In this study we developed an indirect ELISA to detect antibodies against Minute Virus of Mice (MVM) using an antigen produced from BHK-21 cells infected with a prototype strain of the virus. The optimal antigen concentration and serum dilutions were established. In order to analyze variability in the laboratory, reproducibility and repeatability within and between plates were determined. Then, a panel of 460 sera from conventional facilities and previously classified as positive or negative by the indirect fluorescent antibody assay was analyzed. The cutoff value was determined by a receiver operating characteristic (ROC) curve. The results of the indirect ELISA were compared with those of the indirect fluorescent antibody assay. The ELISA assay showed 100% sensitivity and 99% specificity. ELISA is a useful tool to be developed in standard virology laboratories and can be used for screening animals faster than the traditional indirect fluorescent antibody assay.
Se desarrolló un ELISA indirecto para detectar anticuerpos contra el virus diminuto del ratón (Mice minute virus [MVM]), utilizando un antígeno producido a partir de células BHK-21 infectadas con la cepa prototipo del virus. Se establecieron las diluciones óptimas de antígeno y el suero a utilizar. Para analizar la variabilidad en el laboratorio, se determinaron la reproducibilidad y la repetibilidad dentro de una placa y entre placas. Luego se analizaron 460 sueros provenientes de bioterios convencionales y clasificados previamente como positivos o negativos por inmunofluorescencia indirecta. El valor de corte se determinó mediante una curva ROC. Los resultados se compararon con los obtenidos con la prueba de inmunofluorescencia indirecta. El ELISA mostró 100% de sensibilidad y un 99% de especificidad. Esta técnica demostró ser una herramienta útil para desarrollar en laboratorios de virología estándar y puede utilizarse como prueba tamiz para seleccionar animales de manera más rápida que con la tradicional prueba de inmunofluorescencia indirecta.
The presence of infectious agents represents a serious problem in laboratory animal colonies. Some of these agents can induce clinical signs and subclinical or asymptomatic infections. Minute Virus of Mice (MVM) and Mouse Parvovirus (MPV) are two highly prevalent infectious agents in mice colonies2. MVM is a self-limiting virus and antibodies are detected up to 4 weeks after infection in immunocompetent animals6; however, experimental infection will result in severe damage to multiple organs mainly in developing fetuses and neonates9. Infection with an immunosuppressive strain of MVM can result either in runting or in death, depending on the virus dose and mouse strain3,5. Clinical disease or histological lesions have not been observed in naturally or experimentally infected mice with MPV; however, this parvovirus is considered an important pathogen since it produces T-lymphocyte dysfunction, alters graft patterns and causes tumor rejection3. MPV persists for 9 weeks in immunocompetent murine tissues and seems to occur in a low prevalence within mouse colonies9. MVM and MPV can modify physiological parameters and produce significant changes in experimental results8,10. They can directly reduce the tumor growth rate, prevent its development or alter the modulation of the immune response against tumoral cells5. In addition, in immunological studies, MVM and MPV can interfere with the modulation of lymphocytes and the generation of ascites. These viruses interfere in infectious diseases and cell biological studies, and can also alter patterns of rejection of skin allografts10. Since MVM and MPV are resistant to inactivation there is a great risk of transmission by feces, urine and contaminated supplies (cages, litters, beds, food, etc.). Like all parvoviruses, MVM and MPV replicate in proliferating cells. For all these reasons, the development of sensitive detection techniques may allow to know how efficient barrier systems in mice colonies are, and it may also help to obtain the prevalence data of these viruses to know the magnitude of the infection in order to implement the appropriate control measures. The first isolation of MVM was obtained from a mouse adenovirus stock, which was then grown in a mouse embryo culture, plaque purified in mouse fibroblasts without forced adaptive passages and renamed MVM prototype (MVMp)13. Other three MVM strains have been described: MVMi (an immunosuppressive variant isolated from transplantable mouse lymphoma), MVMc (a cutter strain isolated as a contaminant of BHK-21 cells) and MVMm (a Missouri strain isolated from naturally infected B-cell-deficient mice). MVMp and MVMi are 97% homologous at the genomic level and serologically indistinguishable and the most studied strains. Recent studies indicate that several factors, including age, viral dose, mouse strain and for some strains even gender, influence the serological response4. Five strains of MPV (MPV-1, 2, 3, 4 and 5) have been described and several genotypic variants (MPV-1a, 1b, 1c, 1d, 1e and 1UT) of MPV1 were recognized. MPV-1 is most closely related genetically to MVM strains5. MVM and MPV are composed of two structural proteins, capsid proteins VP2 and VP1, and of two non-structural-proteins, NS1 and NS2. VP1 and VP2 vary between both viruses and determine the serogroup for a particular strain while NS1 and NS2 are well conserved between these viruses. For example, between NS1 of MVM and MPV there is more than 90% identity6. Currently, MVM and MPV are monitored by serological detection of antiviral antibodies in the host6. The hemagglutination inhibition test was the first technique used for antibody detection but requires large amounts of viral antigen. More recently the two most used serological techniques are the indirect fluorescent antibody assay (IFA) and the enzyme-linked immunosorbent assay (ELISA)1.
Antigens used for IFA and some antigens for ELISA are produced with MVM-infected cells and contain NS proteins as well as VP. For that reason it is difficult to distinguish whether mice are infected with MVM or MPV. Antigens used for conventional ELISA and produced with MVM virions mainly contain VP and also include small amounts of NS proteins. The IFA is a specific and useful method to analyze a small number of serum samples. ELISA is a good screening technique and its use could be systematized as a routine control for a higher number of samples6.
In this work we describe the development of an indirect ELISA (iELISA) that would be useful for MVM screening in mice colonies, using an antigen of easy production from BHK-21 cell cultures infected with the virus.
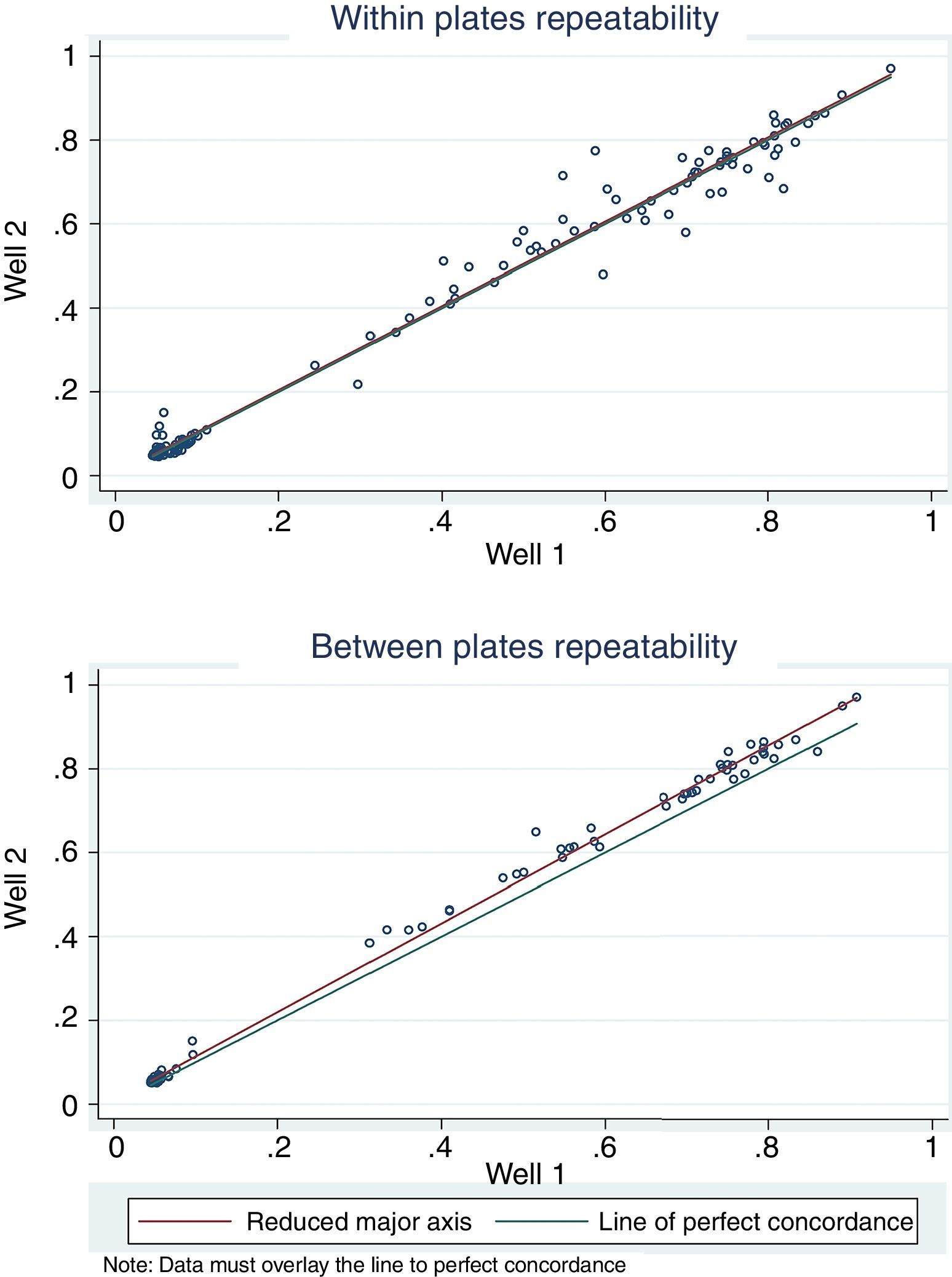
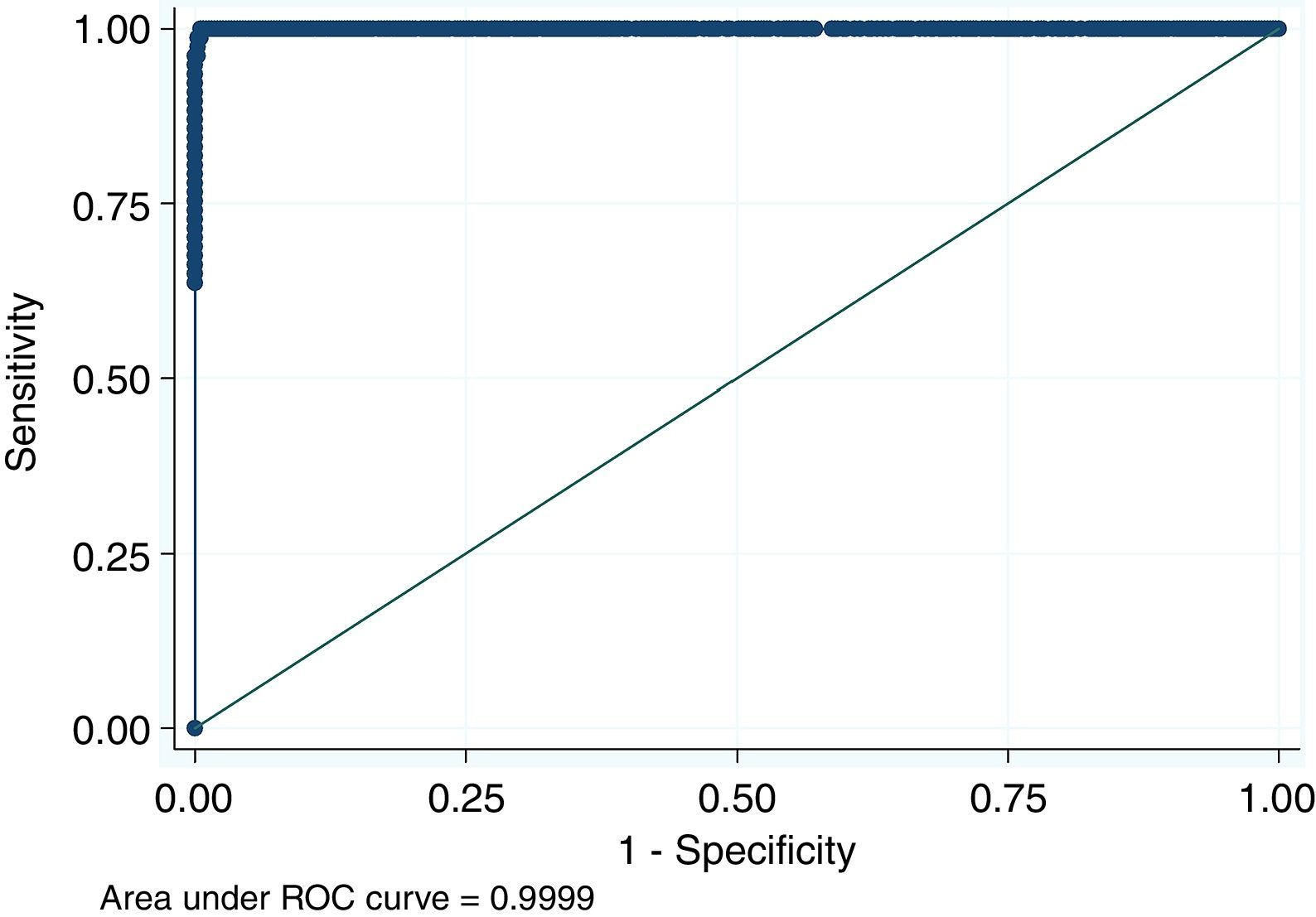
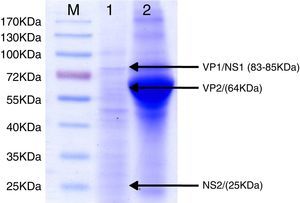
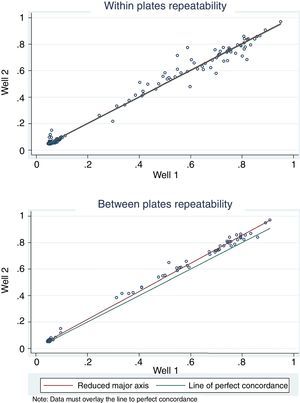
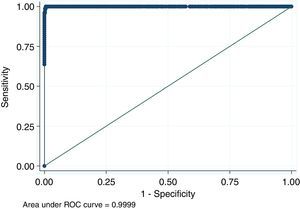
For antigen preparation, MVMp (ATCC® VR-1346) was provided by the Multidisciplinary Center for Biological Research on Laboratory Animal Science (CEMIB, University of Campinas of Brazil). The strain was propagated on baby hamster's kidney (BHK-21) cells (Argentine Cell Bank Association, Argentina), grown in Minimum Essential Medium (MEM) (Gibco, Invitrogen, USA) supplemented with 2mM glutamine (Gibco, Invitrogen, USA), 100IU/ml penicillin, 100μg/ml streptomycin (Ritchet, Buenos Aires, Argentina), 100IU/ml nystatin (Parafarm, Buenos Aires, Argentina) and 10% fetal calf serum, which was reduced to 2% for maintenance of cells. BHK-21 cells preserved in liquid nitrogen were thawed and used for no more than three passages. Cells were harvested with trypsin (0.25% (w/v))–Ethylene Diamine Tetraacetic Acid–EDTA (0.2% (w/v)) (Sigma–Aldrich, USA). Then, individual T-75 flasks of BHK-21 cells (3.75×106 cells in 20ml of growth MEM) were infected (“Inf”) with 300μl of viral stock solution (hemagglutination (HA) titer of 1:128) at three different times: 0h (“Inf 0”), 12h (“Inf 12”) and 24h (“Inf 24”) of development of the cell monolayer. In “Inf 0” cells, a cytopathic effect was observed at 24h post-infection and the supernatant that was collected at 36h of culture was measured by HA. In the other two experiments (“Inf 12” and “Inf 24”) a cytopathic effect was not observed and the HA test was negative from culture supernatants developed daily until 5 days post-infection. The experiments were repeated three times in order to confirm the results. Five hundred ml of infectious supernatant (HA titer of 1:2048) of “Inf 0” cells were used for antigen preparation. Cell debris was clarified by centrifugation at 4000×g for 30min and then the viral particles were concentrated by centrifugation at 25000×g for 5h at 4°C. The pellet was suspended in 1ml of phosphate buffered saline (PBS) before being layered on a discontinuous 20–60% (w/w) sucrose gradient and centrifuged as a concentration step. The visible band was collected (∼2ml), diluted in the same volume of PBS, concentrated by centrifugation as previously described, and then the pellet was diluted in 500μl of PBS in order to determine protein concentration (Fluorometer Qubit 2.0, Invitrogen, Life Technology, Japan). An assay of SDS–PAGE was developed with first infectious supernatant and with the final antigen, confirming the presence of bands of molecular weight similar to viral proteins in the antigen (Fig. 1). The optimal antigen dilution was established by a checkerboard titration with positive and negative reference serum provided by CEMIB, University of Campinas of Brazil. The antigen was coated in 96-well ELISA plates (NUNC Maxisorp, Hamburg, Germany) ranging in concentration from 6.24 to 0.003μg/ml in a final volume of 100μl/well of 50mM in carbonate/bicarbonate buffer (pH 9.6). The plates were incubated overnight at 4°C. After removing excess unbound antigen, 100μl of blocking solution (PBS – 0.1% bovine serum albumin) were added to each well and incubated for 1h at 37°C. Plates were washed five times with PBS plus 0.5% Tween-20 (PBST). Positive and negative reference serum samples were diluted serially from 1:5 to 1:640 (50μl/well) in PBST plus 5% skim milk powder and incubated for 1h at laboratory under proper controlled conditions (22–24°C). Then, plates were washed five times with PBST. Anti-mouse IgG peroxidase (Sigma–Aldrich, St Louis, MO, USA) was used as secondary antibody according to the manufacturer's instructions and incubated for 1h in the laboratory under proper controlled conditions (22–24°C). ABTS (30mg) substrate (2,2′-azino-di-[3-ethyl-benzothiazoline-6-sulphonic acid]) (Sigma–Aldrich, St Louis, MO, USA) was diluted in 50ml of citrate acid buffer (0.1M) plus 50ml of sodium phosphate (0.2M) and 10μl of 30% H2O2. Optical density (OD) values were determined using an ELISA reader (Thermo Scientific Multiskan FC, Vantaa, Finland) at 405nm at room temperature after 30, 40 and 60min of incubation. Dilutions of antigen 1:1600 (195ng/ml) and 1:3200 (97.5ng/ml) were selected since they gave the maximum difference of OD between positive and negative reference serum samples. Optimal dilution of serum was obtained by analysis of these two dilutions of antigen with 2-fold serial dilutions of an initial 1:5 dilution of positive and negative reference serum and four sera (two negative and two positive) from conventional facilities and classified by IFA. In order to investigate variability in the laboratory, reproducibility and repeatability within and between ELISA plates were determined. To this purpose, four trials on different days with forty four mice sera from conventional facilities and two MPV positive control sera provided by CEMIB, University of Campinas of Brazil, were analyzed twice each by duplicate using a working dilution previously determined12. These sera were previously classified as positive or negative using the IFA as gold standard test. The dispersion of the mean of OD values of each serum was analyzed by a coefficient of variation (CV) with 20% tolerance. The concordance correlation coefficient (CCC) was used to evaluate repeatability. All the analysis was carried out using the statistical package Stata11®. The CV for all plates ranged between 4.84% and 17.92%. Repeatability within and between plates was 0.998 (CI 95% 0.997–0.998) and 0.996 (CI 95% 0.993–0.998), respectively (Fig. 2) indicating acceptable values according to the standard empirical criteria suggested by Jacobson (1998)4. Finally, the 1:3200 dilution of antigen (97.5ng/ml of total proteins) was used considering that the same serum showed higher OD than with 1/1600 dilution. A panel of 460 sera from conventional facilities of Argentina and classified as positive or negative by the IFA was analyzed in a previously determined 1:10 dilution. The blocking and washing solutions were the same as those already described. An incubation time with substrate of 40min was selected for all analysis. The results were normalized by using the following formula: OD=OD−Nt/Pst−Nt, where OD is the mean OD value of each simple serum determined by duplicated wells, and Pst and Nt are the mean OD values of the positive and negative control serum samples, respectively11. The cutoff value of 0.3 was determined by the ROC curve. The results of the ELISA assay were compared with those of the IFA as the gold standard technique. The iELISA showed 100% sensitivity and 99% specificity (Table 1, Fig. 3). Three negative sera by the IFA were detected as positive by ELISA. As the reading of OD values for each serum has been statistically corroborated by CCC and CV we think that those results were indeed due to the conjunction of possible variations in both techniques. A percentage of 18.75 and 18.13% of the analyzed sera were found to be positive by iELISA and IFA, respectively.
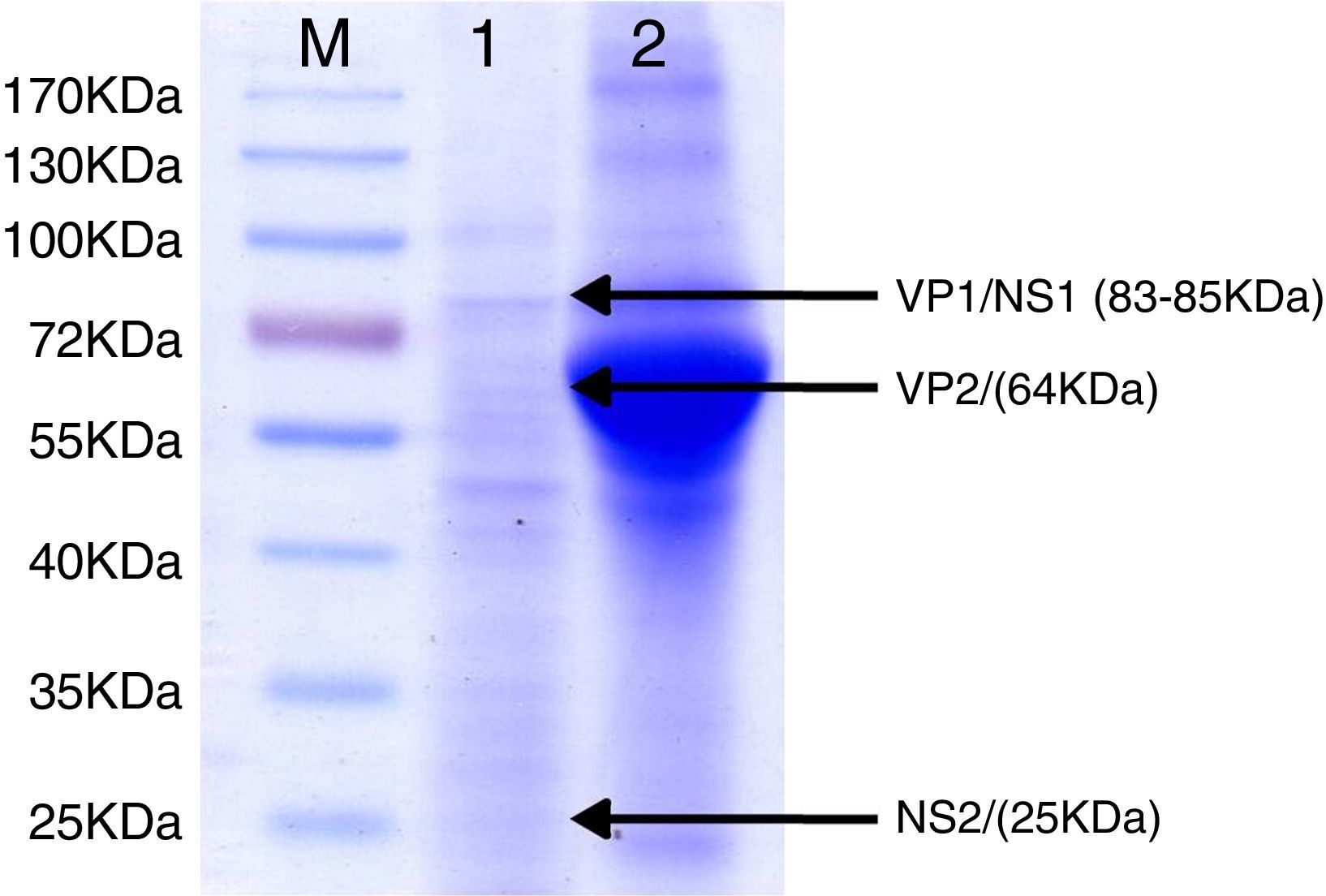
SDS–PAGE analysis of ELISA antigen. Lane M: molecular weight marker proteins with the sizes indicated. Lane 1: 1:100 dilutions of final antigen for ELISA. Arrows indicate bands of viral proteins and molecular weight in brackets: VP1/NS1 (83–85kDa), VP2 (64kDa) and NS2 (25kDa). Lane 2: supernatant of 36h of culture of BHK-21 cells infected simultaneously with an MVM prototype strain. A broad band of 66kDa corresponding to bovine serum albumin can be observed.
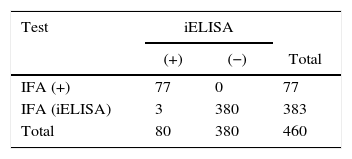
Comparison of results obtained from 460 mice sera of conventional facilities analyzed by the indirect fluorescent antibody test (IFA) and the indirect enzyme-linked immunosorbent assay (iELISA)*
| Test | iELISA | ||
|---|---|---|---|
| (+) | (−) | Total | |
| IFA (+) | 77 | 0 | 77 |
| IFA (iELISA) | 3 | 380 | 383 |
| Total | 80 | 380 | 460 |
MVM and MPV are the most prevalent viruses in mouse colonies; therefore, routine screening is always needed. The IFA was used for several years in many countries with satisfactory results; however, it is a laborious technique on a high number of samples. In this work we developed an iELISA with an antigen yielder and easy to obtain. Growth of MPV in cell cultures is difficult; however MVM can grow in specific cell lines6. We used a MVMp strain and BHK-21 cells for antigen production in contrast with other antigens prepared from murine-infected cell cultures (L929), since BHK-21 showed a fast growing cell line in the first 12h. Furthermore, the method to obtain the virions was simple, and high speed centrifugation for purification was not necessary. The best antigen was achieved when trypsinized cell cultures were seeded in bottles and infected with MVMp simultaneously, possibly since most of the cells were in the S-phase of cell cycle interphase. In the other two experiments the lack or decrease of cells in the S-phase did not cause productive viral replication and for that reason the cytopathic effect was not observed and HA activity was not detected from the culture supernatants. In the last years, alternative ELISA were developed in several laboratories for the detection of specific antibodies against MVM or MPV by using recombinant VP and NS proteins. Some authors highlight the fact that ELISA with recombinant NS protein lacks sensitivity for serodiagnosis and for detection of infection. Therefore, they proposed recombinant VP2 and NS1 proteins of MVM with VP2 protein of MPV combinations3,6,8. The use of baculovirus-expressed recombinant proteins for antigen production is not always reproducible in standard virology diagnostic laboratories; this is the reason why our first step to develop this iELISA was to obtain an antigen of easy production using infected cell cultures. It could be obtained in less than 72h using basic virology methods and an infected cell line. The iELISA test developed here has shown to be specific, making it possible to rapidly test large number of samples to detect MVM antibodies in murine colonies. One preliminary assay analyzing two MPV control positive sera by IFA that were also detected positive by this iELISA could indicate cross-reactivity. This finding may be due to the fact that although conventional antigens contain high amounts of VP, they also include small amounts of NS proteins that could be responsible for the cross-reaction6. To confirm this first result, a higher number of sera should be analyzed comparatively with more specific antigens for MPV7 and our iELISA. The serological results obtained are consistent with those previously reported7 and, are probably the result of the lack of implementation of barrier systems in most conventional facilities of Argentina. In contrast, in the United States of America and some European countries, the percentage of positive animals (1–3 and 2–8%, respectively), is lower surely because most institutions produce rodents free of specific pathogens5,11. In addition, other authors also suggested that seroprevalence is variable among different murine strains3,4,6. Parvoviruses are one of the most common laboratory rodent infectious agents. Serological results indicate that MVM is circulating in conventional facilities of Argentina. With this iELISA the surveillance of mice colonies in Argentina could be increased to know their real health status. In addition, it will contribute to prevent the transmission among animal facilities and to minimize its interference in research studies. Serological techniques remain the most effective methods of screening mouse populations for viral infections. Several factors contribute to the problem of controlling MVM infections, including the existence of different virus strains as well as factors associated with the host (e.g. genetic background, age, gender)4. MVM infections continue to be a problem in laboratory mice facilities; therefore, sensitive and specific techniques must be continuously developed.
Ethical disclosuresProtection of human and animal subjectsThe authors state that the procedures followed conformed to the ethical standards of the responsible human experimentation committee and in agreement with the World Medical Association and the Declaration of Helsinki.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe author(s) declared no potential conflicts of interest respecting to the research, authorship, and/or publication of this article.
We thank with affection, Dr. R Giglioli from Multidisciplinary Center for Biological Research on Laboratory Animal Science (CEMIB), University of Campinas, Brazil (UNICAMP- Brazil), for providing the MVMp strain, positive and negative reference serum to MVM and positive to MPV. We also thank The Japan International Cooperation Agency (Project PROVETSUR) for providing Stata License. We would like to thank the technicians Mr Claudio A. Leguizamón and Mrs Adriana Conde for their supporting help.
This study was supported by grants from the Department for Science and Technology of the National University of La Plata (Project 11V/225) and the Scientific Research Commission of Buenos Aires Province (Act 1395/13), Buenos Aires, Argentina.