Chagas disease, caused by the protozoan Trypanosoma cruzi, is an important public health concern in areas extending from South America northward into the southern United States of America. Although this hemoflagellate has many wild and domestic mammalians reported as reservoir hosts, studies on this subject are scarce in Nuevo León state, a region located in northeastern Mexico. This cross-sectional study showed that the general prevalence of T. cruzi infection in Nuevo León state was 14.5% (35/241), this percentage matching the ones determined by PCR and traditional diagnostics. Localities and infected mammals did not significantly differ (χ2=6.098, p=0.192); however the number of infected animals was highly correlated with mammalian species (p=0.009). Striped skunks (Mephitis mephitis) were found to be the most infected overall (11/34, 32.3%), while dogs (Canis familiaris) had the lowest prevalence. In conclusion, although the prevalence of T. cruzi infection in small mammals was lower in Nuevo León than in other states of Mexico, our results provide new locality records, including striped skunks, opossums (Didelphis marsupialis) and dogs, and extend the recorded area to woodrats (Neotoma micropus).
La enfermedad de Chagas, causada por el protozoario Trypanosoma cruzi, es un problema importante para la salud pública en una vasta región que se extiende en dirección norte desde Sudamérica hasta el sur de los Estados Unidos de América. Aunque este hemoflagelado tiene muchos mamíferos silvestres y domésticos reportados como reservorios, los estudios sobre este tema son escasos en el estado de Nuevo León, localizado en el noreste de México. Se efectuó un relevamiento de la prevalencia de T. cruzi en pequeños mamíferos cubriendo 9 municipios del estado de Nuevo León y 3 tipos de asentamiento (rural, suburbano y urbano). Se observó una prevalencia general de la infección del 14,5% (35/241) usando PCR y diagnóstico tradicional para detectarla. No se determinó una asociación estadísticamente significativa entre las localidades relevadas y las especies de mamíferos infectados (χ2=6,098, p=0,192); sin embargo, el número de animales infectados se correlacionó con la especie de mamífero (p=0,009). La mayor prevalencia de T. cruzi se detectó en los zorrillos (Mephitis mephitis) (11/34; 32,3%); la menor (13/136; 9,5%), en los perros (Canis familiaris). La prevalencia de la infección por T. cruzi en pequeños mamíferos fue más baja en Nuevo León que en otros estados de México. Estos resultados proveen nuevos registros de localidad incluyendo zorrillos, zarigüeyas o tlacuaches (Didelphis marsupialis) y perros, y también amplían el área registrada para las ratas de campo (Neotoma micropus).
Chagas disease, caused by the hemoflagellate parasite Trypanosoma cruzi, is a widespread and endemic parasitosis extending from South America northward into the southern United States of America. It is currently a major cause of morbidity and mortality, with 6–7 million people infected and approximately 21000 deaths per year32. Trypanosoma cruzi is a multihost parasite that affects more than 180 mammalian species; it displays huge intraspecific heterogeneity and a complex transmission cycle, which may exhibit local peculiarities that occur because the distribution of T. cruzi infection is not homogeneous among houses, localities, and/or biomes25.
In northeastern (NE) Mexico, including Nuevo León state, reports of wild and domestic reservoir hosts of T. cruzi are deficient. In contrast, with numerous descriptions from southern and central Mexico of wild mammals of epidemiological importance including nine-banded armadillos (Dasypus novemcinctus), opossums (Didelphis marsupialis and D. virginiana), rodents (Sigmodon hispidus, Mus musculus), white-nosed coati (Nasua narica) and raccoon (Procyon lotor)16,30,31. Moreover, dogs (Canis familiaris) are considered the predominant domestic reservoir hosts for T. cruzi in many areas of endemicity. This infection has been poorly studied in Mexico, with some dispersed studies in Jalisco, Mexico, Morelos, Puebla, and Yucatán6,9,14,23,28. Furthermore, some records have been published from southeastern USA (Louisiana, Oklahoma, Georgia and Texas) where the epidemiological role of domestic dogs as urban and rural mammalian hosts of T. cruzi is becoming increasingly important, as indicated by their high seroprevalence3,13; despite this condition (of high seroprevalence) all dogs display low parasitemia, and no hemoculture has been positive for the parasite in some municipalities of Brazil33.
Northeastern Mexico shares two items with southeastern (SE) USA (Texas and New Mexico): a border and an epidemiological profile, in which peridomestic and wild triatomines, Triatoma gerstaeckeri (Stål), are associated with wild mammals7,18. However, in NE Mexico only the Southern plains woodrat (Neotoma micropus) has been reported11. The objective of this study was to assess the prevalence of T. cruzi in domestic canines and wild small mammals in Nuevo León, based on the responses to previous questionnaires regarding vector recognition and domestic dogs from inhabitants of this state19, where they have been reported as an important risk factor when they are infected with T. cruzi and live in close contact with the human population.
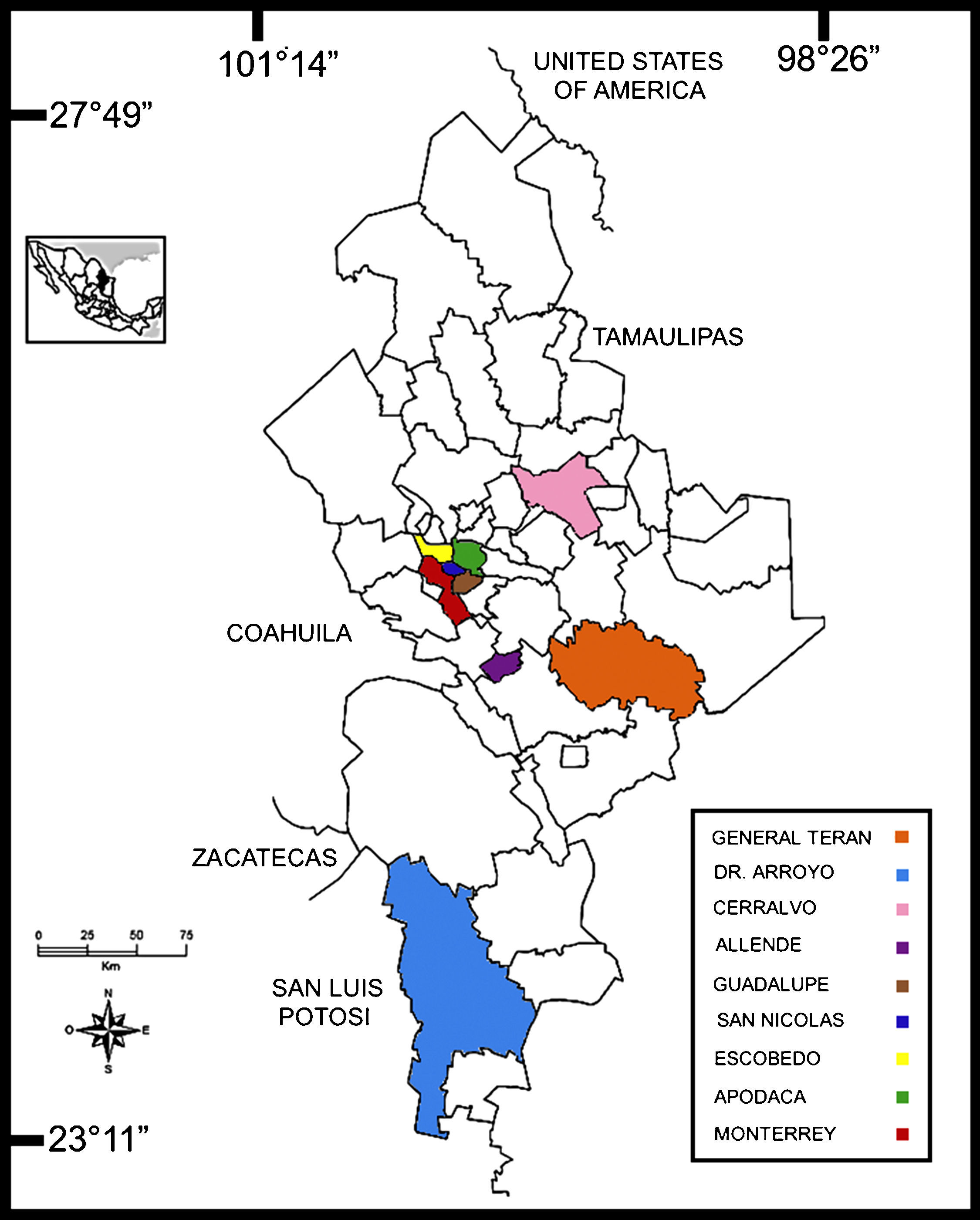
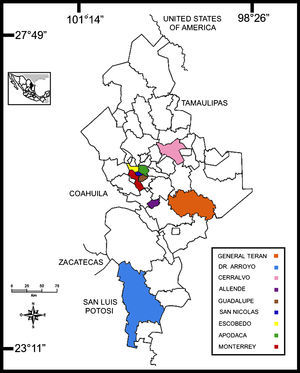
Materials and methodsStudy areaNuevo León state is located in the foothills of the Sierra Madre Oriental mountain range (27°49′N, 23°11′S and 98°26′E, 101°14′W). This study was conducted from March 2011 to November 2013 in nine municipalities, which correspond to: a) a rural area comprising General Terán, Dr. Arroyo and Cerralvo; b) a suburban county located in Allende; and c) an urban area comprising Guadalupe, San Nicolás de los Garza, Escobedo, Apodaca and Monterrey, the Capital of the state (Fig. 1). These localities were selected according to a recent report by Molina-Garza et al19.
Study design. Capture and handling of small wild mammalsThis is a cross sectional study. Wild mammal hosts were collected using live traps (H.B. Sherman Traps, Tallahassee, FL, and Havahart Traps, Lititz, PA) baited with peanut butter and/or sardines during 5 days per month in each municipality to perform a probabilistic sample stratified in three different localities, rural, suburban and urban, where the number of samples equally represented in a sample size calculated among 204–322 individuals, based in an estimated prevalence of T. cruzi infection of 15.8–30% reported in Mexican states6,9, with an absolute precision of 1.96, a confidence level of 95%, and E=5%19.
Fifty traps were placed at an interval of 2-m each in four linear transects of 100m each during the afternoon. Another fifty traps were specifically set for N. micropus, following the track of cacti (locally known as “nopaleras”, Opuntia spp.), pads bitten by the rodents and typical stool pellets found at the base of nests built out of dry sticks7. The total capture effort was standardized to 250traps/night. Traps were set during the afternoon and checked the following morning, as specified by the guidelines of the Mexican Secretary of Environment and Natural Resources20. Captured animals were anesthetized by an intramuscular injection of ketamine (100mg/kg of body weight; Fort Dodge Laboratories, Inc., Fort Dodge, IA, USA)12. Woodrats were euthanized in cervical dislocation and skunks and opposums by intracardiac injection with an overdose of sodium pentobarbital (Butler Company, Columbus, OH), followed by exsanguination7. Small mammals were taxonomically identified, classified as juveniles or adults based on weight and tooth wear, and sexed during sample collection by personnel of the Department of Zoology, UANL.
Dog surveysDogs were sampled in the presence and with the consent of their owners during domiciliary visits and at veterinary clinics and hospitals. Between two and 10 housing blocks were selected for sampling from maps, according to the density of each locality (rural, suburban or urban). The selection procedure for the houses to be sampled involved the enumeration of each house in the block, and the selection of five houses on each side, with the previous consent of the householder. Participation in the study was random and voluntary, with the sample size required described above.
A questionnaire completed by the dogs’ owners and veterinarians ascertained information regarding birthplace, sex, age (puppies=1–4 months; young or juvenile dogs=6–24 months; adult dogs=>2years) and general body condition2. Several of the dogs were found admitted to veterinary hospitals, and in the case of death, organ donation was requested.
Sample collection and parasitological diagnosisBlood samples from both small mammals and dogs were collected in EDTA vacutainer tubes (Beckton, Dickinson and Company, Franklin Lakes, NJ, USA) by puncturing the cephalic vein. Blood smears were prepared directly in situ with freshly sampled blood; smears were stained with Giemsa stain. The remaining blood samples were stored at −20°C for PCR22. Tissue samples (heart, skeletal muscle, smooth muscle, liver, spleen, lung, kidney, pancreas and brain) from each necropsied wild animal were fixed in 10% buffered formalin for processing. Routine histopathology was carried out using the hematoxylin and eosin (H & E) staining technique and the samples were closely examined for the presence of amastigote nests7. Representative photomicrographs were digitized using a Leica photomicroscope linked to a DFC 480 digital camera (Image Driving Software, LW Scientific, Inc., Tucker, GA, USA).
Identification of T. cruzi by PCRTotal DNA was extracted from whole blood using the standard DNAzol technique following the manufacturer's instructions (Invitrogen, San Diego, CA, USA). The extracted DNA pellet was resuspended in 50μl of deionized sterile water and stored at −20°C until use. Presence of T. cruzi DNA was confirmed by amplifying a region of the kinetoplastid minicircle (kDNA), using the oligonucleotide primers KNS1 (5′-GGG GTT CG A TTG GGG TTG GTG TA-3′) and KNS2 (5′-AAA (G/T)TT GAA CGC CCC TCC CAA A-3′), annealing in the conserved microregion of the minircicles and yielded products of 310bp, as described elsewhere1,18.
Statistical analysisReservoirs were considered infected by T. cruzi when one traditional parasitological diagnostic or PCR was positive8. Comparisons of the trypanosome infection rates for different age classes, sex and species of small mammals and dogs by communities (rural, suburban, and urban) were calculated using 2×2 contingency tables and the chi-squared (χ2) or the Fisher's exact test when appropriate. Data were analyzed using SPSS software, version 17 (Chicago, IL).
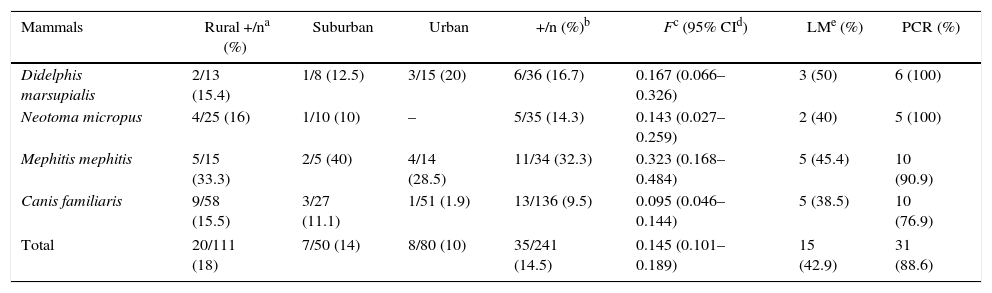
ResultsA total of 241 mammals from four different species were analyzed, included 136 dogs (117 mixed-breed dogs and 19 purebreds, 86.0% and 13.9% respectively), 36 opossums (D. marsupialis), 35 woodrats (N. micropus) and 34 striped skunks (M. mephitis). The number of infected animals highly differed between the species (χ2=11.54, degree freedom [df]=3, p=0.009). Striped skunks were found to be the most infected wild reservoir hosts in all the different localities (11/34, 32.3%, confidence intervals [CI]=0.168–0.484), followed by opossums (16.7%, CI=0.066–0.326) and woodrats (14.3%, CI=0.027–0.259, Table 1).
The prevalence of Trypanosoma cruzi infection among different study species of wild mammals and dogs in Nuevo León, México
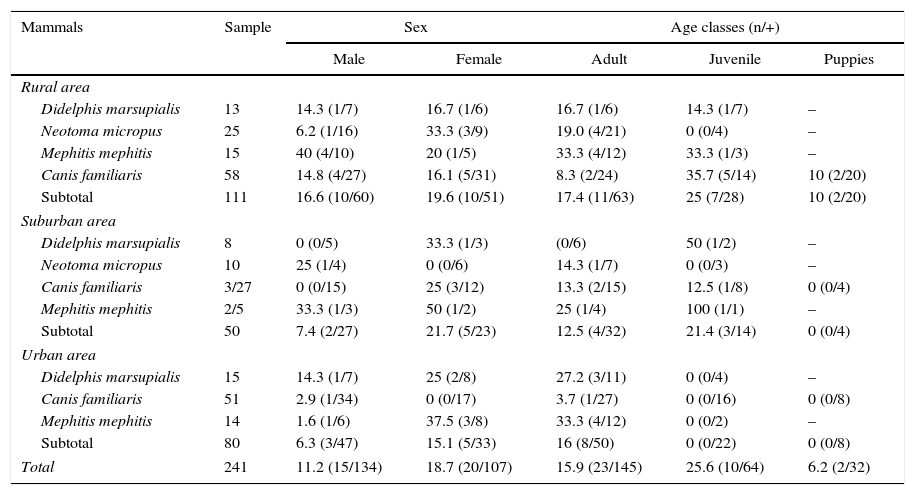
| Mammals | Rural +/na (%) | Suburban | Urban | +/n (%)b | Fc (95% CId) | LMe (%) | PCR (%) |
|---|---|---|---|---|---|---|---|
| Didelphis marsupialis | 2/13 (15.4) | 1/8 (12.5) | 3/15 (20) | 6/36 (16.7) | 0.167 (0.066–0.326) | 3 (50) | 6 (100) |
| Neotoma micropus | 4/25 (16) | 1/10 (10) | – | 5/35 (14.3) | 0.143 (0.027–0.259) | 2 (40) | 5 (100) |
| Mephitis mephitis | 5/15 (33.3) | 2/5 (40) | 4/14 (28.5) | 11/34 (32.3) | 0.323 (0.168–0.484) | 5 (45.4) | 10 (90.9) |
| Canis familiaris | 9/58 (15.5) | 3/27 (11.1) | 1/51 (1.9) | 13/136 (9.5) | 0.095 (0.046–0.144) | 5 (38.5) | 10 (76.9) |
| Total | 20/111 (18) | 7/50 (14) | 8/80 (10) | 35/241 (14.5) | 0.145 (0.101–0.189) | 15 (42.9) | 31 (88.6) |
Number of infected animals and species with a significance of χ2=11.5, df=3, p=0.009.
With regard to habitats, one hundred eleven mammalian hosts were analyzed from rural municipalities, 50 from suburban areas, and 80 from urban municipalities (Table 1). The capture success prevalence for the different localities was 8.8%, 4% and 6.4%, respectively, and was highly associated by area and the number of small mammals species (χ2=30.5, df=2, p=0.002). A total of 35 animals (35/241, 14.5%) were found infected with T. cruzi (Fig. 2A), 20 from rural (the highest prevalence, 20/111, 18%), seven from suburban (14%) and eight from urban (10%) localities. Although the infection prevalence by species of mammal reservoir hosts did not significantly differ between the localities (χ2=6.09, df=4, p=0.192), the infection prevalence by locality and sex was highly dependent (χ2=206.5, df=12, p=0.000). The highest prevalence of infected animals were observed in striped skunks from rural (33.3%), suburban (40%) and urban areas (28.5%), whereas dogs showed the lowest prevalence in these three habitats (Table 1).
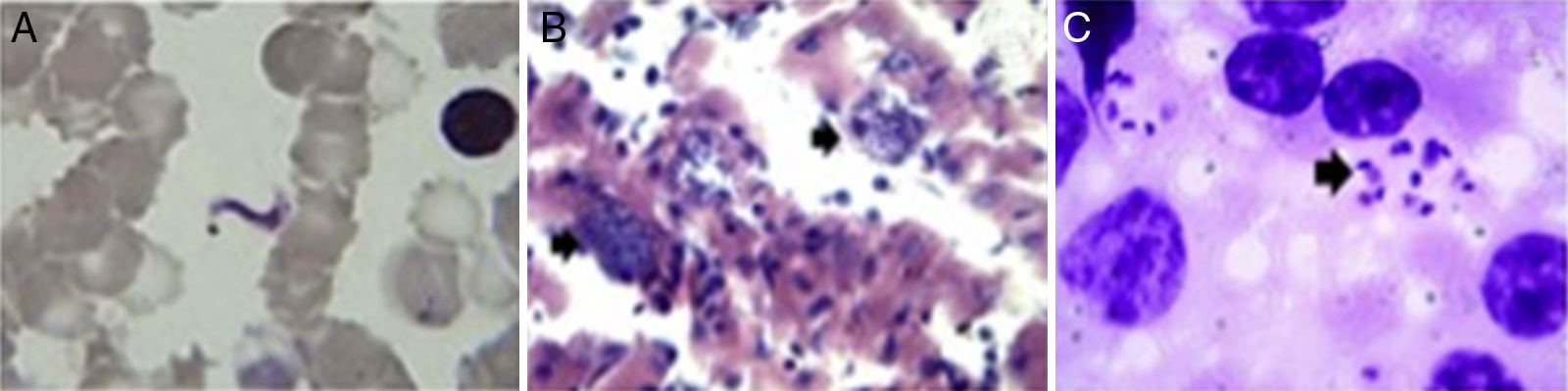
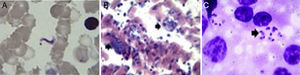
Trypanosoma cruzi from wild mammals and dogs. (A) Giemsa stained blood smear from an opossum showing trypomastigotes of Trypanosoma cruzi. (B) Histopathological lesions with amastigote nests in the cardiac muscle of T. cruzi infected dogs (arrow indicates amastigote nests). (C) Amastigote forms (arrow) of T. cruzi observed in two mixed-breed puppies.
The prevalence of T. cruzi infection by age, in suburban area was the highest in juvenile striped skunks (100%) followed by juvenile opossums (50%). There is statistical dependence among infection rate, age class and animal species (χ2=47.8; df=15, p=0.000). With regard to dog infection by age, in rural areas juveniles reached the highest (35.7%, 5/14) followed by adults (13.3%, 2/15) and juveniles (12.5%, 1/8) in the suburban area; only one male adult dog was found to be infected (3.7%, 1/27) in the urban area (Table 2).
The prevalence of infection by T. cruzi in the small mammalian fauna and dogs from Nuevo León, México by sex and age classes
| Mammals | Sample | Sex | Age classes (n/+) | |||
|---|---|---|---|---|---|---|
| Male | Female | Adult | Juvenile | Puppies | ||
| Rural area | ||||||
| Didelphis marsupialis | 13 | 14.3 (1/7) | 16.7 (1/6) | 16.7 (1/6) | 14.3 (1/7) | – |
| Neotoma micropus | 25 | 6.2 (1/16) | 33.3 (3/9) | 19.0 (4/21) | 0 (0/4) | – |
| Mephitis mephitis | 15 | 40 (4/10) | 20 (1/5) | 33.3 (4/12) | 33.3 (1/3) | – |
| Canis familiaris | 58 | 14.8 (4/27) | 16.1 (5/31) | 8.3 (2/24) | 35.7 (5/14) | 10 (2/20) |
| Subtotal | 111 | 16.6 (10/60) | 19.6 (10/51) | 17.4 (11/63) | 25 (7/28) | 10 (2/20) |
| Suburban area | ||||||
| Didelphis marsupialis | 8 | 0 (0/5) | 33.3 (1/3) | (0/6) | 50 (1/2) | – |
| Neotoma micropus | 10 | 25 (1/4) | 0 (0/6) | 14.3 (1/7) | 0 (0/3) | – |
| Canis familiaris | 3/27 | 0 (0/15) | 25 (3/12) | 13.3 (2/15) | 12.5 (1/8) | 0 (0/4) |
| Mephitis mephitis | 2/5 | 33.3 (1/3) | 50 (1/2) | 25 (1/4) | 100 (1/1) | – |
| Subtotal | 50 | 7.4 (2/27) | 21.7 (5/23) | 12.5 (4/32) | 21.4 (3/14) | 0 (0/4) |
| Urban area | ||||||
| Didelphis marsupialis | 15 | 14.3 (1/7) | 25 (2/8) | 27.2 (3/11) | 0 (0/4) | – |
| Canis familiaris | 51 | 2.9 (1/34) | 0 (0/17) | 3.7 (1/27) | 0 (0/16) | 0 (0/8) |
| Mephitis mephitis | 14 | 1.6 (1/6) | 37.5 (3/8) | 33.3 (4/12) | 0 (0/2) | – |
| Subtotal | 80 | 6.3 (3/47) | 15.1 (5/33) | 16 (8/50) | 0 (0/22) | 0 (0/8) |
| Total | 241 | 11.2 (15/134) | 18.7 (20/107) | 15.9 (23/145) | 25.6 (10/64) | 6.2 (2/32) |
n=sample size by mammalian species. +=positive.
With regard to the sex of the sampled animals, 134 were males (55.6%) and 107 females (44.4%). Twenty females tested positive (18.7%), indicating a higher prevalence of infection with T cruzi in females than in males (15/134, 11.2%). However this difference was not significant (χ2=0.155, df=3, p=0.984). Furthermore, the prevalence of infection was highest in female striped skunks (50% in the suburban area), followed by male in the rural region (40%); however, the analyses among prevalence of infection, species of mammalian reservoir hosts and sex were significant (χ2=15.32, df=6, p=0.018) (Table 2).
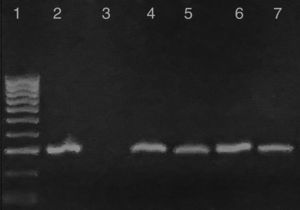
Questionnaire resultsClinical signs suggestive of infection reported by pet owners were confirmed microscopically and by molecular diagnostics. One mixed-breed puppy from the rural area (Cerralvo) and one adult from the metropolitan area showed lethargy, anorexia and fever. Gross necropsy indicated that T. cruzi infection was preferentially located in the adipose tissue, skeletal muscle and cardiac muscle (Fig. 2B). There was a high prevalence of intracellular amastigote nests and interstitial inflammation, accompanied by perivasculitis and adipose degeneration. The kidney and spleen (Fig. 2C) had moderate sinusoidal congestion; however, no pathological changes were found in the brain, lung and pancreas and the parasite did not appear to be present in these tissues. The diagnosis was confirmed by PCR (Fig. 3) and a few weeks later they both succumbed to the infection, showing evidence of the classical histopathological lesions (Fig. 2B).
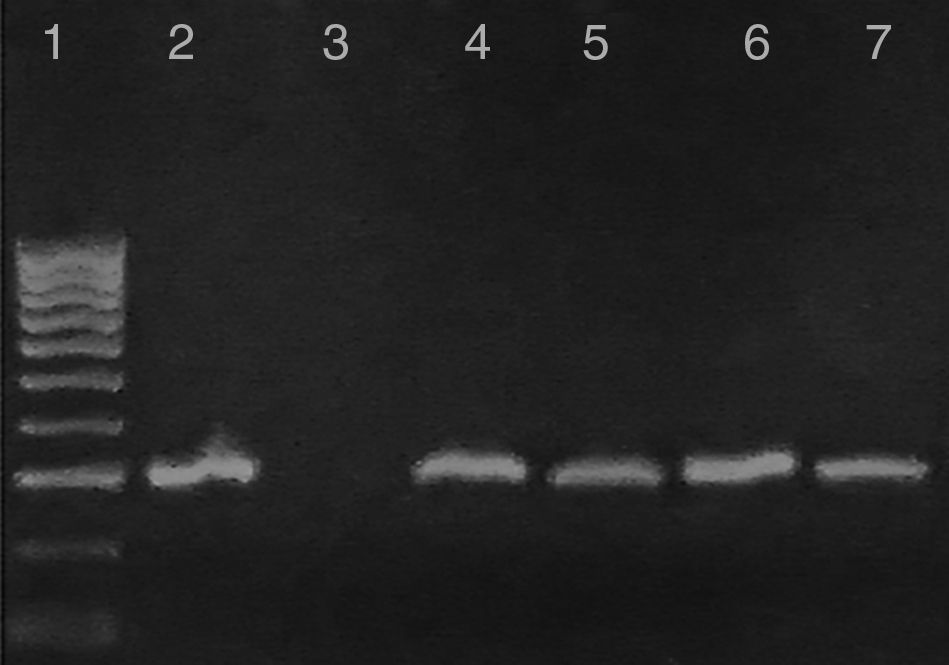
Characterization of kDNA-PCR-positive samples from infected reservoirs: Lane 1: 100-bp DNA ladder; 2: T. cruzi positive control; 3: negative control; 4 and 5: representative amplicons from DNA extracted from a T. cruzi-infected adult dog and a mixed-breed puppy in acute phase from metropolitan and rural areas, respectively; 6: representative sample of positive PCR from woodrats; 7: PCR product from a striped skunk.
This study provided clear evidence of the active cycles of T. cruzi transmission in NE Mexico and demonstrated the first autochthonous cases in the state of Nuevo León, with direct microscopic examination and PCR in striped skunks, dogs and opossums. Therefore, the dogs can be used as sentinels of the local transmission of Chagas disease; the regular screening of domestic dog populations can be used to identify houses or clusters with high risk of transmission5,25. We have reported two acute cases (dogs from Cerralvo and urban area) of infection among 13 positive dogs with moderate to high parasitemia levels; these findings support the reports from USA (Texas) and Argentina (Chaco), where the most frequent clinical signs were an enlarged heart, lethargy and anorexia in acute infection and death in puppies younger than one year old5,13,21, suggesting a fatal course of infection in the dogs sampled, similarly to those reported in cases from Colombia29.
The distribution of T. cruzi infection was not homogeneous among municipalities, i.e., the highest infection prevalence was observed in dogs from rural areas, followed by the suburban and urban areas of Nuevo León, where the prevalence varied from 1.9% to 15.5%. This profile was also observed in Brazilian biomes (the Amazon, Caatinga, and Pantanal), where the distribution of T. cruzi infection ranged from 0%–42% to 11%–89%24,33. Previous reports also identified domestic pets as an important risk factor associated with Chagas disease in human populations from Toluca, Puebla and Morelos, Mexico9,23,28; however, the most serious infection prevalence in dogs was documented in Texas, USA, with 20% PCR-positive cases13, suggesting that the infection is steadily maintained in these reservoir hosts and opportunistic vertebrates (opossums, striped skunks and woodrats), which are used to live in close proximity to human dwellings3. Our results involved direct microscopic examinations (blood smears and histopathology) and PCR assays, showing a percentage of matches between the two analyses of 42.9 and 88.6% respectively (Table 1). Moreover, results of 11–22% with parasitological methods (hemoculture, xenodiagnosis and xenoculture), 67–100% by PCR have been reported1, showing lower levels of prevalence than those reported with serological tests, which yielded infection rates of up to 100%24,25. However, a high association between parasitological and serological results has been reported. Furthermore, PCR1,14 allows to avoid some cases of false positive or false negative results, which could rise from Leishmania spp. or T. rangeli8.
A difference between our study area and the Amazon region is observed. In Brazil dogs have an elevated prevalence of contact with the wild environment, because houses are practically located inside wild forest areas, and it is difficult to delimit peridomestic and wild areas, and dogs are involved in hunting activities24, whereas in NE Mexico the scenario is completely different, because human residences are built after the destruction of the wild environment. This could be the cause that our results did not show any correlation between T. cruzi infection in dogs and small wild mammals as described33.
When we examined the prevalence of infection in dogs by age, the age–prevalence curve increased from 10% in puppies to 35.7% in juvenile dogs, which had the highest T. cruzi infection rate. A similar scenario is described in Panama27, which highlights the importance of puppies and juvenile dogs in the continuous life cycle of T. cruzi in the rural region of Nuevo León and also the epidemiological importance of the single positive adult recorded in an urban area. This dog was involved in camping activities around the mountains close to the state of Nuevo León, recognized as a non-endemic region for T. cruzi infection10, which indeed does not have a Chagas disease vector control program such as those existing in some South American countries.
Furthermore, there are no records of T. cruzi infection prevalence in striped skunks in Mexico, and no systematic studies of its occurrence in small wild mammals. In this study, the highest prevalence of infection was found in the striped skunk in suburban, rural and urban areas, suggesting that this species is an important host for T. cruzi infection, as has been demonstrated in the southeast of the USA4. Several studies have reported the role of opossums as natural wild reservoir hosts of T. cruzi in dwellings and peridomiciles in Chiapas, Yucatán, Jalisco and Mexico state15,26,30,31. In the southern United States and Argentina, the opossum is considered to be the main wild mammal host species as a synanthropic reservoir4,7,21; nevertheless our results reported opossums as the second most important wild reservoir host for T. cruzi. In this study, opossums and striped skunks were collected close to highways near a metropolitan area that had grown substantially in the last 10 years. As a result of the deforestation caused by the building of new residential areas, these vertebrate reservoirs have been strongly forced to visit peridomestic habitats in search of food and to increase the interaction between the sylvatic and domestic cycles; the consequence of this process is the increased opportunity for contact among humans, domestic animals and wildlife33.
Regarding woodrats, previous studies in General Terán, NL (a rural county) reported that the prevalence of T. cruzi infection had been lower than in this study11; the triatomine T. gerstaeckeri, has been found to be associated to N. micropus nets naturally infected with T. cruzi (33%–59.6%)17,18, although some reports have described T. gerstaeckeri as a human and livestock pest species because the adults are frequent invaders of rural houses in southwestern USA3, highlighting their importance by their vectorial capacity in this region. Although the genotyping of T. cruzi was not performed in this work, other investigations have documented different T. cruzi strains in the reservoirs described, the most abundant being TcI, which is distributed by triatomine vectors and is associated with sylvatic and domestic cycles34.
In conclusion, although the prevalence of T. cruzi infection was lower in reservoirs in Nuevo León than in other states of Mexico, this plays an important role in the maintenance and transmission cycles of T cruzi due to the close proximity of the reservoir species with human domiciles. The results of this study provide new locality records for striped skunks, opossums and dogs in rural, suburban, and urban counties in NE Mexico and extend the localities reported to the woodrat previously recorded only in General Terán18 to other rural and suburban counties including Dr. Arroyo, Cerralvo and Allende. It would be necessary to consider all the results obtained and complemented with other epidemiological reports to classify Nuevo León state from a non-endemic to an endemic region, since our current and previous results support this change10,19.
Ethical statementAll procedures involving animals were evaluated and approved by the Ethical Principles of Animal Experimentation established by the Committee of Ethical of Animal Experimentation of the Universidad Autónoma de Nuevo León, in agreement with the international ethical standards of the American Society of Mammalogists12.
Ethical responsibilitiesProtection of people and animalsThe authors state that the procedures followed conformed to the ethical standards of the responsible human experimentation committee and in agreement with the World Medical Association and the Declaration of Helsinki.
Confidentiality of dataThe authors state that no patient data appears in this article.
Right to privacy and informed consentThe authors state that in this article there are no patient data
FundingThe project Epidemiology of American Trypanosomiasis, was supported by Seretaría de Educación Pública-Programa de Desarrollo Profesional Docente (SEP-PRODEP) for the “Integración de Redes Temáticas de Colaboración Académica 2015-UANL-CA-278” and Consejo Nacional de Ciencia y Tecnología (CONACyT), grant number INFRA 2015-253336.
Conflicts of interestThe authors declare that they have no conflicts of interest
We thank Vida Mariel Molina Garza and Abraham Galaviz Molina for their support throughout proofreading and editing process.