Mango fruits (Mangifera indica L.) are highly perishable, causing postharvest losses and producing agroindustrial waste. In the present work, native yeasts were used to evaluate ethanol production in overripe mango pulp. The two isolated strains showed similar sequences in the 18S rDNA region corresponding to Kluyveromyces marxianus, being different to the data reported in the NCBI database. Values of up to 5% ethanol (w/v) were obtained at the end of fermentation, showing a productivity of 4g/l/day, a yield of up to 49% of ethanol and a process efficiency of 80%. These results represent a viable option for using the surplus production and all the fruits that have suffered mechanical injury that are not marketable and are considered as agroindustrial waste, thus achieving greater income and less postharvest losses.
Las frutas de mango (Mangifera indica L.) son altamente perecederas, lo cual causa pérdidas poscosecha y produce desechos agroindustriales. En el presente trabajo, se utilizaron 2 levaduras nativas para evaluar la producción de etanol en pulpa de mango senescente. Las 2 cepas aisladas mostraron similitud en la región 18S ADNr, correspondiente a Kluyveromyces marxianus, la cual es diferente a lo reportado en la base de datos del NCBI. Se obtuvieron valores de hasta el 6% de etanol (v/v) al final de la fermentación, con una productividad de hasta 4g/l/día, un rendimiento de hasta 49% de etanol y una eficiencia en el proceso fermentativo del 80%. Esto representa una opción viable para utilizar excedentes de producción o frutos que han sufrido daño mecánico y no son comercializables, al lograr más ingresos y menos pérdida poscosecha.
Mango fruits (Mangifera indica L.) are highly perishable and, under tropical conditions, ripen within 6–7 days and become overripe and spoiled within 15 days after harvest13. The overripe mangoes, characterized by over softening, desiccation and microbial infection are not marketable and cause postharvest losses12.
The edible pulp makes up 33–85% of the fresh fruit, while the peel and the kernel corresponds with 7–24% and 9–40%, respectively15. Thus it can take advantage of comprehensive manner of the fruit pulp due to the lack of treatment of overripe mangoes which are considered as agricultural waste. Due to the 18–20%8 of sugar content, alcoholic fermentation is a viable alternative to use surplus mangoes. There is no information about the microbial flora associated with the mango fermentation process. The aim of this work was to evaluate ethanol production from overripe mangoes by fermentation using native yeasts, previously isolated from the fruit itself.
Overripe mangoes (M. indica L.) cv. ‘Haden’ and ‘Tommy Atkins’ were obtained from a local market in Mexico City, Mexico. Mangoes were peeled, ground and the juice was extracted using a Turmix® extractor. Mango juice (20ml) was placed in reactors and incubated for 24h at 27±2°C. Six samples (0.1ml) were taken from each reactor after 24h of culture time. All samples were diluted (10−3), inoculated in Potato Dextrose Agar (PDA, Bioxon®) and incubated at 27°C for 72h. Two different colonies were isolated, purified and conserved in a cryoprotective medium (glycerol and skimmed milk) at −50°C.
The strains designated KM1 and KM3 (isolated from Haden and Tommy-Atkins mangoes, respectively) were propagated in yeast extract peptone dextrose (YPD-Bioxon®) at 30°C for 72h under stirring (200rpm). Biomass was recovered by filtration (Whatman #44) and the recovered cells were used for DNA extraction using the protocol described by Ausubel et al.1 DNA integrity was evaluated by agarose gel electrophoresis (1.5%). PCR primers PN3 (5′-CCGTTGGTGAACCAGCGGAGGGATC-3′) and PN10 (5′-TTCGCTTATTGATATGCTTAAG-3′) were used to amplify a 600bp fragment of the 18S rDNA gene. PCR experiments were performed using a PCR Thermal Cycler Px2 (Thermo Electron) system. A hundred (100) ng of template DNA were used for 25μl PCR reaction, prepared by using Taq DNA polymerase (Invitrogen) according to the manufacturer's instructions in biological triplicates. The DNA fragment was sequenced using the Taq FS Dye Terminator Cycle Sequencing Fluorescence-Based method in an automated model 3730 capillary sequencer (Applied Biosystems, IBT-UNAM, Mexico). The obtained sequences were aligned (MAFFT V6, http://mafft.cbrc.jp/alignment) along with others previously reported. Phylogenetic and molecular evolutionary analyses were done using the MEGA 5 software by neighbor-joining analysis of Tamura-3 parameter distance estimates. The tree robustness was determined by bootstrap analysis (1000 replicates). Homology searching was performed using the BLAST algorithm in the NCBI database.
For ethanol production, KM1, KM3 (isolated in this study) and Saccharomyces cereviseae (Viticulture and Enology Center of Galicia, Spain, EVEGA) were used. The strains were propagated in 25ml of YPD medium, incubated at 27°C (48h), collected and counted in a Neubauer chamber. The juice obtained from overripe Haden (H) and Tommy Atkins (T) mango pulp was used as a substrate for ethanol production. In order to release the sugars present in the mango juice, 0.1% (v/v) of the Novoferm® 61 (Novozymes A/S, Denmark) enzyme complex was added. Sugar content was adjusted at 20°Brix and pH at 4.6. To inhibit bacterial growth, 70mg/l of SO2 were added; 400ml of juice were placed in a 500 ml-glass reactor inoculated (106cell/ml) and incubated at 16°C. °Brix, pH and temperature were monitored. Fermentation was stopped when the °Brix value was stable for three days. Samples were centrifuged (4000rpm/15min) and filtered (0.45μm, Millipore®) for analytical determinations. Sugars (glucose and dextrose) and ethanol quantification were carried out by High Performance Liquid Chromatography (HPLC Varian ProStar) with a refractive index detector (RI ProStar 350) under the following conditions: MetaCarb 67H Organic Acids column (300mm×6.5mm), flow rate of 0.6ml/min, 10μl of sample, 40°C in column and 35°C in the detector for 30min. The mobile phase was 0.04N H2SO4. Glucose, dextrose, ethanol and cocktail stock solution (0–2000ppm) were prepared for the construction of calibration curves. Productivity (g/l/day) was defined as the ratio between the maximum ethanol concentration (g/l) and culture time (day). Ethanol yield (Yp/s) was defined as the ratio between ethanol concentration (g/l) and sugar consumption (g/l). Efficiency percentage was defined as the ratio between ethanol concentration (g/l) and maximum theoretical yield (g/l) multiplied by 100.
To evaluate the effect of the strains used on productivity and efficiency in ethanol production, a completely randomized design with factorial arrangement 2×3×9 was established. Levels were: mango variety with two levels [Haden (H) and Tommy Atkins (T)], strains with three levels [S. cerevisiae (S), Kluyveromyces marxianus KM1 and KM3] and time with nine levels (0, 2, 4, 6, 8, 10, 12, 14 and 16 days). ANOVA was carried out for data analyses using SAS 9.0.
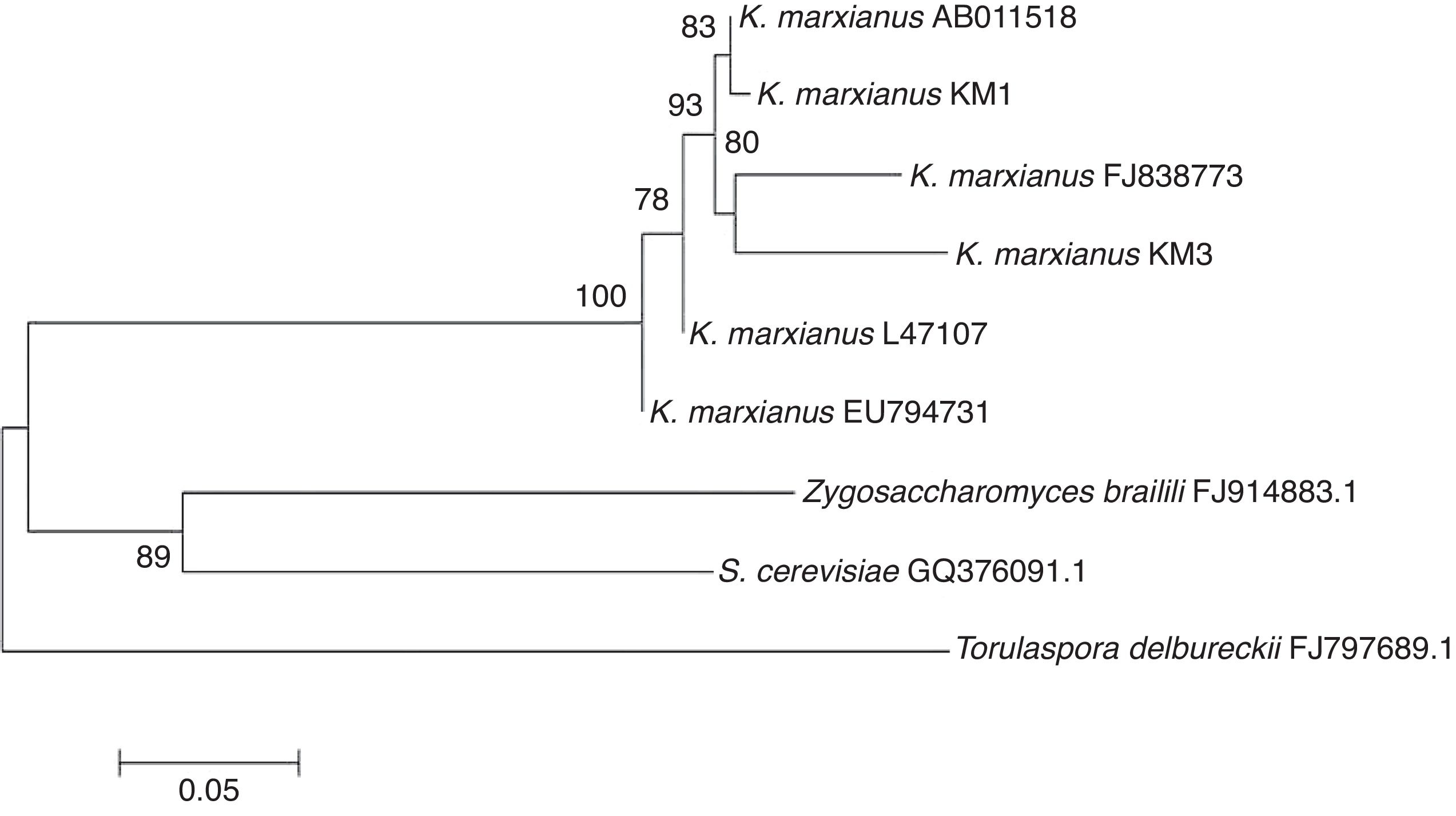
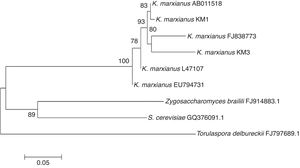
A 600bp fragment of the 18S rDNA was amplified and sequenced from each yeast strain isolated from mango pulp. In this case, the primers used to amplify the 18S rDNA conserved region were universal. A 99% of similarity was observed in the KM1 sequence with that reported for K. marxianus (NCBI accession number AY939806) isolated by Leinberger et al.4, while KM3 had 98% similarity with K. marxianus (access number AY233348) isolated by Millar et al.6
In the present work, all the isolated yeast strains obtained belong to the same genus and species. K. marxianus is also known as Candida kefir, Kluyveromyces bulgaricus, Kluyveromyces fragilis, Candida macedoniensis and Candida tropicalis. The isolated yeasts are similar to those reported by Suresh et al.11 in mango fermentation, who found Candida strains (Candida krusei, Candida sorbosa, Candida tropicalis, Candida diversa) but did not find S. cereviseae.
In another work, Stringini et al.10 reported that S. cereviseae was the predominant species in palm wine fermentation, followed by Saccharomyces ludwigii and Zigosaccharomyces bailli. The presence of K. marxianus in our samples is attributed to sampling time, because it was done in the early stage of fermentation, when the juice had bubbles on the surface as a consequence of CO2 production. Other non-Saccharomyces strains are predominant (Kloeckera, Candida, Pichia, Hansenula, Hanseniaspora and Metschnikowia) in the first steps of the fermentation process, being responsible for secondary metabolite production that modified the organoleptic characteristics of the final product.
The neighbor-joining analysis (Fig. 1) showed that K. marxianus strains were closely related and formed a separate clade in comparison with other non-Kluyveromyces yeasts present during the fermentation process (Zygosaccharomyces, Saccharomyces and Torulaspora). However, genetic differences among the Kluyveromyces strains were found due to the mango varieties. In the first clade, it is interesting to note that K. marxianus KM1 was highly related to K. marxianus AB011518, a strain isolated from deep-seawater in Japan7. In the next clade, the KM3 strain could be observed, which was closely related to K. marxianus FJ838773 isolated from mezcal3. These clades were directly related to K. marxianus L47107, a strain isolated from the oral mucose14. Furthermore, another K. marxianus, strain EU794731, which was isolated from fermented camel milk2, could be observed.
Although KM1 and KM3 were isolated from mango pulp, they have differences in their sequences. These genetic differences suggest the presence of more differences in the yeast genome, which may contribute to different organoleptic characteristics of the fermented product. Strains belonging to K. marxianus have been isolated from several habitats, which suggest metabolic diversity. K. marxianus is a food-grade yeast, and it had been used in several biotechnological applications such as ethanol production fermentation5.
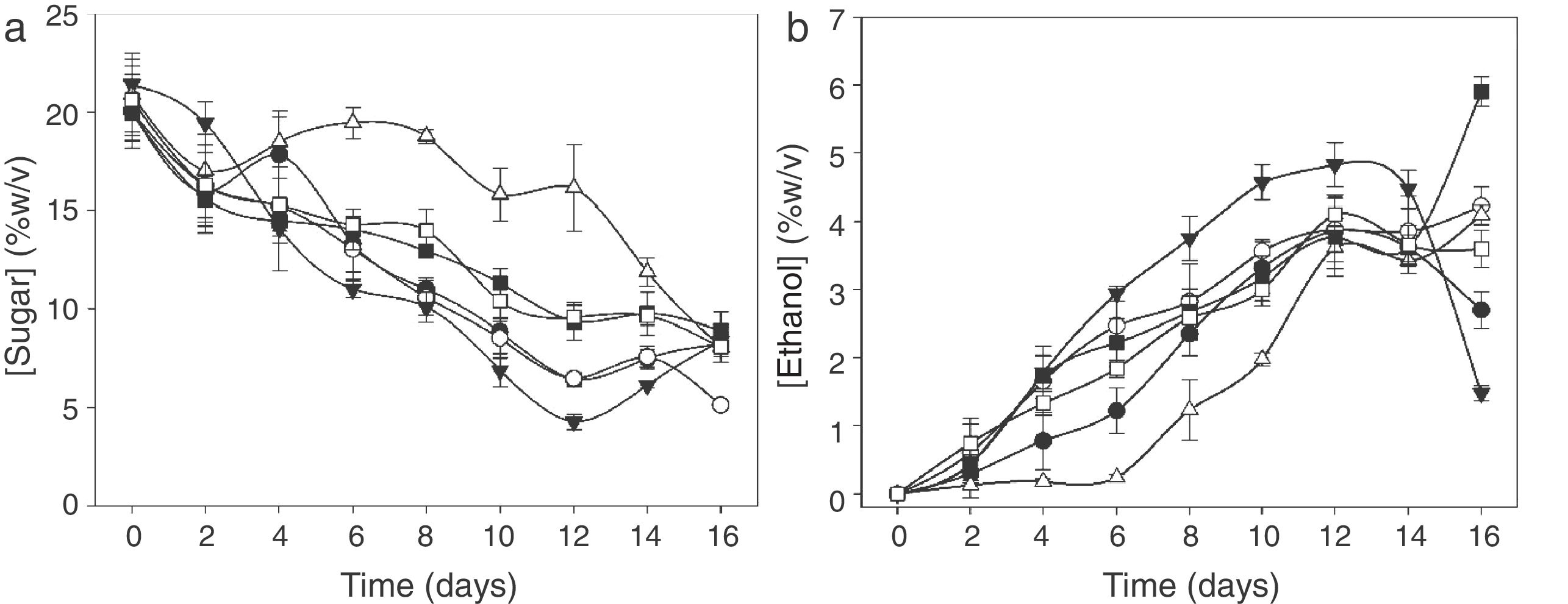
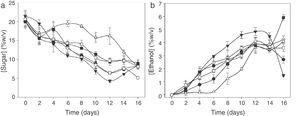
During the time course of the fermentation, a slight decrease in pH value from 4.65 to 4.15 at day 8 (data not shown) was observed. After that, pH value changes were not significant (p≤0.05). Total soluble solids (°Brix) continuously decreased until day 16 when they became stable. At that time, the fermentation process was finished, which agrees with the sugar uptake obtained by HPLC (Fig. 2A).
In all treatments, a continuous reduction in sugar content was observed, reaching the minimum on day 12 of fermentation (Fig. 2A), except for T-KM3, which reached the minimum on day 16. The highest sugar uptake was obtained with H-KM3 on day 12 of culture time.
With regard to ethanol production (Fig. 2B), values increased rapidly, obtaining 5% (w/v) in 12 days with H-KM3 treatment and 6% (w/v) with T-KM1 on day 16 of culture time. H-KM1 and H-KM2 treatments showed an alcohol decrease after 14 days, probably due to ethanol oxidation to acetic acid by the presence of Acetobacter spp.
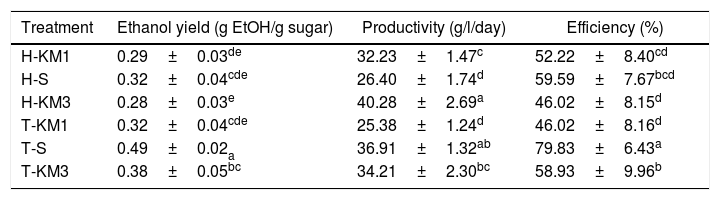
The highest productivity was obtained with H-KM3 (40.28g/l/h) and T-S (36.91g/l/h), with no significant differences among them (Table 1). Lower productivity values were found in H-KM1 and T-KM1 treatments (32.23 and 25.38gl/day, respectively). With respect to process efficiency, the highest value (80%) was observed in T-S, while the rest of the treatments had no significant differences, regardless of the mango variety, showing efficiency values of 46 to 60%. Conversely, Rocha et al.9 reported higher values during banana fermentation. However, they reported the addition of nitrogen and vitamin during the fermentation process, which could influence efficiency.
Values of ethanol yield, productivity and efficiency obtained after 12 days of fermentation process
| Treatment | Ethanol yield (g EtOH/g sugar) | Productivity (g/l/day) | Efficiency (%) |
|---|---|---|---|
| H-KM1 | 0.29±0.03de | 32.23±1.47c | 52.22±8.40cd |
| H-S | 0.32±0.04cde | 26.40±1.74d | 59.59±7.67bcd |
| H-KM3 | 0.28±0.03e | 40.28±2.69a | 46.02±8.15d |
| T-KM1 | 0.32±0.04cde | 25.38±1.24d | 46.02±8.16d |
| T-S | 0.49±0.02a | 36.91±1.32ab | 79.83±6.43a |
| T-KM3 | 0.38±0.05bc | 34.21±2.30bc | 58.93±9.96b |
*Media with the same letter are not significantly different according to the Turkey's test (p<0.01).
Maximum yield was obtained by T-S and T-KM3 (0.49 and 0.38g EtOH/g sugar, respectively). The other treatments had a similar profile, showing a yield ranging from 0.29 to 0.32g EtOH/g sugar. Limtong et al.5 reported an efficiency of 77.5–86% using K. marxianus strains. The highest efficiency value in ethanol production was reached with S. cereviseae (80%).
Regarding productivity, efficiency and yield values, S. cereviseae in Tommy-Atkins mango juice (T-S) was the best treatment to produce ethanol. However, K. marxianus KM3 in Tommy Atkins mango juice (T-KM3) showed promising results, considering that the culture conditions were not the best for strain reproduction, as reported in other studies7. The results obtained with K. marxianus confirm that this strain is not suitable for overripe mango fermentation under the conditions evaluated. However, further studies are needed to find the optimal conditions to improve the fermentation process.
Using the strains previously isolated, purified and identified as K. marxianus, a fermented product from overripe mango pulp containing 5% (w/v) of ethanol, alcohol yields up to 49% with an efficiency of 80% was obtained. The application of biotechnological processes in the production of yeast, enzyme complex and ethanol represent an alternative to optimize the use of overripe mango, representing a viable option to use the surplus production and all the fruits that have suffered mechanical injury and cannot be sent to the market. Thus, these agroindustrial wastes can generate more income for the farmers and less environmental pollution.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed the protocols of their research center on the publication of data generated within the frame of a collaborative agreement between both institutions.
Right to privacy and informed consentThe authors declare that no patient data are included in this article.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Authors thank to the Mexican Council of Science and Technology (CONACyT) for the scholarship assigned for their postgraduate studies in the program of Food Science and Technology, UAdeC.