,32,37 and solid substrates4,23; and in particular, was appropriate for representing the inactivation of A. acidoterrestris vegetative cells and spores by emerging technologies3,6,39.
The biphasic linear model adequately characterized the survival curves as shown by the high Radj2 values obtained, ranging between 94.0% and 96.0% (data not shown) and low RMSE values (Table 1). The kinetic parameter kmax1 was always significantly greater than parameter kmax2, indicating a very high inactivation rate during the first seconds of treatment. A negative correlation (Pearson correlation coefficient: −0.76) in the inactivation rate of the sensitive subpopulation (kmax1) was observed by a decreased IS. Whereas, no changes in the proportion between sensitive and resistant subpopulations (f) neither inactivation rate kmax2 were recorded with an increased IS.
Based on the RMSE values, the Weibull and biphasic models presented equivalent adequacies. The biphasic model showed best performance with two smaller RSME, AIC and BIC values of the three systems analyzed.
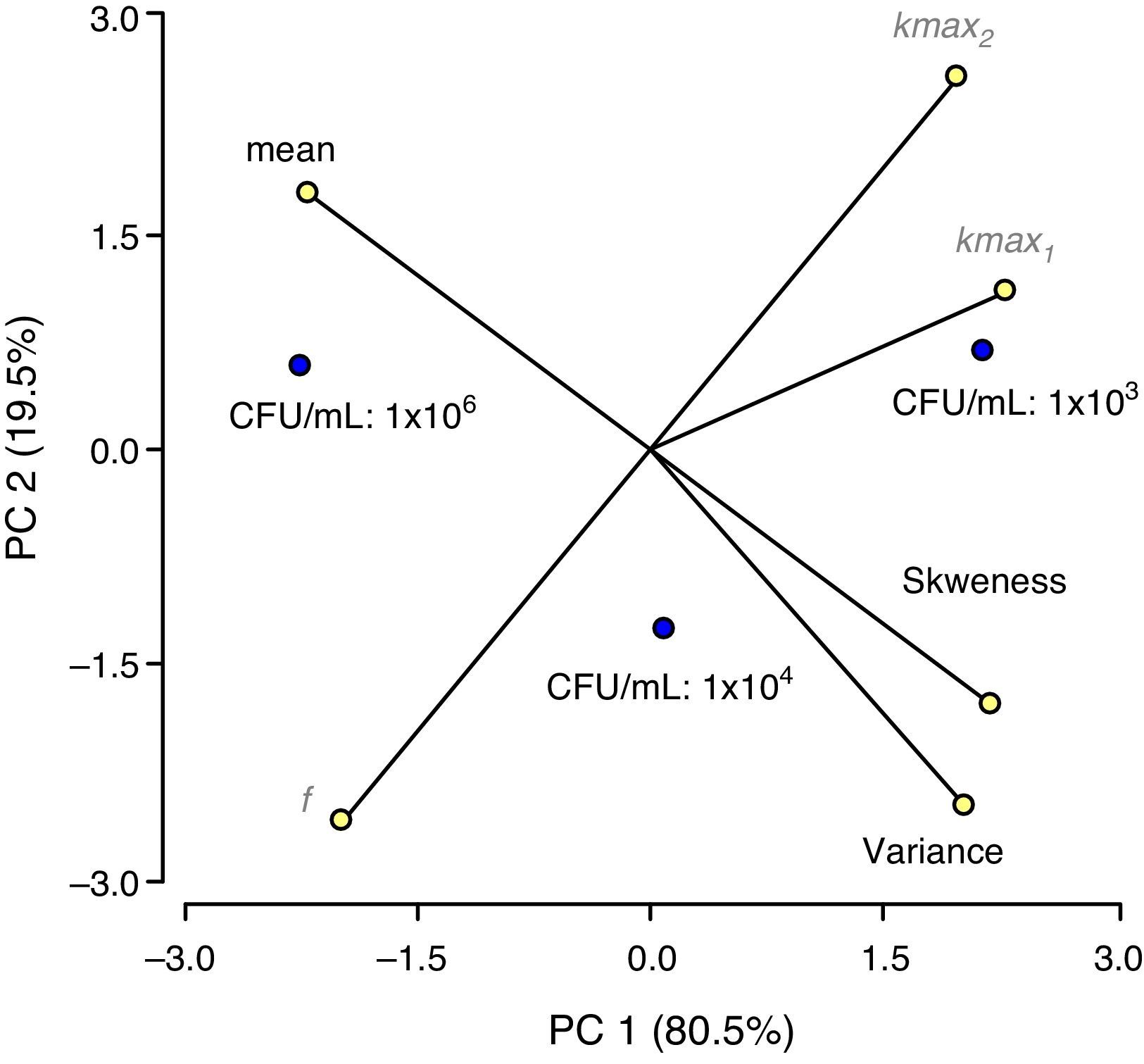
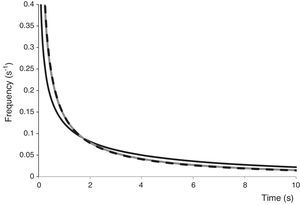
Principal component analysis (PCA) analyzing the interdependence between IS and model parametersA multivariate approach to data analysis by principal components (PCA) showed the spatial relationships among estimated parameters or statistics arising from the Weibull and biphasic models fitting to PL inactivation curves with different IS. The two-dimensional representation (PCA bi-plot) is presented in Figure 3 for both model parameters. The CCC value was 1.00, showing that an accurate reduction was achieved with the analysis, and only the first two principal components (PC1 and PC2) were retained as they explained 100% of the total variance. PC1 was positively associated with kmax1, kmax2, variance and skewness, and negatively with f and mean, while PC2 separated f and variance (negatively associated) from kmax2 (positively associated). PCA showed that the lowest IS assayed (1×103CFU/ml) was strongly associated with kmax1 and displayed high kmax2 and skewness while exhibiting the lowest mean values; followed by IS of 1×104CFU/ml, and finally by 1×106CFU/ml. That is, the lower the IS value, the higher the inactivation rates of both the sensitive and the resistant subpopulations; while their frequency distribution of resistances displayed lower mode and higher skewness values.
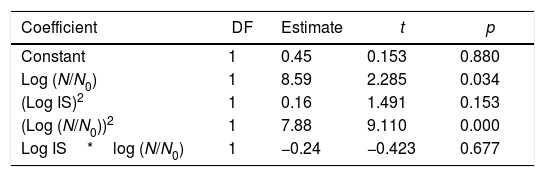
Multiple regression analysisThe effect of initial log IS and required log (N/N0) of A. acidoterrestris spores on the predicted PL time was expressed by means of a second order polynomial equation. The proposed model was adequate to describe experimental data as satisfactory values of Radj2 (97.0%) were obtained. The quadratic term log (N/N0)2 turned out to be the most significant parameter influencing PL treatment time (p<0.0001) impairing a remarkable curvature to the response surface, followed by the linear term log (N/N0) (p<0.05) and the quadratic term (log IS)2 (p<0.05) (Table 2). The best second grade polynomial equation predicting the necessary PL treatment time with coded initial A. acidoterrestris spore log concentration (log IS) and required log reductions (log N/N0) is described as follows:
Values of the second-order polynomial regression coefficients representing the relationship between the necessary PL treatment time and the independent variables log initial inoculum size (log IS) and required log reductions (log (N/N0)) of Alicyclobacillus acidotrerrestris ATCC 49025 spores in apple juice
| Coefficient | DF | Estimate | t | p |
|---|---|---|---|---|
| Constant | 1 | 0.45 | 0.153 | 0.880 |
| Log (N/N0) | 1 | 8.59 | 2.285 | 0.034 |
| (Log IS)2 | 1 | 0.16 | 1.491 | 0.153 |
| (Log (N/N0))2 | 1 | 7.88 | 9.110 | 0.000 |
| Log IS*log (N/N0) | 1 | −0.24 | −0.423 | 0.677 |
DF, degree of freedom; t, Student's statistic; p, significance.
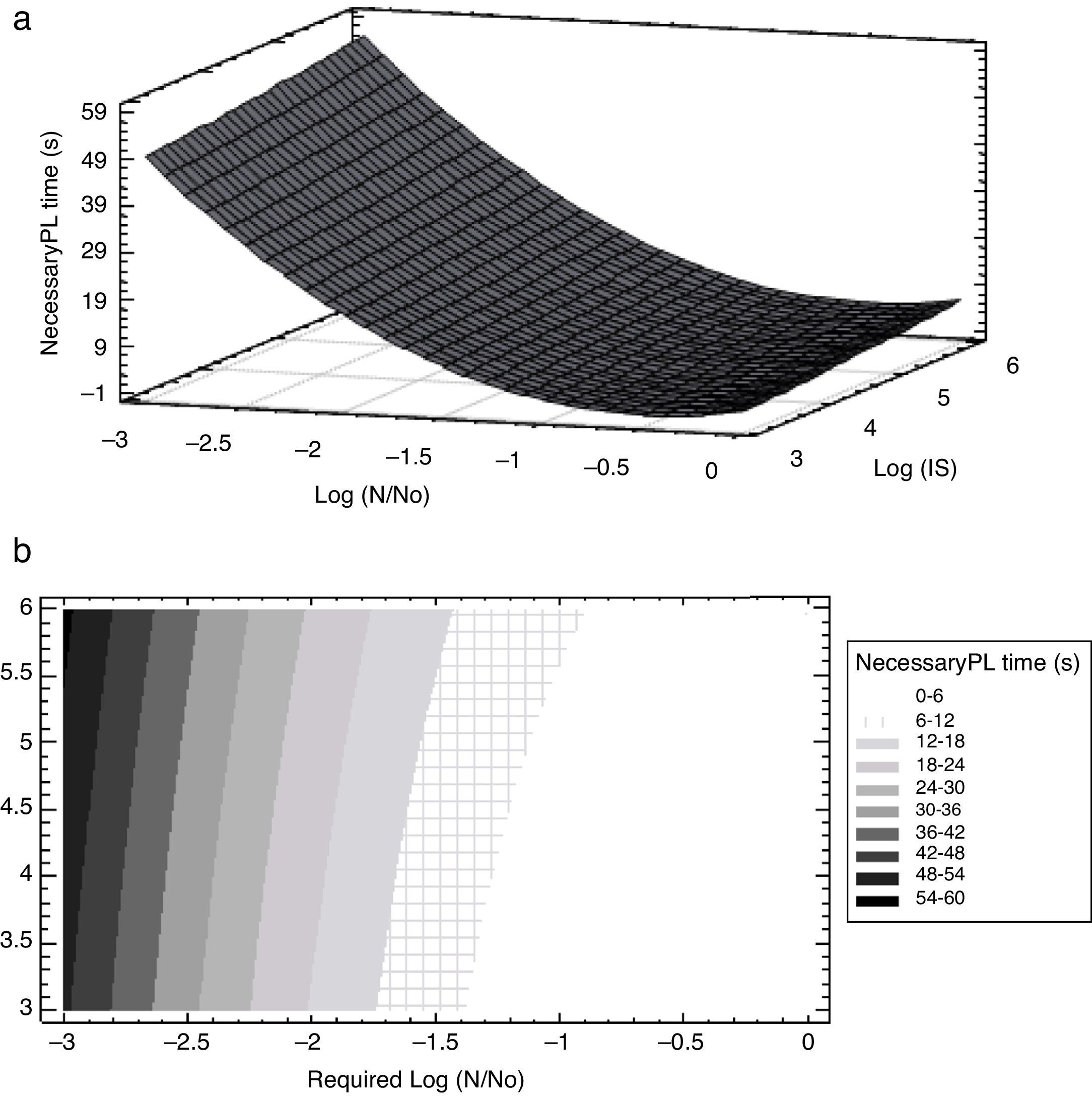
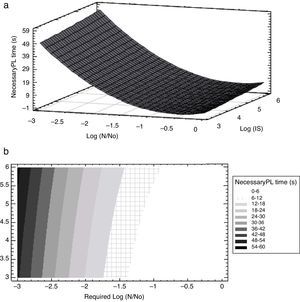
The analysis of variance showed that the linear log N/N0, and quadratic terms (log N/N0)2 and (log IS)2 mostly influenced PL treatment time whilst the cross-product term log IS*log (N/N0) was non-significant (Table 2). Additionally, the analysis of the sum of squares from all the contributions of each variable on PL treatment time showed that the required reduction, log (N/N0), was the most important factor influencing the response, PL treatment time. Figure 4 illustrates the response surface and the corresponding contour plot which relate the initial concentration of A. acidoterrestris spores (log IS) in apple juice, the required log reductions log (N/N0) and the response, the necessary PL treatment time. Required microbial reductions higher than 1.4 log cycles involved a steep ascent on PL treatment time, highlighting the relevance of the quadratic terms in the predictive equation (Fig. 4a). In order to reach a desired inactivation, a lower treatment time was required by reducing inoculum size (log IS). Nevertheless, log (N/N0) was the most important factor predicting PL treatment time (Fig. 4a). Particularly, less than 6s of PL treatment were needed to reach 1 log spore reduction despite the IS value. However, in order to reach between 1.1 and 3.0 log reductions, PL treatment time increased fast and depended on the considered IS value (Fig. 4b). Therefore, the highest dependence of PL treatment time on IS was observed at low required spore reductions, i.e. less than 6s were needed to reach 1.4 log reductions when IS was 1×103CFU/ml while 6–12s were required for higher inoculum concentrations; whereas, in order to reach 2.9 log reductions 42–48s or 48–54 were needed for 1×103–1×104CFU/ml or 1×106CFU/ml concentrations, respectively.
The present work contributed to understanding the influence of initial spore contamination on PL disinfection efficacy. The Weibull and biphasic models adequately characterized A. acidoterrestris ATCC 49025 spore inactivation by PL in apple juice with different IS values. The Weibull model, which considers that the whole population exhibits a distribution of sensitivities toward an inactivation stress factor, allowed to know the frequency distribution of resistances to the PL treatment. Likewise, the biphasic model gave additional information regarding the inactivation rates corresponding to the sensitive and resistant subpopulations. A secondary polynomial model showed that the initial A. acidoterrestris spore contamination and the required log reduction achieved in apple juice contributed to the prediction of the necessary PL treatment time. This study contributed to a better understanding of PL inactivation, which is expected to lead to a better appreciation of this technology by potential users.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Conflict of interestThe authors declare that they have no conflicts of interest.
The authors would like to acknowledge the financial support from Universidad de Buenos Aires (2013-X045 Project), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) (2012-289 Project) and Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) (2011-0288 Project) of Argentina and from Banco Interamericano de Desarrollo (BID).