Preliminary bioassays with whole cultures (WC) of 124 Bacillus thuringiensis strains were performed with neonate larvae of Anthonomus grandis, a major cotton pest in Argentina and other regions of the Americas. Three exotic and four native strains were selected for causing more than 50% mortality. All of them were β-exotoxin producers. The native strains shared similar morphology of parasporal crystals, similar protein pattern and identical insecticidal gene profiles. These features resembled Lepidoptera-toxic strains. Furthermore, these strains showed a Rep-PCR pattern identical to lepidoptericidal strain HD-1, suggesting that these strains may belong to serovar kurstaki. However, some differences were observed in the plasmid profiles and in the production of β-exotoxin. To determine the culture fractions where the insecticidal metabolites were present, bioassays including resuspended spore-crystal pellets, filtered supernatants (FS) were compared with those of WC. Both fractions tested showed some level of insecticidal activity. The results may suggest that the main toxic factors can be found in FS and could be directly correlated with the presence of β-exotoxin. Based on the bioassays with FS and autoclaved FS, the participation of thermolabile virulence factors such as Cry1I in toxicity is neither discarded. In the selected strains, β-exotoxin would be the major associated virulence factor; therefore, their use in biological control of A. grandis should be restricted. Nevertheless, these strains could be the source of genes (e.g., cry1Ia) to produce transgenic cotton plants resistant to this pest.
Se realizaron ensayos preliminares con cultivos completos de 124 cepas de Bacillus thuringiensis utilizando larvas neonatas de Anthonomus grandis, una plaga principal del algodón en Argentina y otras regiones de América. Se seleccionaron 3 cepas exóticas y 4 nativas por producir mortalidad superior al 50%, todas ellas productoras de β-exotoxina. Las cepas nativas presentan la misma morfología de cristales, un perfil de proteínas similar y los mismos genes insecticidas. Estas características hacen que se parezcan a cepas tóxicas para lepidópteros. Además, mostraron un perfil de Rep-PCR idéntico al de la cepa lepidoptericida HD-1, lo que indica que podrían pertenecer al serovar kurstaki. Sin embargo, se observaron diferencias en el perfil plasmídico y en la producción de β-exotoxina. Para determinar en qué fracción del cultivo se encontraban los metabolitos responsables de la toxicidad, se compararon los resultados de bioensayos en los que se utilizó biomasa, sobrenadante filtrado (SF) o cultivos completos. Ambas fracciones mostraron cierto grado de toxicidad. Los resultados indican que los principales factores tóxicos se encuentran en el sobrenadante y estarían directamente relacionados con la presencia de β-exotoxina. De acuerdo con los bioensayos de SF y SF autoclavado, no se descarta también la participación en la toxicidad de factores de virulencia termolábiles, como Cry1Ia. En las cepas seleccionadas, el principal factor de virulencia es la β-exotoxina, por lo que su uso debería restringirse para el control biológico de A. grandis. No obstante, estas podrían ser fuente de genes (p. ej., cry1Ia) para la producción de plantas de algodón transgénicas resistentes a dicha plaga.
The cotton boll weevil, Anthonomus grandis Boh. (Coleoptera: Curculionidae), is one of the main pests of cotton crops in the northern region of Argentina and other regions of the American continent3,5. The adults feed and lay their eggs in the squares (floral buds) and bolls, causing the falling of squares, fruit abscission, and reduce linter production and quality12.
Chemical insecticides and cultural controls are currently used in the management of this pest in Argentina31. The high price of the products used in these control tactics, the increase of resistant insect populations and other negative impacts arising from their use (e.g., biological imbalance, cotton fiber waste and environmental pollution) have promoted the development of new technologies to control boll weevils8,19,24. Among these technologies, microbial control agents, such as Bacillus thuringiensis, are environmentally-friendly alternatives. This gram-positive, spore-forming bacterium is characterized by the production of a set of virulence factors with toxicity to different insect orders and to some species of the Phylum Nematoda, Protozoa and Acari26. This set of virulence factors include proteinaceous crystal parasporal inclusions (Cry/Cyt proteins), which are produced during sporulation, and Vegetative insecticidal proteins (Vip), which are secreted during vegetative bacterial phase of growth26. In addition, some strains can synthesize a non-proteinaceous toxin called β-exotoxin or thuringiensin that is secreted during vegetative growth26. Unlike proteinaceous toxins, β-exotoxin is an unspecific thermostable compound which also affects mammals and persists in the environment1. Therefore, the presence of β-exotoxin in commercial B. thuringiensis pesticide formulations has been banned by the United States Environmental Protection Agency25.
The objectives of this study were:
- 1)
to select Argentine and exotic B. thuringiensis strains toxic to A. grandis;
- 2)
to characterize the most active native strains;
- 3)
to identify the virulence factors involved in their pathogenicity.
One hundred and twenty four different B. thuringiensis strains maintained at the Bacterial Collection of Instituto de Microbiología y Zoología Agrícola of Instituto Nacional de Tecnología Agropecuaria (IMYZA-INTA) were used in this work. One hundred strains were collected from stored product dust, spider webs, leaves and soils from different regions of Argentina. The remaining 24 strains comprise exotic strains kindly provided by different stock collections around the world (Table 1).
Toxic activity of whole cultures of exotic Bacillus thuringiensis strains against neonate larvae of Anthonomus grandis
| B. thuringiensis strain | Mortalitya (%) | β-exob | Sourcec | B. thuringiensis strain | Mortalitya (%) | β-exob | Sourcec |
|---|---|---|---|---|---|---|---|
| aizawai HD-137 | 19.6±3.1 (2) | − | A | morrisoni san diego | 8.7±6.1 (2) | − | B |
| alesti HD-4 | 21.7±0.0 (2) | − | A | morrisoni tenebrionis | 18.8±1.9 (2) | − | D |
| asturiensis 4BQ1 | 23.9±9.2 (2) | − | B | morrisoni 4K1 | 40.2±10.7 (2) | + | B |
| canadensis 4H2 | 33.5±4.3 (2) | − | B | pakistani 4P1 | 28.3±3.1 (2) | − | B |
| entomocidus HD-110 | 39.5±7.6 (2) | − | A | roskildiensis 4BG1 | 29.5±3.2 (2) | − | B |
| galleriae T05001 | 34.8±6.1 (2) | − | C | sotto HD-6 | 11.4±3.2 (2) | − | A |
| israelensis IPS-82 | 2.3±0.0 (2) | − | D | thompsoni HD-542 | 71.7±6.8 (12) | + | A |
| japonensis 4AT1 | 25.0±3.2 (2) | − | B | thuringiensis HD-2 | 86.7±6.3 (4) | + | A |
| kenyae HD-5 | 7.1±3.1 (2) | − | A | tochigiensis 4I1 | 19.6±3.1 (2) | − | B |
| kumamotoensis 4W1 | 19.6±6.1 (2) | − | B | tolworthi HD-125 | 66.6±6.2 (8) | + | A |
| kurstaki HD-1 | 22.7±6.4 (2) | − | A | toumanoffi 4N1 | 47.7±3.2 (2) | − | B |
| kyushuensis 4U1 | 8.7±6.1 (2) | − | B | wuhanensis 4AT1 | 32.5±10.6 (2) | − | A |
To insure standardized B. thuringiensis cultures for the experiments, 50μl of a highly concentrated spore stock suspension of each strain was inoculated into 50ml of BM medium (2.5g NaCl; 1g KH2PO4; 2.5g K2HPO4; 0.25g MgSO4·7H2O; 0.1g MnSO4·H2O; 5g glucose; 2.5g starch and 4g yeast extract, in a total volume of 1l of water, pH 7.2), and was grown during 72h or until complete autolysis, at 29°C, in a rotatory shaker at 250rpm. Twelve milliliters of whole cultures were conserved for preliminary bioassays. Unpurified spore-crystal complexes were obtained by centrifugation of the rest of the culture at 12000g and 4°C for 15min, and pellets were resuspended in 38ml of distilled water. The supernatants were collected and filtered through 0.2-μm-pore-size filter. Portions of filtered supernatants were autoclaved at 121°C for 30min. These fractions and spore-crystal suspensions were kept at −20°C until further use.
Insect culturesInsects used in the bioassays were obtained from laboratory colonies reared in IMYZA-INTA laboratories.
Boll weevil bioassaysBioassays against neonate larvae of A. grandis were conducted by the diet incorporation method. Four milliliters (ml) of different B. thuringiensis preparations (whole culture/resuspended spore-crystal pellet/filtered supernatants/autoclaved filtrate supernatants) were incorporated into 36ml of thermostatized (60°C) artificial diet for A. grandis (33.3g Pharmamedia; 50g wheat germ; 50g yeast extract; 83.33g soy protein; 50g sugar and 10g agar, in a total volume of 1l of water). The volume of culture preparations used was the highest that could be included without altering the proportion of the diet components.
Six hundred microliters (μl) of these mixtures were poured into each well of a 24-well plate (Nunc 143982). Sterile BM medium or distilled water was added to the natural mortality controls. Twenty four larvae of A. grandis were used for each assay (two replicates at least). Mortality was registered after 7 days at 29°C. Larvae of A. grandis were considered dead if they failed to respond to gentle probing. Schneider-Orelli's formula was used to calculate corrected mortality in comparison to the untreated control. The InfoStat software (Universidad Nacional de Córdoba, version 2014) was used for the statistical analysis, and the statistical significance was set at p<0.05.
Qualitative detection of β-exotoxinTen third-stage Musca domestica larvae were placed into Petri dishes that contained a filter paper (Whatman # 1 of 90mm) embedded with 1ml of an autoclaved culture supernatant and 1ml of distilled water (three replicates). B. thuringiensis serovar thuringiensis HD-2 and kurstaki HD-1 were also used as positive and negative controls, respectively. Petri dishes were incubated in darkness at 30°C and after 8 days the number of emerg adult flies was recorded. A strain is considered to produce β-exotoxin (positive (+) strain) when 20% or less of adults emerge.
Phenotypic and genotypic characterization of B. thuringiensis strainsThe presence of parasporal inclusions was confirmed under phase-contrast microscopy.
The protein composition of crystals of B. thuringiensis strains was determined by SDS-PAGE following standard methods. Plasmid extracts were obtained according to Ibarra et al.13. Plasmid patterns were obtained on 0.6% agarose gel electrophoresis carried out for 12h at 30V. Fingerprinting specific for strains within the Bacillus cereus group using Rep-PCR analysis was carried out on B. thuringiensis strains according to Sauka et al.28. Screening of cry1, cry2, cry9 and vip3A genes and further identification of cry1Aa, cry1Ab, cry1Ac, cry1Ad, cry1B, cry1C, cry1D, cry1F, cry1G and cry1I were conducted following previously described methods27,29.
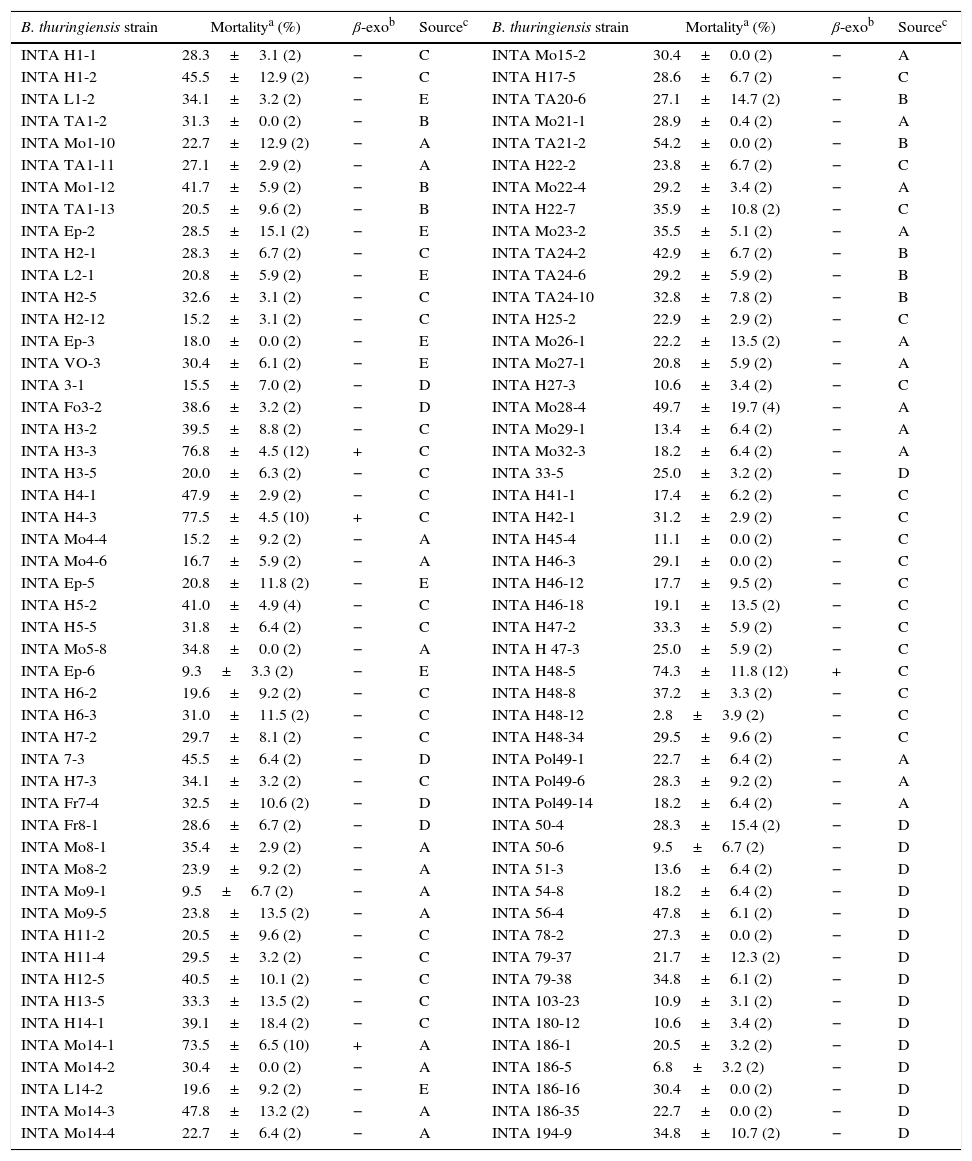
Results and discussionScreening of B. thuringiensis strains against A. grandisPreliminary bioassays with an aliquot of whole culture of B. thuringiensis strains were performed with neonate larvae of A. grandis. Among the 124 B. thuringiensis strains evaluated (Tables 1 and 2), all of them showed some level of toxic activity against this pest. Mortalities from 2.3% to 86.7% and 2.8% to 77.5% were obtained for exotic and Argentine B. thuringiensis strains respectively. Nevertheless, most of the strains showed low virulence with mortality values that ranged under 30.0% (Tables 1 and 2). Just 5.0% (5/100) of the Argentine and 12.5% (3/24) of the exotic B. thuringiensis strains caused more than 50.0% mortality. Similar observations were reported by Martins et al.18, who reported that 2.3% (5/215) of Brazilian B. thuringiensis strains caused greater than 50.0% larval mortality when tested at higher culture volumes than in the present study. These results could be suggesting a higher pathogenic power of Argentine strains against the boll weevil. However, a higher susceptibility of our insect population to B. thuringiensis should not be excluded as being responsible for that difference. It has been found that different insect pest populations may display different susceptibilities to selected B. thuringiensis strains and also to pure preparations of Cry toxins11,22. Some differences were observed when our results were compared with the toxicity data of two exotic strains reported by Monnerat et al.23. In contrast with our results, these authors reported that B. thuringiensis serovar israelensis was significantly more toxic against A. grandis than B. thuringiensis serovar morrisoni strain tenebrionis (Table 1). In addition, spore-crystal suspensions of B. thuringiensis serovar morrisoni strain san diego were reported to be more toxic than strains belonging to serovar morrisoni strain tenebrionis and kurstaki9. Quite different conclusions were reached when we compared the mortality rate of our san diego strain to that of the mentioned serovars (Table 1). Finally, the most active Argentine B. thuringiensis INTA H4-3, INTA H3-3, INTA Mo14-1 and INTA H48-5 strains, and the exotic B. thuringiensis serovar thuringiensis HD-2, thompsoni HD-542 and tolworthi HD-125 were selected for further studies.
Toxic activity of whole cultures of native Bacillus thuringiensis strains against neonate larvae of Anthonomus grandis
| B. thuringiensis strain | Mortalitya (%) | β-exob | Sourcec | B. thuringiensis strain | Mortalitya (%) | β-exob | Sourcec |
|---|---|---|---|---|---|---|---|
| INTA H1-1 | 28.3±3.1 (2) | − | C | INTA Mo15-2 | 30.4±0.0 (2) | − | A |
| INTA H1-2 | 45.5±12.9 (2) | − | C | INTA H17-5 | 28.6±6.7 (2) | − | C |
| INTA L1-2 | 34.1±3.2 (2) | − | E | INTA TA20-6 | 27.1±14.7 (2) | − | B |
| INTA TA1-2 | 31.3±0.0 (2) | − | B | INTA Mo21-1 | 28.9±0.4 (2) | − | A |
| INTA Mo1-10 | 22.7±12.9 (2) | − | A | INTA TA21-2 | 54.2±0.0 (2) | − | B |
| INTA TA1-11 | 27.1±2.9 (2) | − | A | INTA H22-2 | 23.8±6.7 (2) | − | C |
| INTA Mo1-12 | 41.7±5.9 (2) | − | B | INTA Mo22-4 | 29.2±3.4 (2) | − | A |
| INTA TA1-13 | 20.5±9.6 (2) | − | B | INTA H22-7 | 35.9±10.8 (2) | − | C |
| INTA Ep-2 | 28.5±15.1 (2) | − | E | INTA Mo23-2 | 35.5±5.1 (2) | − | A |
| INTA H2-1 | 28.3±6.7 (2) | − | C | INTA TA24-2 | 42.9±6.7 (2) | − | B |
| INTA L2-1 | 20.8±5.9 (2) | − | E | INTA TA24-6 | 29.2±5.9 (2) | − | B |
| INTA H2-5 | 32.6±3.1 (2) | − | C | INTA TA24-10 | 32.8±7.8 (2) | − | B |
| INTA H2-12 | 15.2±3.1 (2) | − | C | INTA H25-2 | 22.9±2.9 (2) | − | C |
| INTA Ep-3 | 18.0±0.0 (2) | − | E | INTA Mo26-1 | 22.2±13.5 (2) | − | A |
| INTA VO-3 | 30.4±6.1 (2) | − | E | INTA Mo27-1 | 20.8±5.9 (2) | − | A |
| INTA 3-1 | 15.5±7.0 (2) | − | D | INTA H27-3 | 10.6±3.4 (2) | − | C |
| INTA Fo3-2 | 38.6±3.2 (2) | − | D | INTA Mo28-4 | 49.7±19.7 (4) | − | A |
| INTA H3-2 | 39.5±8.8 (2) | − | C | INTA Mo29-1 | 13.4±6.4 (2) | − | A |
| INTA H3-3 | 76.8±4.5 (12) | + | C | INTA Mo32-3 | 18.2±6.4 (2) | − | A |
| INTA H3-5 | 20.0±6.3 (2) | − | C | INTA 33-5 | 25.0±3.2 (2) | − | D |
| INTA H4-1 | 47.9±2.9 (2) | − | C | INTA H41-1 | 17.4±6.2 (2) | − | C |
| INTA H4-3 | 77.5±4.5 (10) | + | C | INTA H42-1 | 31.2±2.9 (2) | − | C |
| INTA Mo4-4 | 15.2±9.2 (2) | − | A | INTA H45-4 | 11.1±0.0 (2) | − | C |
| INTA Mo4-6 | 16.7±5.9 (2) | − | A | INTA H46-3 | 29.1±0.0 (2) | − | C |
| INTA Ep-5 | 20.8±11.8 (2) | − | E | INTA H46-12 | 17.7±9.5 (2) | − | C |
| INTA H5-2 | 41.0±4.9 (4) | − | C | INTA H46-18 | 19.1±13.5 (2) | − | C |
| INTA H5-5 | 31.8±6.4 (2) | − | C | INTA H47-2 | 33.3±5.9 (2) | − | C |
| INTA Mo5-8 | 34.8±0.0 (2) | − | A | INTA H 47-3 | 25.0±5.9 (2) | − | C |
| INTA Ep-6 | 9.3±3.3 (2) | − | E | INTA H48-5 | 74.3±11.8 (12) | + | C |
| INTA H6-2 | 19.6±9.2 (2) | − | C | INTA H48-8 | 37.2±3.3 (2) | − | C |
| INTA H6-3 | 31.0±11.5 (2) | − | C | INTA H48-12 | 2.8±3.9 (2) | − | C |
| INTA H7-2 | 29.7±8.1 (2) | − | C | INTA H48-34 | 29.5±9.6 (2) | − | C |
| INTA 7-3 | 45.5±6.4 (2) | − | D | INTA Pol49-1 | 22.7±6.4 (2) | − | A |
| INTA H7-3 | 34.1±3.2 (2) | − | C | INTA Pol49-6 | 28.3±9.2 (2) | − | A |
| INTA Fr7-4 | 32.5±10.6 (2) | − | D | INTA Pol49-14 | 18.2±6.4 (2) | − | A |
| INTA Fr8-1 | 28.6±6.7 (2) | − | D | INTA 50-4 | 28.3±15.4 (2) | − | D |
| INTA Mo8-1 | 35.4±2.9 (2) | − | A | INTA 50-6 | 9.5±6.7 (2) | − | D |
| INTA Mo8-2 | 23.9±9.2 (2) | − | A | INTA 51-3 | 13.6±6.4 (2) | − | D |
| INTA Mo9-1 | 9.5±6.7 (2) | − | A | INTA 54-8 | 18.2±6.4 (2) | − | D |
| INTA Mo9-5 | 23.8±13.5 (2) | − | A | INTA 56-4 | 47.8±6.1 (2) | − | D |
| INTA H11-2 | 20.5±9.6 (2) | − | C | INTA 78-2 | 27.3±0.0 (2) | − | D |
| INTA H11-4 | 29.5±3.2 (2) | − | C | INTA 79-37 | 21.7±12.3 (2) | − | D |
| INTA H12-5 | 40.5±10.1 (2) | − | C | INTA 79-38 | 34.8±6.1 (2) | − | D |
| INTA H13-5 | 33.3±13.5 (2) | − | C | INTA 103-23 | 10.9±3.1 (2) | − | D |
| INTA H14-1 | 39.1±18.4 (2) | − | C | INTA 180-12 | 10.6±3.4 (2) | − | D |
| INTA Mo14-1 | 73.5±6.5 (10) | + | A | INTA 186-1 | 20.5±3.2 (2) | − | D |
| INTA Mo14-2 | 30.4±0.0 (2) | − | A | INTA 186-5 | 6.8±3.2 (2) | − | D |
| INTA L14-2 | 19.6±9.2 (2) | − | E | INTA 186-16 | 30.4±0.0 (2) | − | D |
| INTA Mo14-3 | 47.8±13.2 (2) | − | A | INTA 186-35 | 22.7±0.0 (2) | − | D |
| INTA Mo14-4 | 22.7±6.4 (2) | − | A | INTA 194-9 | 34.8±10.7 (2) | − | D |
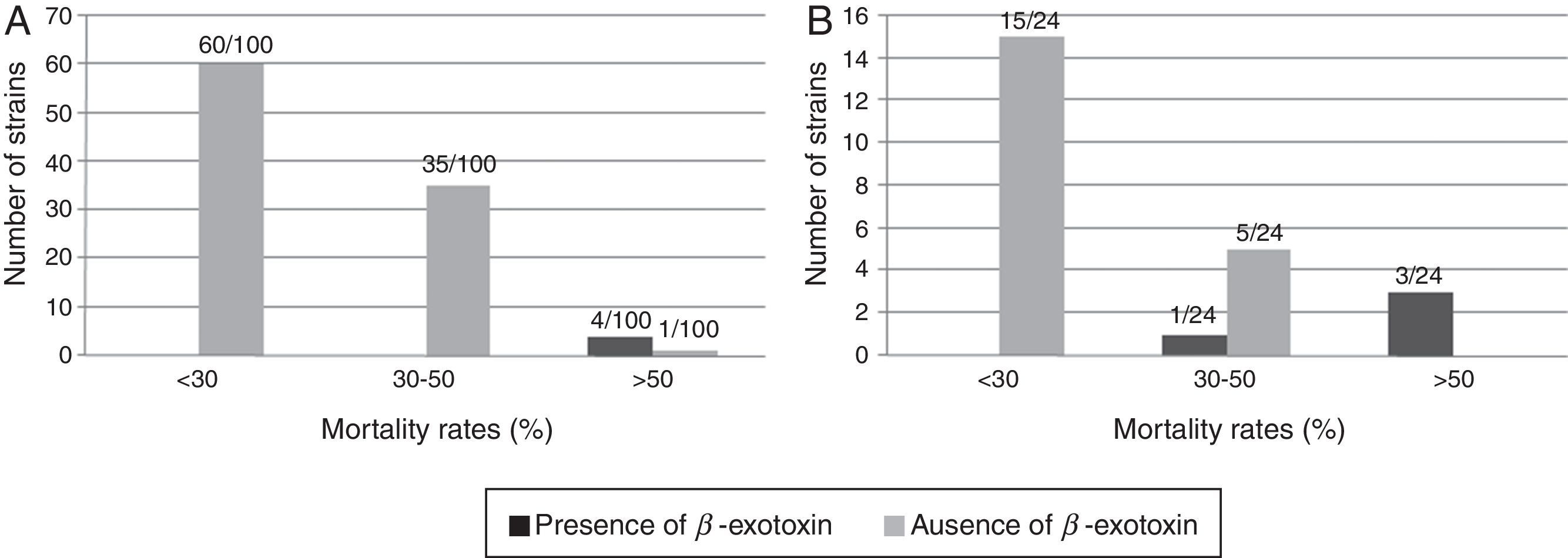
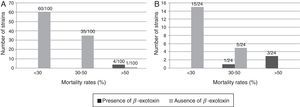
The differentiation of the B. thuringiensis strains according to their toxicity levels and the presence of β-exotoxin are shown in Figure 1.
The information about the β-exotoxin producer strains was obtained from a previous report30. None of the B. thuringiensis strains classified in the category of less than 30.0% mortality showed to be β-exotoxin-producers. Moreover, most of the highly active strains against the boll weevil (mortality rate higher than 50.0%) showed to be β-exotoxin-producer strains. This capacity was corroborated by standard M. domestica bioassays in the four native and in the three exotic most active strains respectively (Table 3). In agreement with other authors7, these results would suggest a strong association between the toxicity against A. grandis and β-exotoxin production in B. thuringiensis. Furthermore, this metabolite has also been reported to be toxic to other coleopteran species. Insecticidal activity was demonstrated in Lasioderma serricorne, Leptinotarsa decemlineata and Diabrotica undecimpunctata4,16,33.
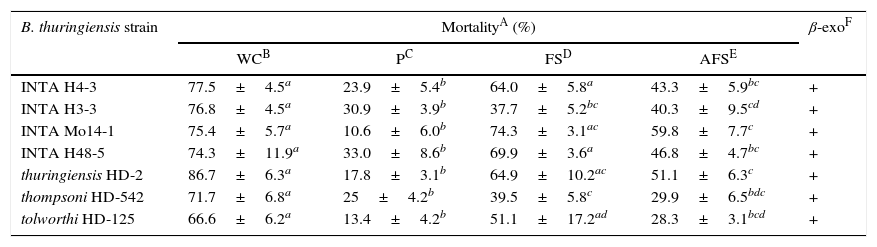
Toxic activity of different culture preparations of selected Bacillus thuringiensis strains against Anthonomus grandis
| B. thuringiensis strain | MortalityA (%) | β-exoF | |||
|---|---|---|---|---|---|
| WCB | PC | FSD | AFSE | ||
| INTA H4-3 | 77.5±4.5a | 23.9±5.4b | 64.0±5.8a | 43.3±5.9bc | + |
| INTA H3-3 | 76.8±4.5a | 30.9±3.9b | 37.7±5.2bc | 40.3±9.5cd | + |
| INTA Mo14-1 | 75.4±5.7a | 10.6±6.0b | 74.3±3.1ac | 59.8±7.7c | + |
| INTA H48-5 | 74.3±11.9a | 33.0±8.6b | 69.9±3.6a | 46.8±4.7bc | + |
| thuringiensis HD-2 | 86.7±6.3a | 17.8±3.1b | 64.9±10.2ac | 51.1±6.3c | + |
| thompsoni HD-542 | 71.7±6.8a | 25±4.2b | 39.5±5.8c | 29.9±6.5bdc | + |
| tolworthi HD-125 | 66.6±6.2a | 13.4±4.2b | 51.1±17.2ad | 28.3±3.1bcd | + |
Mean values from the same row with different letters in superscript are significantly different according to the Tukey's test (p<0.05).
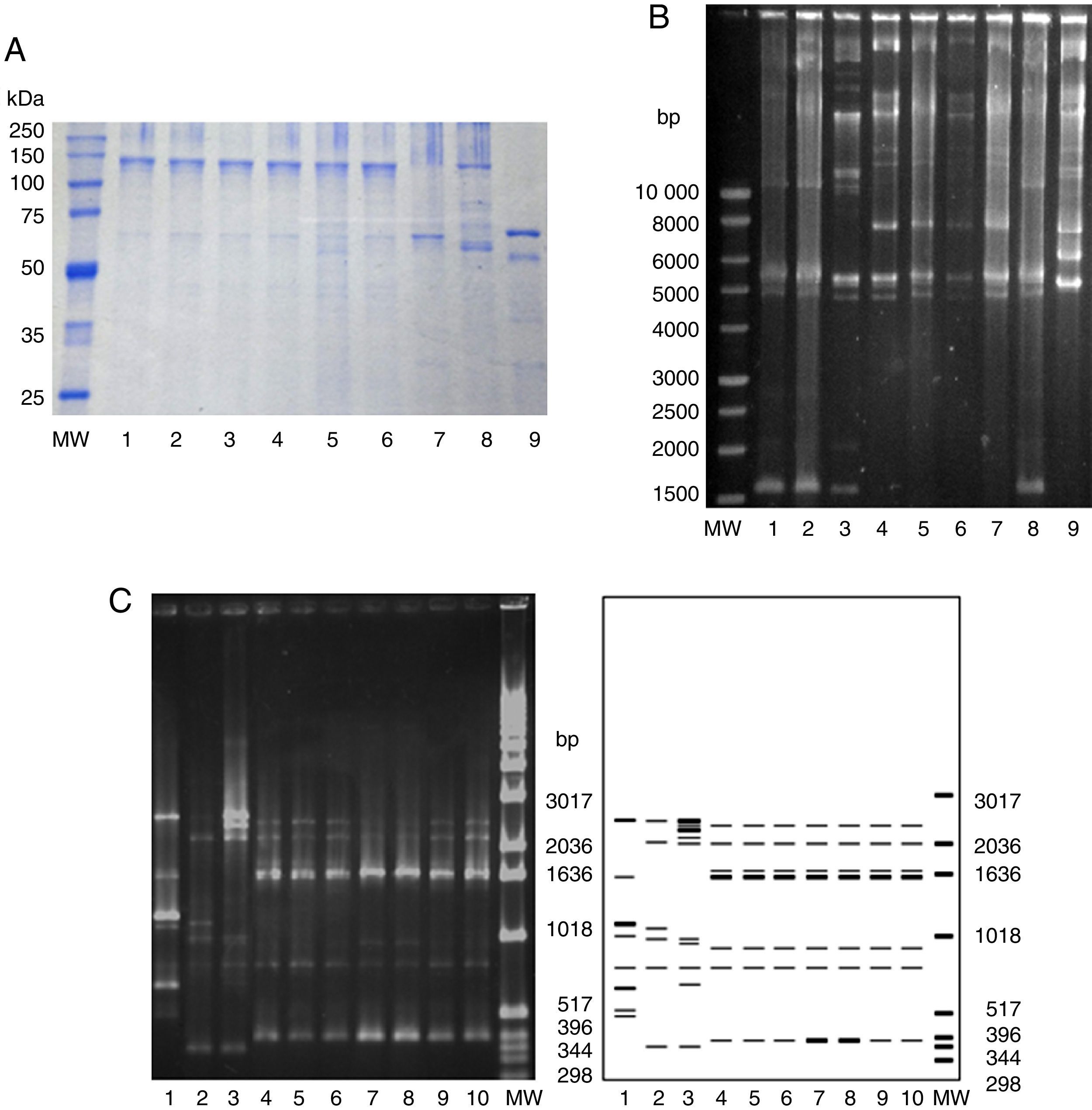
The main phenotypic and genotypic features of the most active native strains against A. grandis are shown in Table 4. B. thuringiensis INTA H4-3, INTA H3-3, INTA Mo14-1 and INTA H48-5 strains shared similar morphology of parasporal crystals as observed by phase-contrast microscopy, similar protein pattern as revealed by SDS-PAGE (Fig. 2A), and identical cry and vip genes identified by PCR. These strains produce bipyramidal and cuboidal crystals and major proteins of ca. 130 and 65kDa consistent with the presence of cry1 and cry2 genes. All these features resembled Lepidoptera-toxic B. thuringiensis rather than coleoptericidal strains26. Evidence supporting this assertion was that these strains showed a Rep-PCR pattern identical to reference strain HD-1 (Fig. 2C), indicating that these native strains may belong to the serovar kurstaki, according to the conclusions drawn during the development of this technique28. B. thuringiensis serovar kurstaki HD-1 is the most widely applied biological pesticide used to control lepidopteran insects that affect agriculture and forestry26. This strain harbors cry1Ab, cry1Ac, cry1Ia, cry2Aa, cry2Ab and vip3Aa genes35, a genetic profile almost identical to that of the four native strains. In contrast, some differences started to appear when plasmid profiles were determined. The plasmid profiling of the most active native strains in agarose gels revealed identical patterns, although different to those of the HD-1 reference strain or to those of other native and exotic strains (Fig. 2B). In spite of that, this observation does not seem strange since plasmid patterns have been widely used in discriminating strains, even within the same serovar10,27.
Main phenotypic and genotypic features of the most toxic native strains
| B. thuringiensis strain | Crystal morphologiesa | Protein size (kDa) | Insecticidal gene profile |
|---|---|---|---|
| INTA H4-3 | B and C | ca. 130 and 65 | cry1Ab, cry1Ac, cry1Ia, cry2Aa, cry2Ab and vip3Aa |
| INTA H3-3 | B and C | ca. 130 and 65 | cry1Ab, cry1Ac, cry1Ia, cry2Aa, cry2Ab and vip3Aa |
| INTA Mo14-1 | B and C | ca. 130 and 65 | cry1Ab, cry1Ac, cry1Ia, cry2Aa, cry2Ab and vip3Aa |
| INTA H48-5 | B and C | ca. 130 and 65 | cry1Ab, cry1Ac, cry1Ia, cry2Aa, cry2Ab and vip3Aa |
Characterization of selected native and exotic Bacillus thuringiensis strains. (A) Electrophoretic analysis of crystal proteins. Lanes: 1, INTA TA21-2; 2, INTA H4-1; 3, INTA H4-3; 4, INTA H3-3; 5, INTA Mo14-1; 6, INTA H48-5; 7, kurstaki HD-1; 8, thuringiensis HD-2; 9, morrisoni strain tenebrionis. MW with sizes indicated on the left (kDa) (Promega). (B) Plasmid profiles. Lanes: 1, INTA TA21-2; 2, kurstaki HD-1; 3, INTA H4-1; 4, INTA H4-3; 5, INTA Mo14-1; 6, INTA H48-5; 7, INTA H3-3; 8, kurstaki HD-1; 9, thuringiensis HD-2. MW with sizes indicated on the left (bp) (Promega). (C) REP-PCR fingerprinting. Lanes: 1, thuringiensis HD2; 2, israelensis HD-567; 3, morrisoni strain tenebrionis; 4, kurstaki HD-1; 5, INTA H3-3; 6, INTA H4-3; 7, INTA H48-5; 8, INTA Mo14-1; 9, INTA TA21-2; 10, INTA H4-1. MW with sizes indicated on the right (bp) (Invitrogen).
In order to determine the culture fractions of the most active native and exotic strains where the insecticidal metabolites were present, bioassays including these fractions were tested against neonate A. grandis larvae. All fractions tested showed some level of insecticidal activity (Table 3).
Firstly, the mortality range of resuspended spore-crystal pellets (P) was compared with that of filtered supernatants (FS) and both with respect to whole cultures (WC). Analysis of variance (ANOVA) with post hoc Tukey's test was used for intergroup comparison. From these results, statistically different groups could be found among the evaluated strains (Table 3). INTA H4-3, INTA Mo14-1, INTA H48-5, HD-2 and HD-125 strains were classified in one major group. These strains showed statistical differences between P and FS, P and WC, but not between FS and WC. Moreover, INTA H3-3 showed statistical differences between P and WC, FS and WC, but not between P and FS. Finally, HD-542 showed statistical differences between the culture fractions and WC. These results may suggest that the main toxic factors can be found in FS due to the high and similar levels of toxicity when compared to WC. In the major group mainly, the high levels of toxic activity of these strains could be directly correlated with the presence of β-exotoxin. As cited previously, this metabolite has been associated as a main virulence factor involved in toxicity against A. grandis7. It has also been reported that combinations of B. thuringiensis serovar kurstaki and β-exotoxin resulted in potentiation on Spodoptera exigua larvae21. However, their participation in toxicity along with other virulence factors such as Cry1I toxins is not ruled out. The studied strains could produce Cry1Ia consistent with the cry1Ia gene detected by PCR (Table 4). This class of proteins does not crystallize in B. thuringiensis, and are secreted during the initial phase of sporulation15. Cry1I has been described as toxic to certain coleopteran and lepidopteran larvae34. Additionally, a recombinant Cry1Ia protein was shown to be highly toxic to neonate A. grandis larvae2,12,19.
In order to test if thermolabile virulence factors against A. grandis exist in supernatants, the mortality range of FS was compared with that of autoclaved filtrate supernatants (AFS) that only conserve the heat-stable β-exotoxin. Again, statistically different groups could be found among the evaluated strains (Table 3). One group contained INTA H4-3 and INTA H48-5 strains, showing statistical differences between FS and AFS. The lower mortality rates of AFS compared to that of FS would demonstrate the existence of thermolabile virulence factors in FS, which would be contributing significantly to the virulence of strains against A. grandis.
INTA H3-3, INTA Mo14-1, HD-2, HD-542 and HD-125 strains were differentiated as a separate group, not showing statistical differences between the compared fractions. These results reflect that thermolabile factors in FS may not play the most important role in the virulence of these particular strains. Moreover, these results also support the fact that toxicity to A. grandis is mainly associated with the production of β-exotoxin in these strains. The obtained differences between the toxic levels of these two groups of strains may be influenced by the concentration of β-exotoxin produced by each strain, by the level of expression of cry1Ia genes, or by other unknown secreted virulence factors.
We also found that all the P tested were at least slightly toxic (Table 3). It could be attributed to the fact that during the seven days of the bioassay spore germination may occur and then the β-exotoxin is secreted causing mortality. Another reason might be due to some of the Cry proteins that form their crystals. Some authors have reported Cry1Ba6, Cry8Ka, Cry4, Cry10Aa, Cry11A and Cyt different toxic activity levels toward the cotton boll weevil6,20,23,24. Since our knowledge, none of the cry genes that harbor the native strains have been reported to encode crystals with coleoptericidal activity (Table 4). Interestingly, Sims32 has reported that Cry1Ac, one of the proteins encoded in the native strains, produced no toxic effect on A. grandis. Individual tests of Cry1Ab, Cry1Ac and Cry2A should be assayed in order to identify which protein or proteins are responsible for P toxicity in native strains. Nevertheless, further work is required to unravel this point.
Moreover, the observed statistical differences between FS and WC in INTA H3-3 and HD-542, but not in the rest of the strains, are not easy to explain. It might be attributed to a strong synergistic activity between the spores and crystals in the WC. Some evidence exists affording that spores in mixtures or with purified cry toxin solutions can act synergistically to increase toxicity in susceptible insect larvae14.
To gain further insight into B. thuringiensis INTA H4-3, INTA H3-3, INTA Mo14-1 and INTA H48-5 toxins, we sequenced the genome of these bacteria (data not shown) and analyzed the genome sequences for the presence of virulence factors. The same cry/vip gene profiles were found in the four strains (cry1Ab, cry1Ac, cry1Ia, cry2Aa, cry2Ab and vip3Aa). As expected, these strains also harbored the β-exotoxin biosynthetic gene cluster or most of their genes at least17. In addition, genes encoding virulence synergistic factors that have the ability to enhance the insecticidal activity of Cry and Vip proteins were also found (e.g., zwittermycin A, enhancin, proteases, enterotoxins, collagenase, chitinase)26. However, no other toxins implicated in specific B. thuringiensis insecticidal activity were found in these native strains26.
In summary, four native and three exotic B. thuringiensis strains were selected as the most active against boll weevil larvae. Despite the good level of insecticidal activity shown, the potential of their use directly in the biological control of A. grandis should be restricted. This assertion is based on the fact that β-exotoxin would be a major associated virulence factor. Anyway, these strains could be the source of genes (e.g., cry1Ia) to produce transgenic cotton plants resistant to this pest.
Ethical responsibilitiesProtection of human and animal subjectsThe authors state that no human or animal experiments have been performed for this research.
Confidentiality of dataThe authors state that in this article there are no patient data.
Right to privacy and informed consentThe authors state that no patient data appears in this article.
Conflict of interestThe authors declare that they have no conflicts of interest.
The work was funded by CVT 2982 INTA-Gobiernos de las Provincias de Chaco, Formosa, Santa Fe y Santiago del Estero “Generar conocimientos y tecnologías para el control del picudo del algodonero”.
MP and MO worked with fellowship from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). DS and MB holds research career award from CONICET.