Interaction between severe acute respiratory coronavirus 2 (SARS-CoV-2) and IIEB remains under investigation. Objective: to compare IIEB incidence before and during COVID-19 pandemic, and assess incidence of coinfection with COVID-19 and case fatality. A cross-sectional study was performed on data from a centralized microbiology laboratory serving a network of healthcare centers comprising 713 pediatric and adult inpatient beds, expanded by 20% during the pandemic. Three periods were evaluated: (1) pre-pandemic: March 1, 2019–February 29, 2020; (2) pandemic year 1: March 1, 2020–February 28, 2021; (3) pandemic year 2: March 1, 2021–July 31, 2021. Descriptive statistical analysis was performed. 56 502 samples (96% blood cultures) from 27224 patients were analyzed. Of these, 54 samples (from 54 patients) were positive for encapsulated bacteria. IIEB incidence was: 167.4, 32.6, and 50.4 per 100000 samples for periods 1, 2, and 3, respectively. Twelve IIEB episodes occurred during the pandemic period: 10 Streptococcus pneumoniae, and 2 Haemophilus influenzae, of which 7 were SARS-CoV-2/S. pneumoniae coinfections, with an incidence of 5.68 per 10000 COVID-19-related hospitalizations (0.056%). IIEB case fatality was 31%, 29%, and 60% for each period, respectively, 3/7 patients with coinfection died (43%). Case fatality for invasive pneumococcal disease (IPD) in patients without COVID-19, was 32.5%. Significant reduction in IIEB incidence was observed during the pandemic, coinciding with implementation of containment measures. The incidence of SARS-CoV-2/S. pneumoniae coinfection was low, with higher case fatality than IPD patients without COVID-19.
La interacción entre SARS-CoV-2 e infecciones invasivas por bacterias capsuladas (IIBC) continúa bajo estudio. Objetivos: comparar la incidencia de IIBC antes y durante la pandemia por COVID-19, evaluar la incidencia de coinfección con COVID-19 y la letalidad. Estudio transversal de registros de un laboratorio centralizado de Microbiología, que asiste a una red de centros asistenciales con 713 camas de internación para adultos y pediátricos, expandida 20% durante la pandemia. Tres periodos evaluados: 1) Pre-pandemia: 1-Marzo-2019 al 29-Febrero-2020; 2) Primer año de Pandemia: 1-Marzo-2020 al 28-Febrero-2021; 3) Pandemia 2021: 1-Marzo-2021 al 31-Julio-2021. Análisis estadístico descriptivo: Se analizaron 56.502 muestras (96% hemocultivos) correspondientes a 27.224 pacientes. De estas, 54 muestras (de 54 pacientes) fueron positivas para bacterias capsuladas. La incidencia de IIBC fue 167,4, 32,6 y 50,4 por cada 100.000 muestras para los periodos 1, 2 y 3, respectivamente. Doce IIBC ocurrieron durante la pandemia: 10 Streptococcus pneumoniae y dos Haemophilus influenzae, siete de ellos corresponden a coinfección SARS-CoV-2/S. pneumoniae, con una incidencia de 5,68 por cada 10.000 internaciones por COVID 19 (0,056%). La letalidad de las IIBC fue de 31, 29 y 60% para los tres periodos, respectivamente, 3/7 coinfectados fallecieron (43%). La letalidad por enfermedad neumocócica invasiva (ENI), sin COVID fue de 32,5%. Se evidenció una reducción significativa de la incidencia de IIBC luego del comienzo de la pandemia, coincidente con la implementación de las medidas sanitarias de contención de la pandemia. La incidencia de coinfección de SARS-CoV-2/S. pneumoniae fue baja y presentó mayor letalidad que las ENI sin COVID-19.
Invasive bacterial infections such as bacteremic pneumonia, empyema, meningitis, and sepsis are life threatening conditions. Encapsulated bacteria, namely Streptococcus pneumoniae, Haemophilus influenzae and Neisseria meningitidis are among the most common pathogens, causing a high burden of disease, especially in children under 5 years of age, adults over 65 years of age and in individuals with comorbidities. Mortality rates reaching up to 36% for invasive pneumococcal disease (IPD) in winter, made prevention of invasive infections a priority for WHO, leading to the development of effective vaccines9,10,12. Bacterial pathogens habitually colonize the respiratory tract, particularly in children, presenting a commensal relationship with the host unless local or systemic immune deficiencies facilitate invasion into sterile tissues26. Although transmission mechanisms are still under investigation, person-to-person spread through exposure to infectious secretions is widely accepted as the main source. Recent animal models have shown airborne transmission also occurs between close contacts, enhanced by coinfection with respiratory viruses27. A paradigmatic example is synergism between influenza viruses and S. pneumoniae contributing to pulmonary superinfection25. Since December 2019, emergence of a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing coronavirus disease 2019 (COVID-19) has threatened global health. On March 3, 2020, the first case was reported in Argentina and on March 11, WHO declared COVID-19 a global pandemic. To control spread of the virus, compulsory containment measures were established in Argentina on March 20, including discontinuation of non-essential services such as schools, public or private social gatherings, and restricted use of public transport. Essential workers (in healthcare, security services, among others) were excluded2,7. Social distancing, use of face masks and adequate ventilation of indoor spaces were recommended. Individuals over the age 60 and/or immunocompromised individuals were encouraged to stay at home2,23. Although adherence to these measures declined as the epidemic evolved, use of face masks was sustained over time.
Large-scale application of containment measures, as shown by various studies, led to a significant reduction in incidence of other viral infections such as influenza, Respiratory Syncytial Virus and Enterovirus4,6,11,14,22,28. Interaction between SARS-CoV-2 and encapsulated bacteria is still under investigation, and frequency of coinfection has yet to be established. Other questions of interest include, whether health strategies for pandemic containment and mitigation influenced incidence of invasive infections due to encapsulated bacteria (IIEB), as occurs with other viral pathogens, and whether coinfection increased case fatality rates. The main objective of this study was to evaluate IIEB rates during the COVID-19 pandemic and compare incidence levels to the ones reported during the year prior to the pandemic. As a secondary objective, incidence of COVID-19/IIEB coinfection and case fatality rate were also examined.
MethodsA cross-sectional study was performed. Data from a centralized microbiology laboratory serving a network of privately administered healthcare institutions in Buenos Aires City (CABA) and the greater Buenos Aires Metropolitan Area (BAMA) were collected. The network includes 713 inpatient beds for children and adults, which were expanded by 20% during the pandemic. Three periods were evaluated: (1) pre-pandemic: March 1, 2019–February 29, 2020; (2) pandemic year 1: March 1, 2020–February 28, 2021; and (3) pandemic year 2: March 1, 2021–July 31, 2021.
Biological samples collected from sterile sites (blood, cerebrospinal fluid, pleural fluid, synovial fluid, etc.) of patients attending the emergency departments, general wards and intensive care units of the network centers were analyzed. Samples positive for S. pneumoniae, H. influenzae, and N. meningitidis were identified, and clinical characteristics, laboratory results, and outcome of patients with IIEB collected from medical records. During the second and third period, occurrence of COVID-19 (confirmed by positive RT-PCR) and IIEB coinfection was investigated and considered primary (when simultaneous to IIEB) or secondary (diagnosed at least 3 days after hospital admission). In-hospital case fatality rate of IIEB was determined.
Descriptive statistical analysis was conducted. Categorical variables were expressed as absolute and relative frequencies. Continuous variables were characterized with measures of central tendency (mean and median) and measures of dispersion (ranges).
The study was approved by the Institutional Review Board. Sensitive patient data were protected by confidentiality and coding. Only investigators directly related to the research and competent authorities had access to the information.
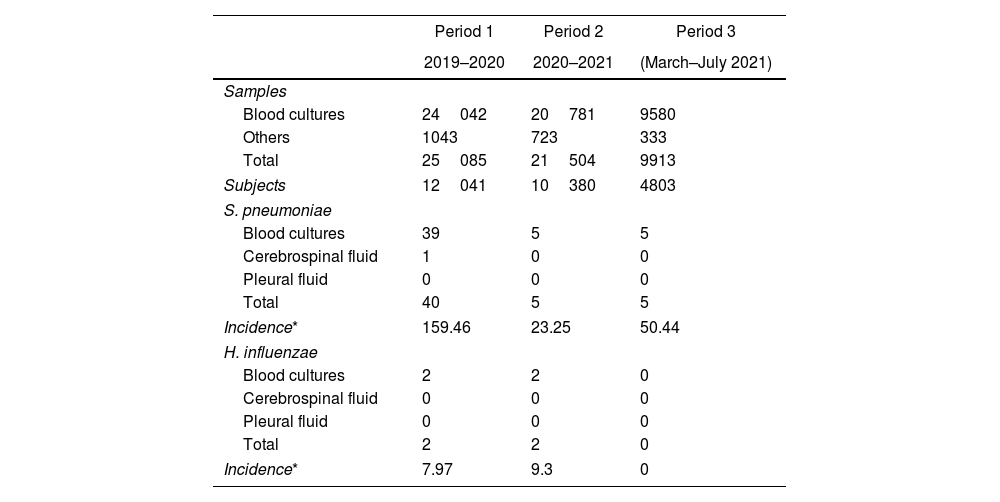
ResultsBetween March 1, 2019 and July 31, 2021, a total of 56502 biological samples belonging to 27224 patients were assessed; 54403 (96%) were blood cultures and 2099 (4%) from other sterile sites. Fifty-four samples from 54 subjects were positive for encapsulated bacteria, of which 50 were S. pneumoniae and 4 H. influenzae. N. meningitidis was not found during the study period (Table 1).
Samples and culture results according to time period.
| Period 1 | Period 2 | Period 3 | |
|---|---|---|---|
| 2019–2020 | 2020–2021 | (March–July 2021) | |
| Samples | |||
| Blood cultures | 24042 | 20781 | 9580 |
| Others | 1043 | 723 | 333 |
| Total | 25085 | 21504 | 9913 |
| Subjects | 12041 | 10380 | 4803 |
| S. pneumoniae | |||
| Blood cultures | 39 | 5 | 5 |
| Cerebrospinal fluid | 1 | 0 | 0 |
| Pleural fluid | 0 | 0 | 0 |
| Total | 40 | 5 | 5 |
| Incidence* | 159.46 | 23.25 | 50.44 |
| H. influenzae | |||
| Blood cultures | 2 | 2 | 0 |
| Cerebrospinal fluid | 0 | 0 | 0 |
| Pleural fluid | 0 | 0 | 0 |
| Total | 2 | 2 | 0 |
| Incidence* | 7.97 | 9.3 | 0 |
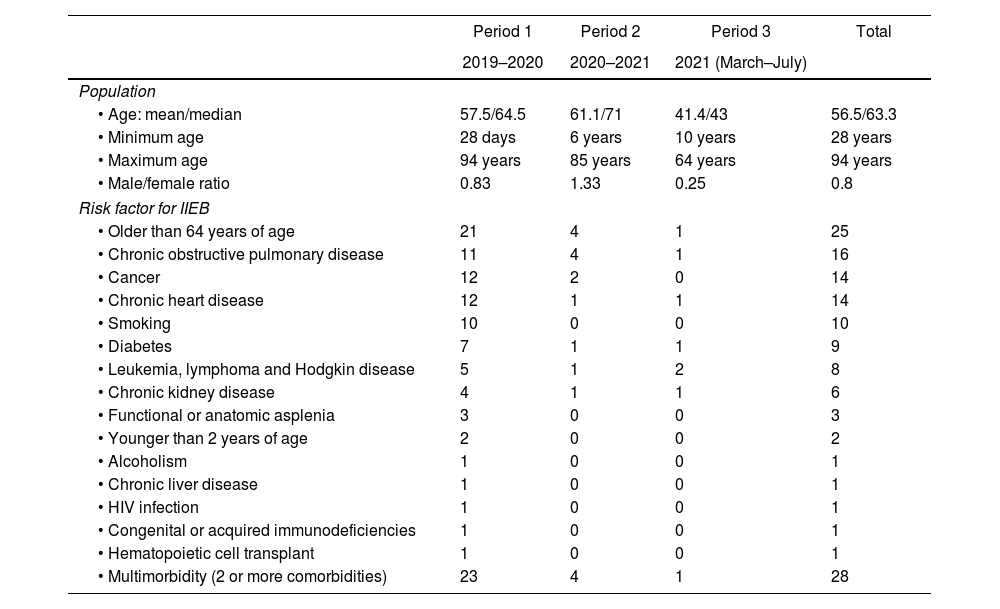
Demographics of the study population and risk factors for IIEB are shown in Table 2.
Characteristics of the study population by time period.
| Period 1 | Period 2 | Period 3 | Total | |
|---|---|---|---|---|
| 2019–2020 | 2020–2021 | 2021 (March–July) | ||
| Population | ||||
| • Age: mean/median | 57.5/64.5 | 61.1/71 | 41.4/43 | 56.5/63.3 |
| • Minimum age | 28 days | 6 years | 10 years | 28 years |
| • Maximum age | 94 years | 85 years | 64 years | 94 years |
| • Male/female ratio | 0.83 | 1.33 | 0.25 | 0.8 |
| Risk factor for IIEB | ||||
| • Older than 64 years of age | 21 | 4 | 1 | 25 |
| • Chronic obstructive pulmonary disease | 11 | 4 | 1 | 16 |
| • Cancer | 12 | 2 | 0 | 14 |
| • Chronic heart disease | 12 | 1 | 1 | 14 |
| • Smoking | 10 | 0 | 0 | 10 |
| • Diabetes | 7 | 1 | 1 | 9 |
| • Leukemia, lymphoma and Hodgkin disease | 5 | 1 | 2 | 8 |
| • Chronic kidney disease | 4 | 1 | 1 | 6 |
| • Functional or anatomic asplenia | 3 | 0 | 0 | 3 |
| • Younger than 2 years of age | 2 | 0 | 0 | 2 |
| • Alcoholism | 1 | 0 | 0 | 1 |
| • Chronic liver disease | 1 | 0 | 0 | 1 |
| • HIV infection | 1 | 0 | 0 | 1 |
| • Congenital or acquired immunodeficiencies | 1 | 0 | 0 | 1 |
| • Hematopoietic cell transplant | 1 | 0 | 0 | 1 |
| • Multimorbidity (2 or more comorbidities) | 23 | 4 | 1 | 28 |
The most common causes for hospitalization were: pneumonia (28 cases) and diarrhea (2) during period 1; suspected SARS-CoV-2 infection (3), pneumonia (1), acute-on-chronic kidney disease (1) and febrile neutropenia and abdominal pain (1) during period 2; and SARS-CoV-2 infection (4) in period 3.
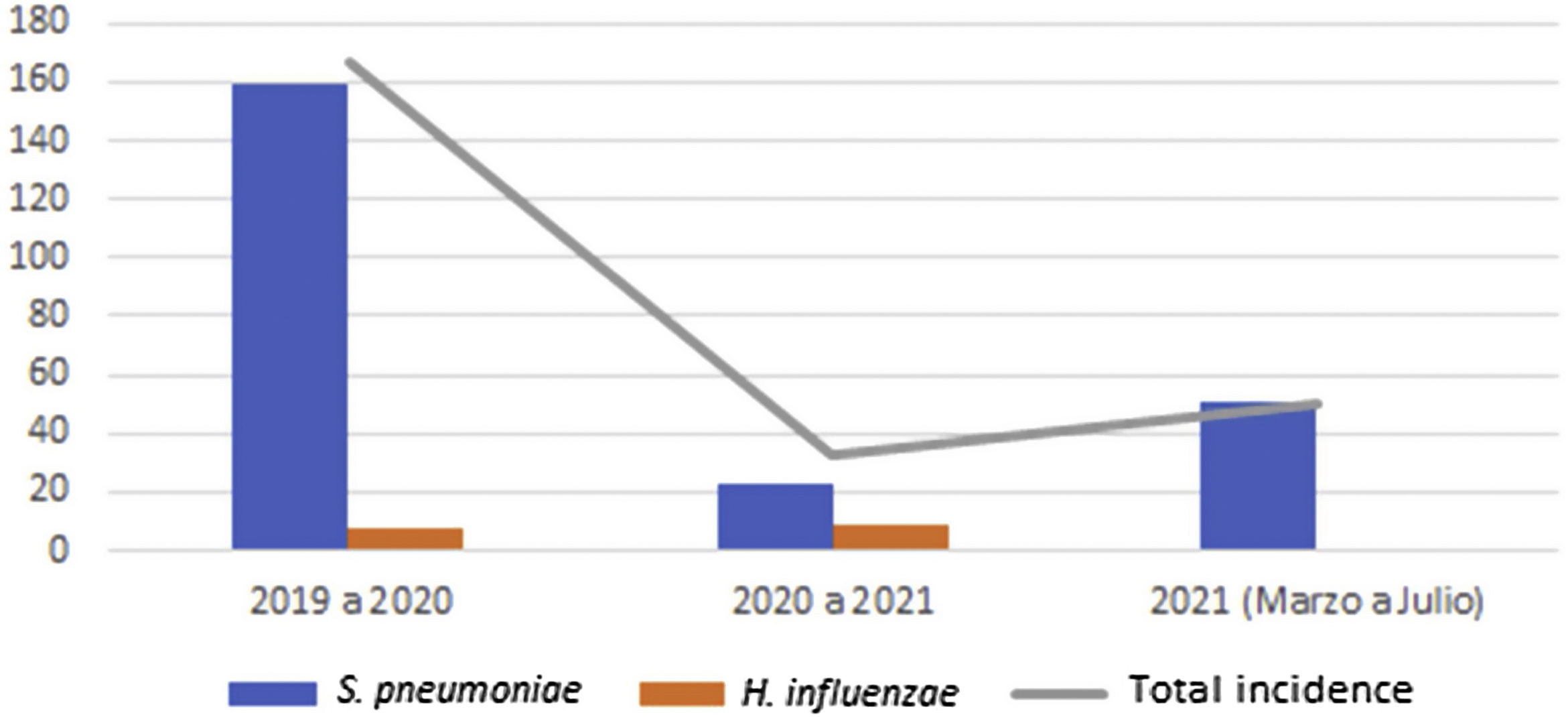
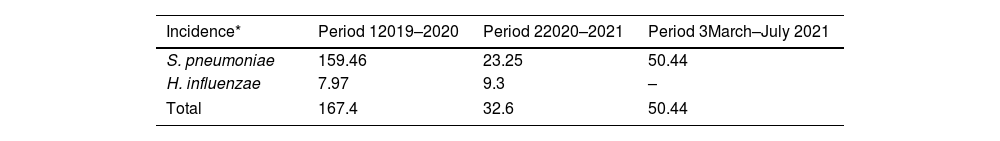
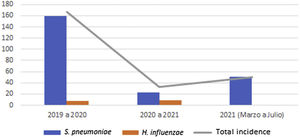
Global cumulative incidence of IIEB was 95.57 per 100000 samples. Comparative analysis showed incidence varied by period (Table 3 and Fig. 1).
IIEB incidence according to time period and pathogen.
| Incidence* | Period 12019–2020 | Period 22020–2021 | Period 3March–July 2021 |
|---|---|---|---|
| S. pneumoniae | 159.46 | 23.25 | 50.44 |
| H. influenzae | 7.97 | 9.3 | – |
| Total | 167.4 | 32.6 | 50.44 |
Between the first and second period, cumulative incidence of S. pneumoniae infections decreased by 136.21 cases per 100000 samples (relative decrease 0.15). However, between the second and third, 27.19 more cases per 100000 samples (relative increase 2.17) were observed.
Cumulative incidence of H. influenzae infections between periods 1 and 2 showed an increase of 1.33 cases per 100000 samples (relative increase 1.17).
During periods 2 and 3, total number of COVID-19 hospitalizations was 12328; 12 IIEB occurred, of which 10 were due to S. pneumoniae and 2 to H. influenzae. Seven cases of SARS-CoV-2/S. pneumoniae coinfection were identified, 2 primary and 5 secondary. Coinfection rate was 0.056% in patients hospitalized for COVID-19, corresponding to an incidence of 5.68 per 10000 hospitalizations for COVID-19. However, in a subanalysis of patients hospitalized with IPD, we found that 7 of 10 subjects were co-infected with SARS-COV-2.
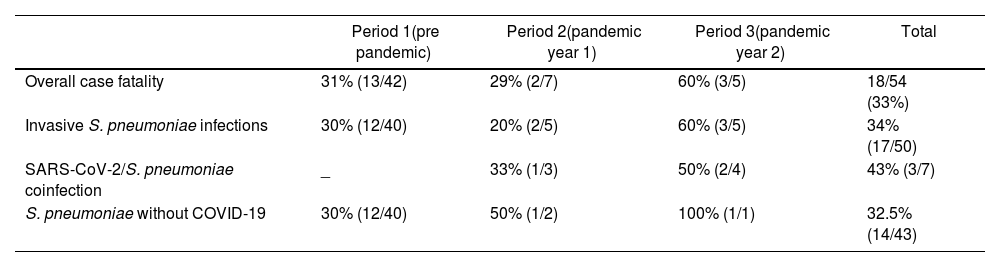
Overall case fatality in IIEB patients was 33%, (31%, 29%, and 60% for periods 1, 2 and 3 respectively) (Table 4).
Case fatality of IIEB according to pathogen and time period.
| Period 1(pre pandemic) | Period 2(pandemic year 1) | Period 3(pandemic year 2) | Total | |
|---|---|---|---|---|
| Overall case fatality | 31% (13/42) | 29% (2/7) | 60% (3/5) | 18/54 (33%) |
| Invasive S. pneumoniae infections | 30% (12/40) | 20% (2/5) | 60% (3/5) | 34% (17/50) |
| SARS-CoV-2/S. pneumoniae coinfection | _ | 33% (1/3) | 50% (2/4) | 43% (3/7) |
| S. pneumoniae without COVID-19 | 30% (12/40) | 50% (1/2) | 100% (1/1) | 32.5% (14/43) |
All deaths occurred in patients with S. pneumoniae infections, except for 1 patient with H. influenzae in period 1. Case fatality of SARS-C0V-2/S. pneumoniae coinfection reached 43% (3 of 7 subjects), whereas case fatality of IPD cases without COVID-19 coinfection was 32.5%.
Overall, all-cause in-hospital mortality in COVID-19 patients between March 1, 2020, and July 31, 2021, was 4% (492 deaths).
DiscussionA significant reduction in incidence of IPD was observed during the period 2020–2021, coinciding with the implementation of large-scale containment measures to mitigate the spread of the COVID-19 pandemic. This reduction was more pronounced during the first year of the pandemic (period 2), probably because more stringent restrictions were enforced. Only 4 cases of invasive H. influenzae infections occurred over the whole study period, with no differences observed between periods.
From March to July 2021, a small increase in the incidence of IPD was observed compared to the first pandemic year, coinciding with relaxing of restrictions. Median age of hospitalized patients with IIEB was younger (43 years) during period 3 than during periods 2 (70 years) and 1 (64 years), in line with the decrease in average age of COVID-19 hospitalized patients in 2021, during the second disease wave in Argentina, and the launch of a vaccination program for healthcare workers and adults over 60 years of age, which began in the first quarter of 2021.
During the study period we observed a reduction in the incidence of IPD. Our findings coincide with those of Brueggemann et al., who conducted a large prospective laboratory surveillance study, in 26 countries and territories on 6 continents, finding a significant and sustained reduction in invasive S. pneumoniae infections, that reached 68% in the first 4 weeks, and 82% in the following 8 weeks after introduction of restrictions on human mobility (measured using Google COVID-19 Community Mobility Reports digital tool)5. The authors suggested interruption of person-to-person transmission of S. pneumoniae as the most plausible explanation for this reduction, in the context of pandemic-related confinement measures. Since the nasopharynx of children is the natural ecological niche of S. pneumoniae, school closures, in addition to strict protection measures in older adults, were the most probable reasons for the fall in IPDs observed. In Tuscany, Italy, Lastrucci et al. reported a significant reduction in the incidence of hospitalizations for community-acquired pneumonia in the elderly, during the first wave of COVID-19, compared to the previous 3 years.19 Less use of antimicrobials in the community was also observed during this period, suggesting patients with pneumonia had not been treated in the outpatient setting. The authors therefore attributed the fall in community acquired pneumonia to the indirect impact of the lockdown. In Japan, Hiroyuki et al. also reported a significant reduction in the number of patients hospitalized for community pneumonia, between August, 2019 and July, 2020.21
Similar observations were made in younger populations. Friedrich et al. analyzed causes for hospital admission in children under 14 years of age in Brazil, finding a significant decrease in hospitalizations for pneumonia in 2020 compared to the period 2015 to 2019, for all age groups, but especially in children under 9 years of age.13 This reduction coincided with the implementation of non-pharmacological measures to contain the pandemic.
Another relevant finding of our study was the low frequency of SARS-CoV-2/S. pneumoniae coinfection; the observed incidence was 5.68 per 10000 hospitalizations for COVID-19. It should be clarified that only invasive S. pneumoniae infections were considered since samples were from sterile sites. Conversely, in patients hospitalized for IPD during the pandemic, SARS-CoV-2 coinfection rate was 70%. The low rate of bacterial coinfections in patients hospitalized for COVID-19 argues in favor of avoiding unnecessary early use of antimicrobial agents, which predisposes to spread of multidrug-resistant gram negative bacteria both in the hospital and in the community.1,8,15,20 Lansbury et al. performed a meta-analysis of 30 studies and found, of a total of 3834 patients hospitalized for COVID-19, 7% had bacterial coinfections, increasing to 14% for patients admitted to the ICU.18 The most frequent pathogens were Mycoplasma pneumoniae (47%), Pseudomonas aeruginosa (12%), and H. influenzae (12%). Another meta-analysis carried out by Langford et al. estimated 3.5% of patients with COVID-19 had a primary bacterial coinfection and 14.3% developed secondary bacterial infections during hospitalization.17 A retrospective study performed by Hughes et al. included 836 patients hospitalized for COVID-19, 27 (3.2%) presented bacterial coinfection early, within 5 days of admission, increasing to 51 (6.1%) during the remaining hospital stay.16 No S. pneumoniae coinfections were found.
With respect to SARS-CoV-2 and S. pneumoniae coinfections, the largest study to date was conducted in England by Amin-Chowdhury et al., between February and June, 2020, monitoring incidence of IPD and COVID-19 during lockdown. The authors reported 30% reduction in the incidence of IPD cases, even after adjusting for age, gender, and bacterial serotype.3 The rate of coinfection was 0.025% for patients diagnosed with COVID-19, and 3.5% in those hospitalized for IPD. Risk of coinfection increased with age, and high blood pressure and dementia were the most common comorbidities.
In our study, case fatality rate of SARS-COV-2/S. pneumoniae coinfection was high (43%), greater than for isolated IPD cases (32.5%), and for in-hospital COVID-19 cases (4%). These findings are similar to the Lansbury meta-analysis, which reported a 5.82-fold increase in the risk of dying in coinfected patients.18 Rodriguez-Nava et al., who analyzed 11 episodes of SARS-CoV-2 and S. pneumoniae coinfection, also found high mortality rate (64%) during hospitalization.24 In the surveillance study conducted by Amin-Chowdhury, 30-day mortality was 3.88 times higher in patients who developed COVID-19 after IPD and 7.75 times higher in those with primary coinfection, compared to those with IPD alone.3
Limitations to our study include the fact that patients originated from the Buenos Aires metropolitan area (CABA and BAMA) exclusively, with access to private healthcare or social security. Although this region has one of the highest incidences of COVID-19 in the country, no patients from the public health system were included. Furthermore, information on pneumococcal vaccination status of patients was not available, and the third study period was shorter than the first two. Finally, because of its observational nature, statistical results presented are descriptive only. Lack of data precluded further analysis since univariate analysis without adjusting for other variables could have led to incorrect conclusions.
ConclusionsThis study is the first in Argentina to assess the incidence of IIEB, particularly due to S. pneumoniae, in the context of the COVID-19 pandemic. Significant reduction compared to the pre-pandemic year was observed, coinciding with implementation of public health strategies for pandemic containment and mitigation. The potential impact of these measures on other health conditions beyond COVID-19, such as IPD, is remarkable. Based on these findings, we believe that people with risk factors for IPD would strongly benefit from continued use of face masks and social distancing, beyond the pandemic, in particular during winter. We also observed low rates of SARS-CoV-2 and invasive S. pneumoniae coinfection, supporting the concept that early antimicrobial treatment in COVID-19 patients is unnecessary. Finally, case fatality was higher in coinfected patients than in those presenting IPD, or in-hospital COVID-19 alone, suggesting a potential adverse synergistic interaction between pathogens, although further studies will be needed to confirm this hypothesis.
FundingNone declared.
Conflicts of interestThe authors declare that there are no conflicts of interest.