Plant Growth-Promoting Rhizobacteria (PGPR) of the genus Bacillus are useful as biological control agents by synthesizing and releasing different metabolic antimicrobial compounds such as surfactin, fengycin, hydrocyanic acid, pyrrolnitrin, dimethylhexadecylamine, phenazines and others; however, the antifungal mechanism and the application of these compounds to protect wood-based materials are still not known4. Wood is a lignocellulosic material that is an important source of carbon for insects, bacteria and fungi which have the potential to degrade it and generate significant economic losses3. In the agricultural system, the control mechanisms offered by the PGPR have been an environmentally sustainable alternative to the use of eco-toxic compounds; nonetheless, this strategy has not been adopted in the wood preservation industry. That is why bacterial metabolites can represent a biotechnological option to control the deterioration caused by decay fungi.
To test the above, we used isolates of xylophagous fungi identified using 18S rRNA gene sequences2. In an in vitro system, we confronted fungal isolates to already identified PGPR Bacillus megaterium UMCV1, B. subtilis BS-MIA02 and other Bacillus spp. (BA, SS and BM), we selected the strain with the highest antagonistic activity and performed a methanolic extraction of the bacterial metabolites1. The extracted compounds were incubated with wood pieces of Pinus pseudostrobus in Petri dishes containing Potato Dextrose Agar/Nutrient Agar medium ratio 1:1 at 28°C in the dark for 10 days5. Using ImageJ software6 we recorded the mycelial growth diameter and the percentage of the area colonized in the wood.
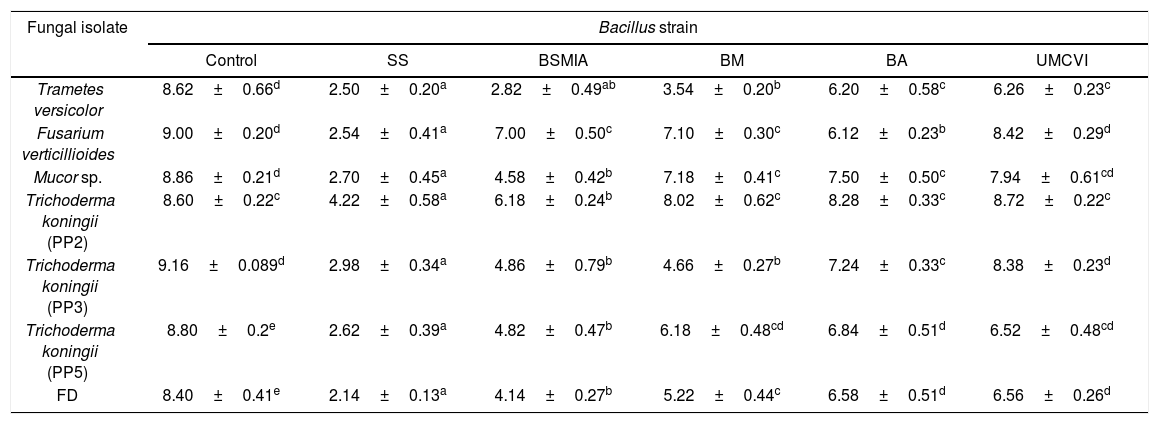
Table 1 shows the growth of fungal isolates confronted to PGPRs and we can observe a strong inhibition of mycelial growth by the five bacteria tested against the six identified fungi and the isolate FD. We also found that of the five bacteria tested, Bacillus sp. SS had the strongest mycelial growth inhibition. Using the metabolic extracts from the strain identified as Bacillus sp. SS, different pieces of wood were impregnated with the extracts and exposed to the fungal isolates. Results in this trial showed that the fungal isolates grew ad libitum on the lignocellulosic material (100% colonization) that did not receive treatment with the metabolic extracts. The pieces of wood that received the protective treatment showed a significant inhibition of fungal development (p<0.05 regarding the untreated pieces), contrary to the percentages of colonization obtained, which were: Trametes versicolor (55.9%), Fusarium verticillioides (1.3%), Mucor sp. (0%), Trichoderma sp. (PP2, PP3 and PP5) (0%) and FD (0%). The metabolic extracts from the Bacillus strains tested in this work inhibited the growth of the xylophagous fungi. Although the metabolomic profile of the PGPRs tested is not known, the results were important and convincing. It has been proven that biological control strategies in field conditions are effective; on the other hand, in forest habitats the exploration is poor. With the information obtained in this work, it can be concluded that there is an interesting profile of metabolic compounds, still uncharacterized, produced by the mentioned tested bacterial isolates, indicating their broad-spectrum antagonism to wood-degrading fungi, as well as the potential or capacity for fungal repression by these metabolites does not show a homogeneous behavior among them. There is still work to do related to the mode of use, minimum repressing concentration, durability in woody tissue, application costs, among others; however, we believe that in the short term the compounds produced by the bacterial strains used here will be identified and used in wood protection susceptible to microbial attack and low durability.
Antagonistic activity of different PGPR strains against isolated xylophagous fungi.
| Fungal isolate | Bacillus strain | |||||
|---|---|---|---|---|---|---|
| Control | SS | BSMIA | BM | BA | UMCVI | |
| Trametes versicolor | 8.62±0.66d | 2.50±0.20a | 2.82±0.49ab | 3.54±0.20b | 6.20±0.58c | 6.26±0.23c |
| Fusarium verticillioides | 9.00±0.20d | 2.54±0.41a | 7.00±0.50c | 7.10±0.30c | 6.12±0.23b | 8.42±0.29d |
| Mucor sp. | 8.86±0.21d | 2.70±0.45a | 4.58±0.42b | 7.18±0.41c | 7.50±0.50c | 7.94±0.61cd |
| Trichoderma koningii (PP2) | 8.60±0.22c | 4.22±0.58a | 6.18±0.24b | 8.02±0.62c | 8.28±0.33c | 8.72±0.22c |
| Trichoderma koningii (PP3) | 9.16±0.089d | 2.98±0.34a | 4.86±0.79b | 4.66±0.27b | 7.24±0.33c | 8.38±0.23d |
| Trichoderma koningii (PP5) | 8.80±0.2e | 2.62±0.39a | 4.82±0.47b | 6.18±0.48cd | 6.84±0.51d | 6.52±0.48cd |
| FD | 8.40±0.41e | 2.14±0.13a | 4.14±0.27b | 5.22±0.44c | 6.58±0.51d | 6.56±0.26d |
Values correspond to the average mycelial growth diameter (cm), different letters show statistical differences according to the Tukey's test (p<0.05, n=8), ± shows the standard deviation.
The authors declare no conflict of interest.
Thanks to CONACYT and UMSNH-CIC for its support in the work.