Fungal metabolites are promising alternatives for the development of biorational pesticides. In this sense, microfungi from tropical regions are valuable sources of natural compounds for pest management. With the aim of broadening the search for new eco-friendly products to manage plant pests, this study was carried out to evaluate the biological activity of 23 tropical fungal extracts on three species of phytophagous insects and a plant parasitic nematode. In addition, the active principles of the most effective extract were identified. The insect deterrent activity of fungal extracts was evaluated on the settling of aphids Myzus persicae and Rhopalosiphum padi, and on the feeding of lepidoptera larva Spodoptera littoralis; the nematostatic activity was evaluated on the mobility of Meloidogyne javanica. Active metabolites from Gliomastix masseei were identified by GC–MS techniques and by comparison with commercial standards. Results showed seven extracts with strong effect on the settling of M. persicae and R. padi (settling inhibition >80%). The calculated median of effective concentration (EC50) values ranged from 8 to 38μg/cm2 for the extracts of Clonostachys rosea and G. masseei, respectively. Bioassay-guided separation of the ethyl acetate extract of G. masseei revealed the presence of fatty acids and their derivatives, where methyl 9-octadecenoate was the most active compound with EC50 values of 16μg and 35μg/cm2 for M. persicae and R. padi, respectively. Extracts of C. rosea and G. masseei could be a promising option in the control of pest aphids in agriculture.
Los metabolitos fúngicos son agentes prometedores para el desarrollo de plaguicidas biorracionales. En este sentido, los hongos microscópicos de zonas tropicales representan una valiosa fuente de compuestos naturales para el manejo de plagas. Con la finalidad de ampliar la investigación en productos amigables con el medio ambiente, en este estudio se evaluó la actividad biológica de 23 extractos de hongos sobre 3 especies de insectos fitófagos y un nematodo fitoparásito; además se identificaron los componentes del extracto más activo. El efecto disuasivo de los extractos fúngicos se evaluó en el asentamiento de Myzus persicae y Rhopalosiphum padi, así como en la alimentación de Spodoptera littoralis; la actividad nematostática se evaluó sobre la movilidad de Meloidogyne javanica. Los metabolitos activos de Gliomastix masseei se identificaron por cromatografía de gases-espectrometría de masas y por comparación con muestras comerciales. Los resultados mostraron 7 extractos con fuerte efecto en la inhibición del asentamiento (> 80%) de M. persicae y R. padi. Los valores de la concentración efectiva media (CE50) estuvieron en el rango de 8 a 38μg/cm2 para los extractos de Clonostachys rosea y G. masseei, respectivamente. El extracto de acetato de etilo de G. masseei se fraccionó por un proceso biodirigido y reveló la presencia de ácidos grasos y sus derivados, donde el 9-octadecenoato de metilo fue el más activo, con una CE50 de 16μg/cm2 para M. persicae y 35μg/cm2 para R. padi. Los extractos fúngicos de C. rosea y G. masseei pueden ser una alternativa promisoria en el control de áfidos que son plaga en la agricultura.
The negative impact of synthetic pesticides on the environment and human health has highlighted the use of natural products as a promising alternative for biorational plant pest management7. Fungal secondary metabolites are considered a valuable source of molecules with a wide variety of biological applications. Various reports have pointed out the potential of these compounds for the control of insects and nematodes. One of the first detailed works on the insecticidal properties of fungal metabolites was published by Cole and Rolinson9. More recently, Asaff et al.4 showed that dipicolinic and oxalic acids, isolated from Paecilomyces fumosoroseus, have strong insecticidal effects on nymphs of Bemisia species. Likewise, Gaich and Mulzer12 showed that the sesquiterpenoid, penifulvin A (Penicillium griseofulvum) has antifeedant effects on Spodoptera frugiperda. In addition, nematotoxic activity has been reported in a wide variety of fungal metabolites, such as cytochalasin B, enniatin B, moniliformin, verrucarin A20, trichodermin36,37, and butyric acid from Paecilomyces lilacinus 602933.
Our research group has undertaken a long-term project on bioprospection of mycodiversity and its biotechnological application. In this sense, the search for microfungi in the tropical regions of Mexico has yielded a fungal collection whose mycelial extracts have been evaluated on several pathogenic bacteria and fungi, as well as the plant parasitic nematode Meloidogyne incognita13,29. None of these native fungi have been assessed against phytophagous insects. Therefore, in the present work, with the aim of broadening the search for new and more eco-friendly agents to control plant pests, twelve saprophytic fungi (Table 1) were selected, based on their biological profile. The fungi were cultured and their organic extracts were evaluated on the root-knot nematode Meloidogyne javanica (Neal) Chitwood (Nematoda: Meloidogynidae), the poligophagous Lepidoptera Spodoptera littoralis Boisduval (Lepidoptera: Noctuidae), and aphids Myzus persicae Sulzer (Hemiptera: Aphididae) and Rhopalosiphum padi L. (Hemiptera: Aphididae). In addition, one of the most active extracts was partitioned and its active constituents were identified through bioassay-guided fractionation.
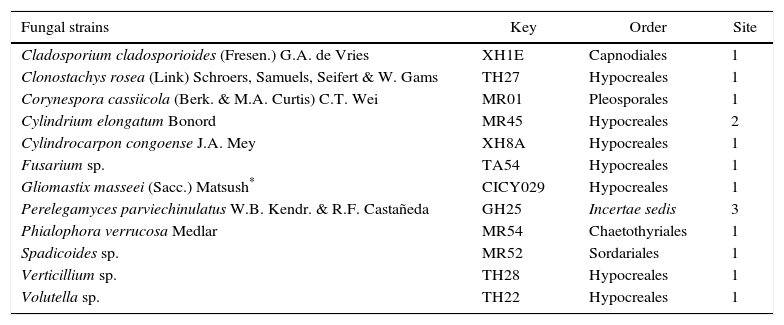
List of Mexican microfungi tested (organic extracts) on insect deterrent and nematostatic activities
| Fungal strains | Key | Order | Site |
|---|---|---|---|
| Cladosporium cladosporioides (Fresen.) G.A. de Vries | XH1E | Capnodiales | 1 |
| Clonostachys rosea (Link) Schroers, Samuels, Seifert & W. Gams | TH27 | Hypocreales | 1 |
| Corynespora cassiicola (Berk. & M.A. Curtis) C.T. Wei | MR01 | Pleosporales | 1 |
| Cylindrium elongatum Bonord | MR45 | Hypocreales | 2 |
| Cylindrocarpon congoense J.A. Mey | XH8A | Hypocreales | 1 |
| Fusarium sp. | TA54 | Hypocreales | 1 |
| Gliomastix masseei (Sacc.) Matsush* | CICY029 | Hypocreales | 1 |
| Perelegamyces parviechinulatus W.B. Kendr. & R.F. Castañeda | GH25 | Incertae sedis | 3 |
| Phialophora verrucosa Medlar | MR54 | Chaetothyriales | 1 |
| Spadicoides sp. | MR52 | Sordariales | 1 |
| Verticillium sp. | TH28 | Hypocreales | 1 |
| Volutella sp. | TH22 | Hypocreales | 1 |
Site: 1: Yucatán, 2: Veracruz, 3: Tabasco.
Fungal strains were obtained from the culture collection of the Unidad de Biotecnología of the Centro de Investigación Científica de Yucatán (Table 1) as previously reported by Gamboa-Angulo et al.13 and Reyes-Estebanez et al.29.
Culture and extraction of fungiFungi were grown in fermented rice (FR) for 40 days at 25±2°C and in light–dark photoperiod of 12:12h. Fungal cultures were lyophilized, fragmented and extracted with ethyl acetate three times (50ml×100g FR) and methanol (50ml×100g FR, at approximately 50°C). The solvents were eliminated by reduced pressure in a rotary evaporator. Ethyl acetate (EA) and methanol (ME) extracts were kept in darkness at 4°C until use29.
Partition and chromatographic fractionation of ethyl acetate extract of Gliomastix masseeiEthyl acetate extract (100mg) of G. masseei was re-suspended in acetonitrile (5ml) and partitioned with hexane three times (2:1, 1:1, 1:1, v/v). Solvents were eliminated under reduced pressure to obtain fractions of low (ARGM-1A, 21%) and medium (ARGM-1B, 5%) polarity, and a precipitate. Both fractions were sub-fractionated by silica gel chromatographic column and eluted with hexane, ethyl acetate, acetone, and methanol mixtures. Extract and fractions were analyzed by thin layer chromatography (TLC) on silica gel (60F254 plates) and detected under UV light and by spraying phosphomolybdic acid as revealing agent.
Preparation of methyl derivativesMethyl derivatives of fatty acids were prepared in the laboratory. Potassium carbonate (50mg) re-suspended in acetone was added to the sample (100mg) and, after 15min of continuous stirring, methyl iodide was added (150μl) and the mixture was left for one night at room temperature. Methylated products were recovered by extraction with ethyl acetate17.
Analytical methodsGas chromatography–mass spectrometry (GC–MS) analyses were performed on an Agilent Technologies 6890N chromatograph coupled to an Agilent Technologies 5975B mass selective detector, with Ultra 1 column [methylsiloxane, 25m long, 0.32mm i.d., 0.52μm film thickness, helium at flow rate=1.0ml/min, T1=150°C, gradient=10°Cmin, T2=280°C (20min)]. Each extract was analyzed using 0.4μl of sample at 2% (w/v). Most components were identified by matching their mass spectra to those of the corresponding compounds in the database of the equipment (NIST 05) and/or by comparison with commercial standards (Sigma–Aldrich).
BioassaysSettling inhibition of M. persicae and R. padiEvaluations of settling inhibition (SI) were performed on adults of M. persicae and R. padi. Aphids were grown on pepper (Capsicum annuum L.) and barley (Hordeum vulgare L.), respectively. Colonies were kept in a growth chamber at 60–70% room humidity, photoperiod of 16:8h (L:D), and temperatures of 21±2°C.
Bioassays were conducted in plastic boxes (3cm×3cm×1.5cm) lined at the bottom with 2% agar. Two leaf pieces (ca. 1cm2) cut from the appropriate host plant were placed on the agar. One piece was coated with 100μg extract diluted in 10μl of either acetone or methanol (treated=T) and the other leaf piece was coated only with 10μl of the respective solvent (control=C). Twenty replicates (plastic boxes) per extract were evaluated, each replicate containing ten aphids. The percentage of aphids settled on each piece of leaf was recorded 24h after exposure. A settling inhibition index (%SI) was calculated for each extract,%SI=[1−(%T/%C)]×100, where%T and%C are the percentage of aphids settled on the treated and control leaf pieces, respectively6. The dose–response experiments for the most active extracts were carried out with the following serial dilutions: 200, 100, 50, 20, and 4μg/cm2.
Feeding inhibition of S. littoralisS. littoralis larvae were reared on an artificial diet as described by Poitout and Bues26. The colony was kept in a growth chamber at 60–70% room humidity, photoperiod of 16:8h (L:D), and temperature of 20±2°C. For the bioassay, sixth instar larvae of S. littoralis were used. Two larvae were placed in a Petri dish (9cm diameter×1cm) containing agar (2%). Four wells were prepared and four discs (1cm2) of fresh bell-pepper leaves were deposited in the wells. Two of the discs were coated with 100μg of the extracts dissolved in 10μl acetone or methanol (T), and the other two discs were coated with the solvent only (C). Larvae were starved for 3h prior to the experiments and subsequently placed on the leaf disc and allowed to consume approximately 75% of the disc area before evaluation. Each treatment was conducted in quintuplicate and repeated two times for the active extracts. The uneaten disc areas were measured with a digital program (Image J Version 1.37r, 2006, http://rsb.info.nih.gov./ij/), and the percentage of feeding inhibition was calculated by the equation%FR=[1−(T/C)]×100, where T and C are the consumption of treated and control leaf discs, respectively6.
Mobility inhibition of M. javanicaEgg masses of M. javanica were obtained from roots of tomato plants (Solanum lycopersicum L.) grown under greenhouse conditions. The eggs were incubated in distilled water at 25°C for 24h to obtain second-stage juveniles (J2). Bioassay with J2 was performed in 96-well plates. Fungal extracts were dissolved in DMSO with 0.6% Tween 20 (20μg/μl). Subsequently, 5μl of the extracts were deposited in each well and 100–150 J2 in 95μl of water were added to each well. The well plates were incubated at 25°C for 72h. Inhibition of mobility (IM) of J2 was obtained as previously described by Hernández-Carlos et al.16.
Data analysisThe effect of an extract is considered strong when SI>70% for aphids15, FR>80% for S. littoralis10 and IM>90% after 48h exposure for Meloidogyne spp.2 Effective concentrations (EC50) were calculated by the probit analysis using the Statistical Analysis System (SAS), version 8.1 for Windows. EC50 values were considered significantly different if confidence intervals did not overlap.
Molecular identification of G. masseeiMolecular identification of the fungal strain MR-36 was performed as described by Moo-Koh et al.22. The mycelial mass of fungal culture was obtained and the genomic DNA was extracted using the Kit ZR Fungal/Bacterial DNA MiniPrep™. Nuclear ribosomal DNA internal transcribed spacer (ITS) regions (ITS1-5.8S rDNA ITS2) were amplified and sequenced using primers ITS1 and ITS435. The amplified products were visualized by electrophoresis in 2% agarose gel (m/v) (Sigma®) and the products of amplification were sequenced in Macrogen (www.macrogen.com). Sequences were edited to eliminate the noise at ends, minus strands were converted into reverse complement, and both strands were aligned. A query of 500bp was used to compare with those in the database of the Gene Bank of the National Center for Biotechnology Information (http://blast.ncbi.nlm.gov), using the BLAST software, and deposited in GenBank (accession number KR069085).
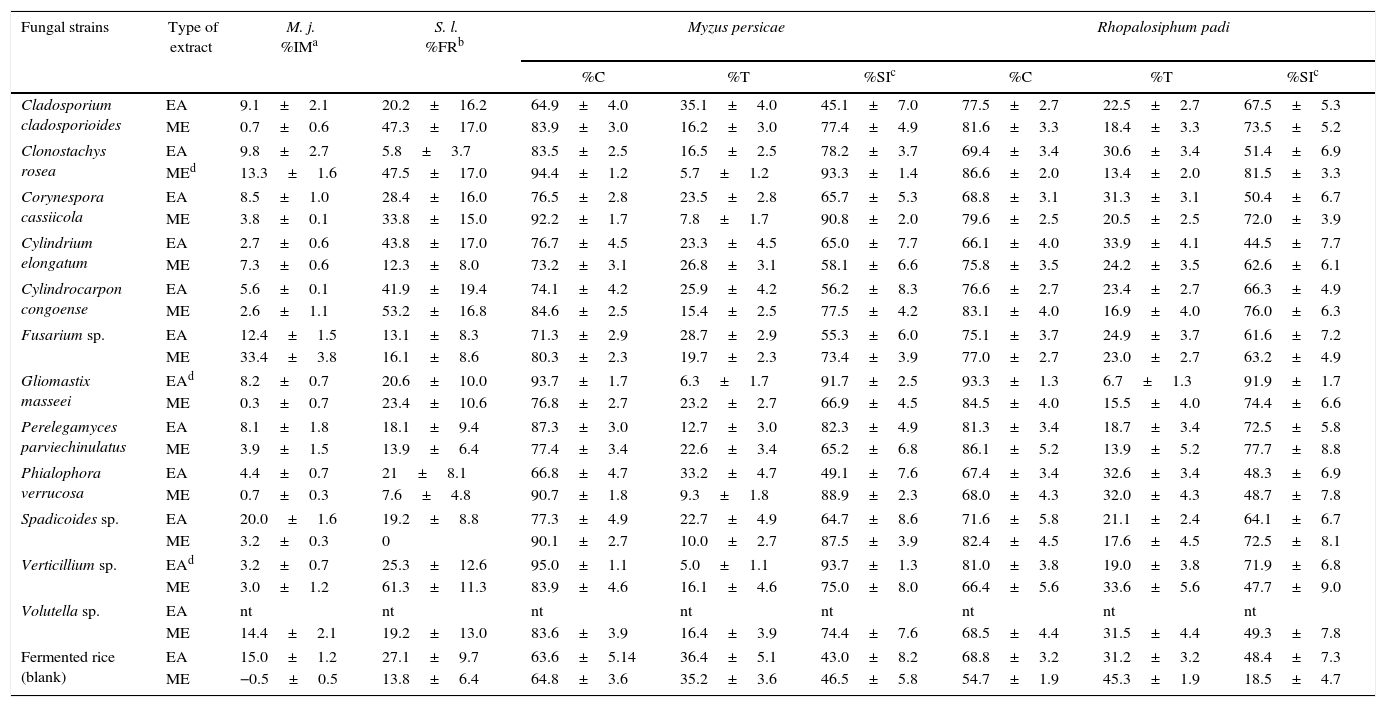
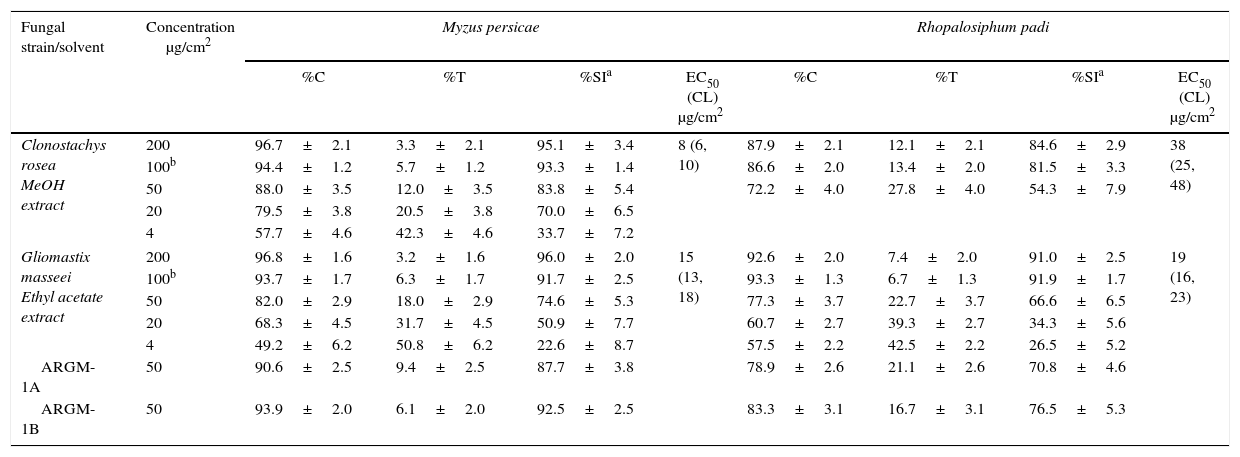
ResultsThe results of settling inhibition, feeding reduction, and nematotoxic activities are shown in Table 2. None of the extracts inhibited the mobility of M. javanica (MI<50%) or the feeding of S. littoralis (FR<70%). In contrast, the EA extracts of G. masseei, Perelegamyces parviechinulatus and Verticillium sp. TH28, and the ME extracts of Clonostachys rosea and Corynespora cassiicola, Phialophora verrucosa, and Spadicoides sp. inhibited the settling of M. persicae and R. padi (SI>80%; Table 2). The extracts of C. rosea and G. masseei caused strong settling inhibition of both aphid species. The dose–response evaluations for these extracts are shown in Table 3. For M. persicae, the ME extract of C. rosea was the most active, with an EC50 value of 8μg/cm2; whereas for R. padi, the EA extract of G. masseei was the most active with an EC50 value of 19μg/cm2.
In vitro evaluation of the organic extracts from microfungi on Meloidogyne javanica (100μg/ml), Myzus persicae, Rhopalosiphum padi, and Spodoptera littoralis (100μg/cm2)
| Fungal strains | Type of extract | M. j. %IMa | S. l. %FRb | Myzus persicae | Rhopalosiphum padi | ||||
|---|---|---|---|---|---|---|---|---|---|
| %C | %T | %SIc | %C | %T | %SIc | ||||
| Cladosporium cladosporioides | EA | 9.1±2.1 | 20.2±16.2 | 64.9±4.0 | 35.1±4.0 | 45.1±7.0 | 77.5±2.7 | 22.5±2.7 | 67.5±5.3 |
| ME | 0.7±0.6 | 47.3±17.0 | 83.9±3.0 | 16.2±3.0 | 77.4±4.9 | 81.6±3.3 | 18.4±3.3 | 73.5±5.2 | |
| Clonostachys rosea | EA | 9.8±2.7 | 5.8±3.7 | 83.5±2.5 | 16.5±2.5 | 78.2±3.7 | 69.4±3.4 | 30.6±3.4 | 51.4±6.9 |
| MEd | 13.3±1.6 | 47.5±17.0 | 94.4±1.2 | 5.7±1.2 | 93.3±1.4 | 86.6±2.0 | 13.4±2.0 | 81.5±3.3 | |
| Corynespora cassiicola | EA | 8.5±1.0 | 28.4±16.0 | 76.5±2.8 | 23.5±2.8 | 65.7±5.3 | 68.8±3.1 | 31.3±3.1 | 50.4±6.7 |
| ME | 3.8±0.1 | 33.8±15.0 | 92.2±1.7 | 7.8±1.7 | 90.8±2.0 | 79.6±2.5 | 20.5±2.5 | 72.0±3.9 | |
| Cylindrium elongatum | EA | 2.7±0.6 | 43.8±17.0 | 76.7±4.5 | 23.3±4.5 | 65.0±7.7 | 66.1±4.0 | 33.9±4.1 | 44.5±7.7 |
| ME | 7.3±0.6 | 12.3±8.0 | 73.2±3.1 | 26.8±3.1 | 58.1±6.6 | 75.8±3.5 | 24.2±3.5 | 62.6±6.1 | |
| Cylindrocarpon congoense | EA | 5.6±0.1 | 41.9±19.4 | 74.1±4.2 | 25.9±4.2 | 56.2±8.3 | 76.6±2.7 | 23.4±2.7 | 66.3±4.9 |
| ME | 2.6±1.1 | 53.2±16.8 | 84.6±2.5 | 15.4±2.5 | 77.5±4.2 | 83.1±4.0 | 16.9±4.0 | 76.0±6.3 | |
| Fusarium sp. | EA | 12.4±1.5 | 13.1±8.3 | 71.3±2.9 | 28.7±2.9 | 55.3±6.0 | 75.1±3.7 | 24.9±3.7 | 61.6±7.2 |
| ME | 33.4±3.8 | 16.1±8.6 | 80.3±2.3 | 19.7±2.3 | 73.4±3.9 | 77.0±2.7 | 23.0±2.7 | 63.2±4.9 | |
| Gliomastix masseei | EAd | 8.2±0.7 | 20.6±10.0 | 93.7±1.7 | 6.3±1.7 | 91.7±2.5 | 93.3±1.3 | 6.7±1.3 | 91.9±1.7 |
| ME | 0.3±0.7 | 23.4±10.6 | 76.8±2.7 | 23.2±2.7 | 66.9±4.5 | 84.5±4.0 | 15.5±4.0 | 74.4±6.6 | |
| Perelegamyces parviechinulatus | EA | 8.1±1.8 | 18.1±9.4 | 87.3±3.0 | 12.7±3.0 | 82.3±4.9 | 81.3±3.4 | 18.7±3.4 | 72.5±5.8 |
| ME | 3.9±1.5 | 13.9±6.4 | 77.4±3.4 | 22.6±3.4 | 65.2±6.8 | 86.1±5.2 | 13.9±5.2 | 77.7±8.8 | |
| Phialophora verrucosa | EA | 4.4±0.7 | 21±8.1 | 66.8±4.7 | 33.2±4.7 | 49.1±7.6 | 67.4±3.4 | 32.6±3.4 | 48.3±6.9 |
| ME | 0.7±0.3 | 7.6±4.8 | 90.7±1.8 | 9.3±1.8 | 88.9±2.3 | 68.0±4.3 | 32.0±4.3 | 48.7±7.8 | |
| Spadicoides sp. | EA | 20.0±1.6 | 19.2±8.8 | 77.3±4.9 | 22.7±4.9 | 64.7±8.6 | 71.6±5.8 | 21.1±2.4 | 64.1±6.7 |
| ME | 3.2±0.3 | 0 | 90.1±2.7 | 10.0±2.7 | 87.5±3.9 | 82.4±4.5 | 17.6±4.5 | 72.5±8.1 | |
| Verticillium sp. | EAd | 3.2±0.7 | 25.3±12.6 | 95.0±1.1 | 5.0±1.1 | 93.7±1.3 | 81.0±3.8 | 19.0±3.8 | 71.9±6.8 |
| ME | 3.0±1.2 | 61.3±11.3 | 83.9±4.6 | 16.1±4.6 | 75.0±8.0 | 66.4±5.6 | 33.6±5.6 | 47.7±9.0 | |
| Volutella sp. | EA | nt | nt | nt | nt | nt | nt | nt | nt |
| ME | 14.4±2.1 | 19.2±13.0 | 83.6±3.9 | 16.4±3.9 | 74.4±7.6 | 68.5±4.4 | 31.5±4.4 | 49.3±7.8 | |
| Fermented rice (blank) | EA | 15.0±1.2 | 27.1±9.7 | 63.6±5.14 | 36.4±5.1 | 43.0±8.2 | 68.8±3.2 | 31.2±3.2 | 48.4±7.3 |
| ME | −0.5±0.5 | 13.8±6.4 | 64.8±3.6 | 35.2±3.6 | 46.5±5.8 | 54.7±1.9 | 45.3±1.9 | 18.5±4.7 | |
EA: ethyl acetate extract, ME: methanol extract, M. j.: Meloidogyne javanica, S. l.: Spodopera littoralis, nt: not tested.
Dose–response evaluation of active fungal extracts from Clonostachys rosea and Gliomastix masseei on settling inhibition of Myzus persicae and Rhopalosiphum padi
| Fungal strain/solvent | Concentration μg/cm2 | Myzus persicae | Rhopalosiphum padi | ||||||
|---|---|---|---|---|---|---|---|---|---|
| %C | %T | %SIa | EC50 (CL) μg/cm2 | %C | %T | %SIa | EC50 (CL) μg/cm2 | ||
| Clonostachys rosea MeOH extract | 200 | 96.7±2.1 | 3.3±2.1 | 95.1±3.4 | 8 (6, 10) | 87.9±2.1 | 12.1±2.1 | 84.6±2.9 | 38 (25, 48) |
| 100b | 94.4±1.2 | 5.7±1.2 | 93.3±1.4 | 86.6±2.0 | 13.4±2.0 | 81.5±3.3 | |||
| 50 | 88.0±3.5 | 12.0±3.5 | 83.8±5.4 | 72.2±4.0 | 27.8±4.0 | 54.3±7.9 | |||
| 20 | 79.5±3.8 | 20.5±3.8 | 70.0±6.5 | ||||||
| 4 | 57.7±4.6 | 42.3±4.6 | 33.7±7.2 | ||||||
| Gliomastix masseei Ethyl acetate extract | 200 | 96.8±1.6 | 3.2±1.6 | 96.0±2.0 | 15 (13, 18) | 92.6±2.0 | 7.4±2.0 | 91.0±2.5 | 19 (16, 23) |
| 100b | 93.7±1.7 | 6.3±1.7 | 91.7±2.5 | 93.3±1.3 | 6.7±1.3 | 91.9±1.7 | |||
| 50 | 82.0±2.9 | 18.0±2.9 | 74.6±5.3 | 77.3±3.7 | 22.7±3.7 | 66.6±6.5 | |||
| 20 | 68.3±4.5 | 31.7±4.5 | 50.9±7.7 | 60.7±2.7 | 39.3±2.7 | 34.3±5.6 | |||
| 4 | 49.2±6.2 | 50.8±6.2 | 22.6±8.7 | 57.5±2.2 | 42.5±2.2 | 26.5±5.2 | |||
| ARGM-1A | 50 | 90.6±2.5 | 9.4±2.5 | 87.7±3.8 | 78.9±2.6 | 21.1±2.6 | 70.8±4.6 | ||
| ARGM-1B | 50 | 93.9±2.0 | 6.1±2.0 | 92.5±2.5 | 83.3±3.1 | 16.7±3.1 | 76.5±5.3 | ||
SI: settling inhibition in percentage±standard error; SI=[1−(%T/%C)]×100; where%T and%C are the percentage of aphids settled on the treated and control leaf pieces, respectively, after 24h; n=20 replicates, 40 replicates.
EC50 (CL): effective concentration (confidence limits) for settling inhibition. EC50 values are considered significantly different if confidence limits do not overlap.
nt=not treated.
Fraction ARGM-1A: hexane fraction after partition of EA extract.
Fraction ARGM-1B: acetonitrile fraction after partition of EA extract.
Molecular identification by using ITS1 and ITS4 primers resulted in a DNA band of ∼650bp. After edition, 561bp were used as query in BLAST, finding the highest homology with G. masseei (first hit accession AB540554.1). Query cover about 97% of known G. masseei ITS fragment, with an E value of 0.0 and 99% of identity.
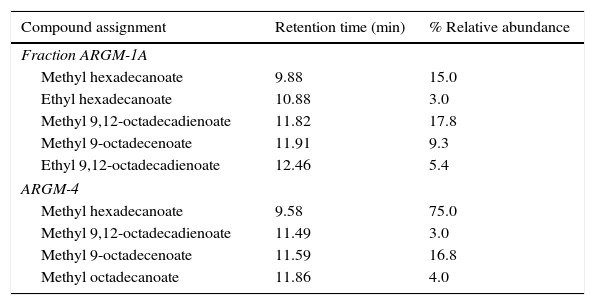
The chemical composition of the EA extract of G. masseei was determined by a bioassay-guided fractionation. The hexane (ARGM-1A) and acetonitrile (ARGM-1B) fractions had strong effects on settling inhibition of M. persicae (>85%) and R. padi (SI>70%) (Table 3). Analysis of fraction ARGM-1A and its methyl derivative (ARGM-4) by GC–MS and by comparison with commercial standards (free and methyl esters) revealed the presence of four fatty acids as major components. Fatty acids included hexadecanoic acid (16:0), octadecanoic acid (18:0), 9-octadecenoic acid (18:1) and 9,12-octadecadienoic acid (18:2) (Table 4). The most abundant components were hexadecanoic acid (74%) and 9,12-octadecadienoic acid (16.8%). In addition, ARGM-1A fraction was methylated and the product, ARGM-4, also had an effect on the settling of both aphid species.
Analysis of the chemical constituents of Gliomastix masseei hexane fraction (ARGM-1A) and its methylated derivative (ARGM-4) by GC–MS techniques
| Compound assignment | Retention time (min) | % Relative abundance |
|---|---|---|
| Fraction ARGM-1A | ||
| Methyl hexadecanoate | 9.88 | 15.0 |
| Ethyl hexadecanoate | 10.88 | 3.0 |
| Methyl 9,12-octadecadienoate | 11.82 | 17.8 |
| Methyl 9-octadecenoate | 11.91 | 9.3 |
| Ethyl 9,12-octadecadienoate | 12.46 | 5.4 |
| ARGM-4 | ||
| Methyl hexadecanoate | 9.58 | 75.0 |
| Methyl 9,12-octadecadienoate | 11.49 | 3.0 |
| Methyl 9-octadecenoate | 11.59 | 16.8 |
| Methyl octadecanoate | 11.86 | 4.0 |
Purification of the second fraction ARGM-1B by silica gel columns yielded a complex mixture of compounds which included ergosterol. This compound was isolated and identified by comparison with a commercial sample (E6510-10G, Sigma–Aldrich). The subfractions were not active on aphid settling.
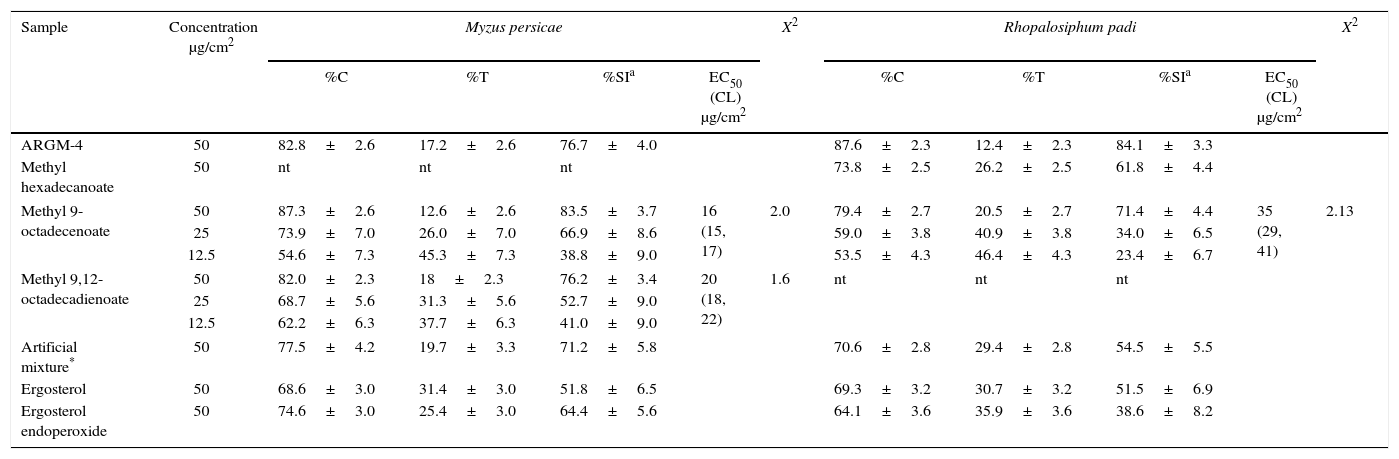
The compounds isolated in this work and commercial standards were evaluated on both aphid species (Table 5). Methyl hexadecanoate had a low effect on the settling of R. padi and was not tested on M. persicae. Methyl 9-octadecenoate strongly inhibited the settling of both aphid species (SI>70%); likewise, methyl 9,12-octadecadienoate only inhibited the settling of M. persicae (SI>70%). Ergosterol showed no activity. Dose–response evaluation of both active pure esters showed that methyl 9-octadecenoate was the most active compound, with an EC50 value of 16μg/cm2 for M. persicae and 35μg/cm2 for R. padi (Table 5).
Effects of methylated fraction (ARGM-4) from Gliomastix masseei, and methyl esters from commercial fatty acids on settling of Myzus persicae and Rhopalosiphum padi and dose–response evaluation of the most active methyl esters
| Sample | Concentration μg/cm2 | Myzus persicae | X2 | Rhopalosiphum padi | X2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| %C | %T | %SIa | EC50 (CL) μg/cm2 | %C | %T | %SIa | EC50 (CL) μg/cm2 | ||||
| ARGM-4 | 50 | 82.8±2.6 | 17.2±2.6 | 76.7±4.0 | 87.6±2.3 | 12.4±2.3 | 84.1±3.3 | ||||
| Methyl hexadecanoate | 50 | nt | nt | nt | 73.8±2.5 | 26.2±2.5 | 61.8±4.4 | ||||
| Methyl 9-octadecenoate | 50 | 87.3±2.6 | 12.6±2.6 | 83.5±3.7 | 16 (15, 17) | 2.0 | 79.4±2.7 | 20.5±2.7 | 71.4±4.4 | 35 (29, 41) | 2.13 |
| 25 | 73.9±7.0 | 26.0±7.0 | 66.9±8.6 | 59.0±3.8 | 40.9±3.8 | 34.0±6.5 | |||||
| 12.5 | 54.6±7.3 | 45.3±7.3 | 38.8±9.0 | 53.5±4.3 | 46.4±4.3 | 23.4±6.7 | |||||
| Methyl 9,12-octadecadienoate | 50 | 82.0±2.3 | 18±2.3 | 76.2±3.4 | 20 (18, 22) | 1.6 | nt | nt | nt | ||
| 25 | 68.7±5.6 | 31.3±5.6 | 52.7±9.0 | ||||||||
| 12.5 | 62.2±6.3 | 37.7±6.3 | 41.0±9.0 | ||||||||
| Artificial mixture* | 50 | 77.5±4.2 | 19.7±3.3 | 71.2±5.8 | 70.6±2.8 | 29.4±2.8 | 54.5±5.5 | ||||
| Ergosterol | 50 | 68.6±3.0 | 31.4±3.0 | 51.8±6.5 | 69.3±3.2 | 30.7±3.2 | 51.5±6.9 | ||||
| Ergosterol endoperoxide | 50 | 74.6±3.0 | 25.4±3.0 | 64.4±5.6 | 64.1±3.6 | 35.9±3.6 | 38.6±8.2 | ||||
ARGM-4: methylated ARGM-1A fraction.
Artificial mixture: methyl hexadecanoate, methyl 9-octadecenoate, and methyl 9,12-octadecadienoate (27:6:1).
Settling inhibition in percentage±standard error; SI=[1−(%T/%C)]×100, where%T and%C are the percentage of aphids settled on the treated and control leaf pieces, respectively, after 24h; n=20 replicates.
EC50 (CL): effective concentration (confidence limits) for settling inhibition. EC50 values are considered significantly different if confidence limits do not overlap.
nt=not tested.
In the present work, the evaluation of organic extracts of microfungi showed that the ME and EA extracts had different effects on the plant pest targets. The extracts had very low or no effect on S. littoralis or M. javanica. However, various extracts displayed a strong effect on the settling of aphids M. persicae and R. padi. To our knowledge, the fungal extracts evaluated in the present work had not been previously assessed on aphid settling. The results showed that 30% of the evaluated fungi produced metabolites that deterred aphids. The most active were the EA extract of G. masseei (EC50, 15–19μg/cm2) and the ME extract of C. rosea (EC50, 8–38μg/cm2).
The molecular analysis identified fungus G. masseei. Minor discrepancies between the query and retrieved sequences were observed, however identity was supported by the congruency of molecular identification with traditional taxonomy performed according to Kiyuna et al.19. Thus, the ITS fragment was enough to identify the fungus and discrepancies can be attributed to usual polymorphism among strains. The fungus G. masseei is a cosmopolitan species that has antimicrobial properties28; however, no studies on identification of secondary metabolites produced by this species have been reported. The primary fractionation (ARGM-1A and 1B) of the extract revealed that G. masseei produces metabolites that affect aphid settling. These metabolites were detected in the low and medium polarity fractions. In the low polarity fraction, fatty acids and their derivatives are the most abundant components. Although hexadecanoic acid (75%) was the major constituent, its effect on the settling of M. persicae was produced only by their derivatives methyl and ethyl esters (5.5–8.7μg/cm2), in agreement with previous reports32. Surprisingly, these compounds showed no effect on the settling of R. padi. Other components of the low polarity fraction were the methyl esters of 9,12-octadecadienoic acid (17.8%) and 9-octadecenoic acid (9.3%). In this case, previous studies have shown that the acid forms of these compounds have anti-aphid properties8. In the present study, methyl 9-octadecenoate was the most active compound with an EC50 of 16 and 35μg/cm2, for M. persicae and R. padi, respectively (Table 5). Methyl 9,12-octadecadienoate, in contrast, only inhibited the settling of M. persicae (EC50=20μg/cm2), but not of R. padi.
During the purification of the extract, we observed that the settling inhibition activity remained in low and medium polarity fractions for M. persicae, but decreased for R. padi, where SI values decreased from crude extract (91.9) to 70 and 76%, respectively. An opposite trend was observed for the natural methylated ARGM-4 fraction (SI=84%). We then verified the activity of the isolated methyl ester mixture and an artificial mixture of commercial standards of the major constituents present in the fraction ARGM-1A. The mixture was prepared with the methyl esters of the hexadecanoic, 9,12-octadecadienoic, and 9-octadecenoic acids (27:6:1). The methyl 9-octadecenoate and natural mixture of hexane fraction inhibited the settling of both aphids (SI>70%); however, 9,12-octadecadienoate and the artificial mixture only inhibited the settling of M. persicae, in the same manner as the saturated methyl hexadecanoate. These data showed that on esterified fatty acids, the pattern for anti-aphid activity is different in comparison with that of the free unsaturated fatty acids8. The difference between the effects on the settling of R. padi caused by the artificial and natural mixtures can be attributed to the presence of other minor unknown constituents in the latter.
The biological activity of fatty acids and their derivatives on pest insects have been previously reported27. For example, 9,12-octadecadienoic acid has shown activity on Aedes aegypti, Callosobruchus maculates, Lymantria dispar, Liposcelis bostrychophila, and Malacosoma disstria; 9-octadecenoic acid on Anopheles aegypti, Anopheles stephensi; 9,12-octadecadienoic, hexadecanoic and octadecanoic acids on S. frugiperda1,18,25. Fatty acids can be extracted from plants and from fungi. In this sense, various studies have found that different species of fungi, such as Aspergillus ustus, Cladosporium sp., Curvularia sp., Penicillium sp., and Rhizopus oryzae produce this type of compounds23,24,31.
The TLC and GC–MS analyses of acetonitrile fraction of G. masseei showed a complex mixture of compounds, where traces of ergosterol, a component of the fungal membrane, were observed. Ergosterol is commonly oxidized in the presence of light into ergosterol endoperoxide. There are various reports on the strong antimicrobial activity of ergosterol endoperoxide, but no data on its effect against insects has been published5,30. Although ergosterol endoperoxide was not detected in the G. masseei extracts, its effect was evaluated on aphid settling, where this compound slightly inhibited the settling of M. persicae and R. padi (Table 5).
It is important to note that the activity of the full extract of G. masseei, compared to that of the fractions, subfractions and commercial standards, suggests that the anti-aphid effect was most likely due to the synergistic effect of the known and unknown minor components; however, further investigation is required in this regard.
The fungus C. rosea is a cosmopolitan species which has shown a wide range of biological activities, in particular as a biocontrol agent against phytopathogenic fungi and nematodes21,34. The activity in this case has been attributed to secondary metabolites, such as gliocladine A-E, verticillin A and 11′-deoxy-verticillin A, Sch52900, and Sch5290111. In previous works, we have found that the EA extract of C. rosea is active against human pathogenic bacteria, plant pathogenic fungi, and plant parasitic nematodes13,14. Surprisingly, in the present work, the EA extract of C. rosea showed no activity on M. javanica.
It is also pertinent to mention that the effect of the most active fungal extracts evaluated in the present work was similar to that of other natural products isolated from microorganisms or plants3.
In summary, the settling inhibition capacity of the fungal extracts screened in this work highlights the potential of these compounds for biorational plant protection. ME extracts of C. rosea and EA extracts of G. masseei showed the highest anti-settling activity on M. persicae and R. padi. Furthermore, we observed that ester derivatives of hexadecanoic, 9-octadecenoic and 9,12-octadecadienoic acids were some of the active principles and the most abundant components in the EA extract of G. masseei, which may have a synergistic effect with other unknown minor constituents. This work also contributes with insights into the biological activity of natural products derived from micromycetes isolated from the tropical regions of Mexico.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data are included in this article.
Right to privacy and informed consentThe authors declare that no patient data are included in this article.
Conflict of interestThe authors declare that there is no conflict of interests regarding the publication of this article.
This research was supported by Conacyt (Grants 47549 and 2009/CB131256, respectively); our thanks also go to Conacyt for the postgraduate scholarship to A.L.R.J. (Conacyt No. 228272). We thank Daniela Hurtado-Cantillo, Miguel Tzec-Sima and Felicia Moo-Koh their assistance in the laboratory.