The objectives of this research were: (1) to determine the occurrence of zoonotic enteroparasites in dog feces from Bahía Blanca, Argentina; (2) to characterize the spatial distribution of the parasites found in association with the quality of life index (QLI) in neighborhoods of Bahía Blanca; and (3) to determine if the presence of a particular parasite genus in a stool sample was facilitated or impeded by the presence of other parasite genera. Samples of dog stools (n=475) were collected between December 2012 and December 2013 in areas with varying QLI. The association between QLI values and the presence of parasites was analyzed using logistic regression. Overall enteroparasite occurrence was 36.6%. Parasitic forms found included nematode larvae, cysts of Blastocystis spp., Giardia spp., and oocysts of Cryptosporidium spp., and eggs of Ancylostoma caninum, Toxocara canis, cestodes and Trichuris spp. For certain enteroparasites, we detected significant associations between their occurrence and QLI. Feces collected in areas with medium and low QLI were 2.46 and 5.43 times more likely, respectively, to contain A. caninum than stools from the high-QLI area. Samples from areas with low QLI were 2.36 times more likely to contain Trichuris spp. than those from the high QLI area. Regarding protozoa, feces from areas with low QLI were 2.4 times more likely to be positive than those from areas with high QLI. We demonstrated that canine zoonotic parasites have a wide distribution in the study area, and that occurrence is higher in neighborhoods with lower QLI.
Los objetivos del presente trabajo fueron los siguientes: a) determinar la ocurrencia de enteroparásitos zoonóticos en heces de perros de Bahía Blanca, Argentina; b) caracterizar la distribución espacial de los parásitos hallados en función del índice de calidad de vida (ICV) en barrios de Bahía Blanca, y c) determinar si la presencia de un género parasitario en particular en las heces fue facilitada o impedida por la presencia de otro/s. Se recolectaron muestras de heces de perro (n=475) durante el período diciembre 2012-diciembre 2013 en áreas con diferente ICV. La asociación entre el ICV y la presencia de parásitos fue analizada mediante regresión logística. La ocurrencia global de enteroparásitos fue del 36,6%. Las formas parasitarias detectadas correspondieron a larvas de nematodos, a quistes de Blastocystis spp., Giardia spp. y Cryptosporidium spp., y a huevos de Ancylostoma caninum, Toxocara canis, cestodes y Trichuris spp. Se hallaron asociaciones significativas entre ocurrencia de algunos enteroparásitos e ICV. Las heces recolectadas en áreas con ICV medio y bajo mostraron una probabilidad de contener A. caninum 2,46 y 5,43 veces mayor, respectivamente, que las recolectadas en la zona de ICV alto. Las muestras de zonas con ICV bajo tuvieron una probabilidad 2,36 veces mayor de contener Trichuris spp. que aquellas de áreas con ICV alto. En lo referido a protozoos, las heces de áreas con ICV bajo tuvieron una probabilidad 2,4 veces mayor de presentar quistes que las heces de áreas con ICV alto. Se demostró que los parásitos zoonóticos caninos tienen una amplia distribución en el área estudiada y que la ocurrencia es más alta en los barrios con menor ICV.
Close relationships between people and animals date from very early in human history. The domestication of the dog (Canis lupus familiaris) some 12,000 years ago has enabled this species to become the most abundant carnivore in the world today, with an estimated population of 500 million. Cultural transformations over the ensuing centuries have seen a remarkable increase in the interaction between humans and domestic animals, and mainly the domestic dog19.
The benefits of contact with domestic animals, such as psychological well-being, are now well recognized. Family pets provide acceptance, joy and companionship for family members, and particularly for young children32,34,45. However, this close relationship between dogs and humans also brings potential problems such as parasitic zoonoses (PZs), which represent a threat to public health. Humans most commonly become infected with these zoonotic parasites through consumption of infected food or water or via direct fecal–oral contamination. The results of these infections may vary from asymptomatic carriage to long-term morbidity and even death. Although data are still scarce, it is clear that these PZs present a significant burden for public health, particularly in poor and marginalized communities30,52 and especially for young children, immunosuppressed and/or malnourished people and pregnant women8,33,37,40,54. Moreover, PZs can lead to significant economic losses, both directly through their adverse effects on human and animal health, and indirectly through the control measures required in the food production chain52.
In recent years, there has been a fairly large amount of research regarding the presence of enteroparasites in domestic dog populations worldwide5,7,22,43. Comparatively, fewer studies exist in Argentina3,12,38,47,56; however, only a minority addressed the spatial distribution of infection26,46.
Several studies reported elevated prevalence of intestinal parasites, mostly among children dwelling in the peripheral and poorest areas of Bahía Blanca13,15,17, in vegetables grown in the area (Visciarelli pers. comm.), and in dog feces collected from the streets6.
Local and updated information is essential to understand the epidemiology of gastrointestinal parasitic infections in dogs and to design preventive strategies at local, national, or regional levels. With this background, the objectives of this study were: (1) to determine the occurrence of zoonotic enteroparasites in dog feces from the city of Bahía Blanca, Argentina; (2) to characterize the spatial distribution of the parasites found in association with the quality of life index (QLI) in neighborhoods of Bahía Blanca; and (3) to determine if the presence of a particular parasite genus in a sample was facilitated or impeded by the presence of other parasite genera.
Materials and methodsStudy area sampling designBahía Blanca (38°44′S, 62°16′W) is the third largest city in Buenos Aires province, Argentina, with a population of a little over 300,000 people and a growth rate of 6% reported for the period 2001–2010. Bahía Blanca presents profound social inequalities leading to heterogeneous spatial structuring of well-being levels41. Temperature extremes are notably frequent, with an average daily mean of 15.1°C (ranges 8.9–21.9°C). Annual precipitation averages 613.7mm.
A prospective study was conducted between December 2012 and December 2013 in different neighborhoods of the city of Bahía Blanca, Argentina. Considering the second objective of this work (to analyze parasite occurrence as a function of quality of life), our sampling scheme was done by following Prieto41, who defined four levels for the quality of life in the city (very high, high, low, and very low) by considering variables such as housing quality, educational level, and indicators of public and environmental health. Considering a dog population of 68,000 dogs14 in the city, an infection prevalence of 50% (the most conservative value) for each of the parasites studied, a relative maximum error of 4.5%, and a 95% confidence level, the minimum sample size calculated was 475 stool samples for the entire study area. Then, fresh dog stool samples were collected in neighborhoods comprising only areas with very high (n=244), high (n=131) and low (n=100) QLI. Because of the safety risks implied, samples were not collected in the area with very low QLI. If two samples were separated by less than 30m, only one was collected. This approach was used to minimize the chances of double sampling feces from the same dog, which could falsely inflate occurrence values.
A complete record of small animal veterinary attention centers (henceforth referred to as VACs) in the city was provided by the District's professional association of veterinarians.
Sample processingStool specimens were collected from the sidewalks and were placed in individual sterile plastic jars with screw caps, transported to the laboratory, and fixed in 10% formalin until assayed. Samples were sieved through a single gauze followed by concentration using the Ritchie method. Finally, they were observed under a light microscope. The presence of Cryptosporidium spp. was assessed using Kinyoun acid-fast stain. All samples were observed during 5min. The eggs, cysts, and oocysts found were identified according to morphological and staining characteristics under light microscopy.
Statistical analysisSince the results were originated from stool samples in the environment, and not from individual dogs, we used the term “occurrence” (expressed as percentage), which we considered more appropriate than “prevalence”. Therefore, overall occurrence represents the proportion of fecal samples that were positive for at least one parasitic form (i.e. eggs, cysts, oocysts, adults), whereas specific occurrence was defined as the proportion of samples that were positive for each of the recorded parasite forms. For overall occurrence, feces were classified as positive if at least one of the subsamples had a parasitic form, and negative if otherwise. The same approach was used to define specific occurrence.
Results for Giardia spp., Blastocystis spp., and Cryptosporidium spp. were grouped into one single category as protozoa. Then, protozoa occurrence was defined as the percentage of samples that were positive for at least one of the protozoan species observed.
The association between the independent variable QLI (categorical: very high, high, low) and the outcome variables (overall occurrence, specific occurrence, protozoa occurrence; all binomial), as well as their interaction, was assessed using generalized linear models (GLM; logistic regression with logit link function). In this study, QLI was the only independent variable included in the model because (1) an unknown proportion of owners take their dogs to VACs outside their corresponding neighborhood (mostly owners from the area with a very high QLI value), and (2) QLI could be used as proxy for other unmeasured and epidemiologically relevant variables (e.g. family income, responsible pet ownership).
The model's goodness of fit was assessed by comparing the full model with the corresponding intercept-only model using the Akaike information criterion (AIC)1 as described in Burnham and Anderson10. A model was considered to provide substantial support of the data if AIC increased by more than 2 units after removing the independent variable QLI (intercept-only model).
The strength of association between exposure and outcome was assessed using the occurrence ratios (OcR) as an approximation to the prevalence ratios frequently reported in cross-sectional studies. For example, if parasite occurrence is higher in the exposed group compared with the unexposed one (e.g. low QLI vs. very high QLI), the ratio will be greater than one; if occurrence is the same, the ratio will be close to one, and if occurrence is lower in the exposed group, the ratio will be less than one. The level of significance for statistical analyses was defined as p <0.05.
To assess if the presence of any particular parasite was facilitated or impeded by the presence of other parasite genera, the frequency distribution of the number of parasitic taxa per sample was tested for a Poisson distribution using a goodness-of-fit test that included samples from all neighborhoods.
All statistical analyses were done using the R and Quantum GIS softwares42,44.
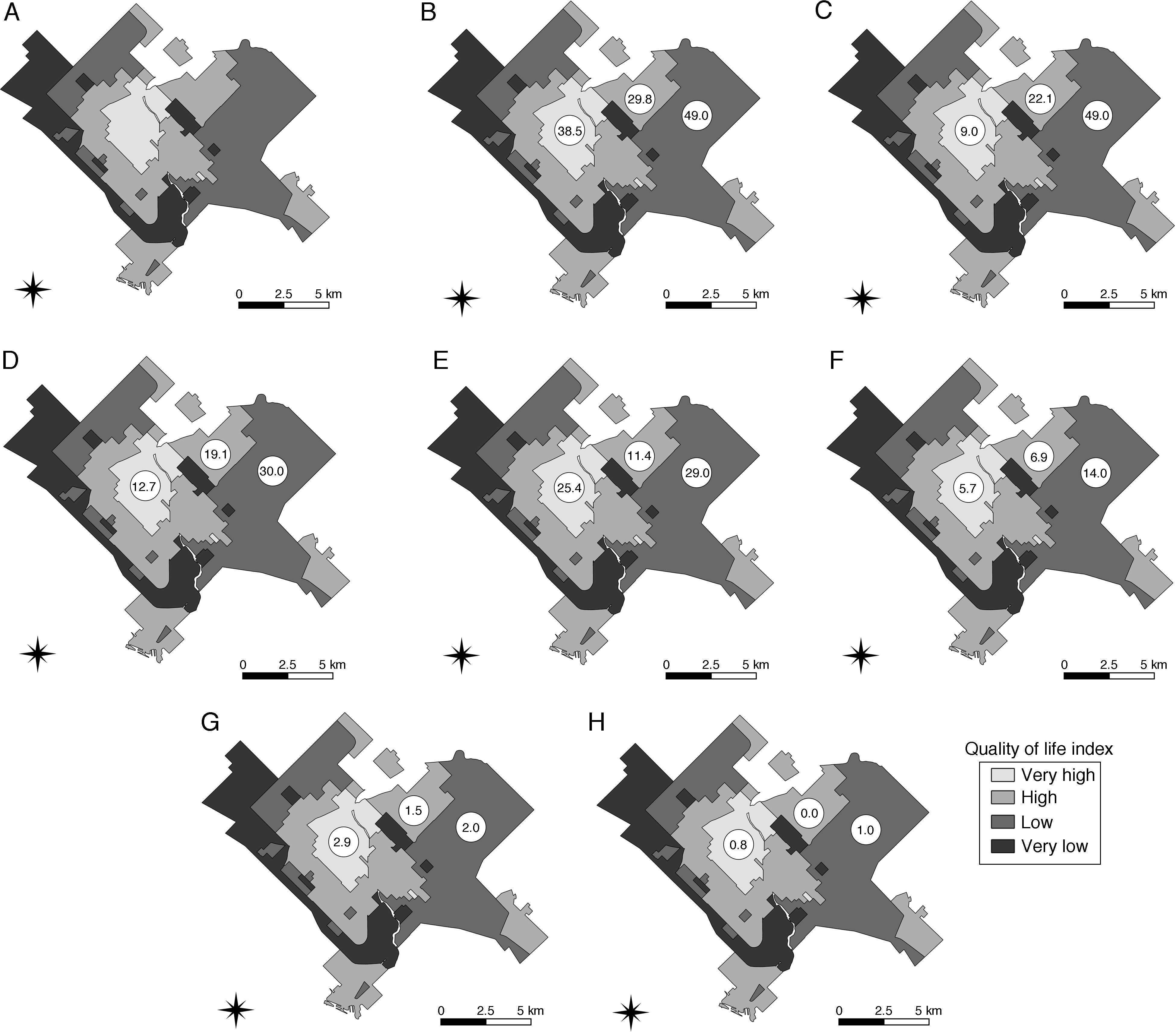
ResultsParasite occurrence and spatial distributionThe occurrence (expressed as percentage) of enteroparasites in dog feces from Bahía Blanca, Buenos Aires, Argentina is shown in Fig. 1.
Maps showing the occurrence (expressed as percentage) of enteroparasites in dog feces from Bahía Blanca, Buenos Aires, Argentina. Images show: neighborhoods and their quality of life index (A), overall occurrence of enteroparasites (B), and specific occurrences of A. caninum (C), Trichuris spp. (D), nematode larvae (E), protozoa (F), Toxocara spp. (G), and cestodes (H).
Overall parasite occurrence was 36.6%. The highest occurrence was recorded for nematode larvae (22.3%), followed by Ancylostoma caninum eggs (21.1%), protozoan cysts (19.8%), trichurid eggs (18.1%), Toxocara canis eggs (2.3%), and cestode eggs (0.6%). When protozoa were analyzed separately (all three areas combined), occurrence was 1.4% (95% CI: 0.1–5.4) for Giardia spp., 2.9% (95% CI: 0.9–7.4) for Blastocystis spp., and 14.7% (95% CI: 0.1–22.3) for Cryptosporidium spp.
No significant differences were detected between QLI and overall occurrence (Table 1). However, for some parasites, occurrence varied significantly among neighborhoods with different QLI values (Table 2).
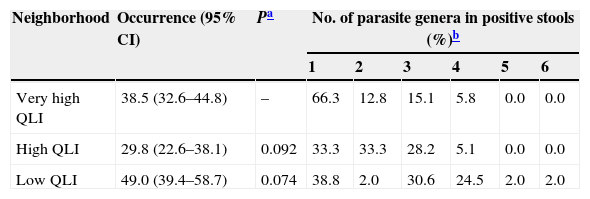
Overall occurrence of parasites (expressed as percentage) followed by 95% CI and the number of parasitic genera are reported for areas with different QLI in Bahía Blanca, Argentina.
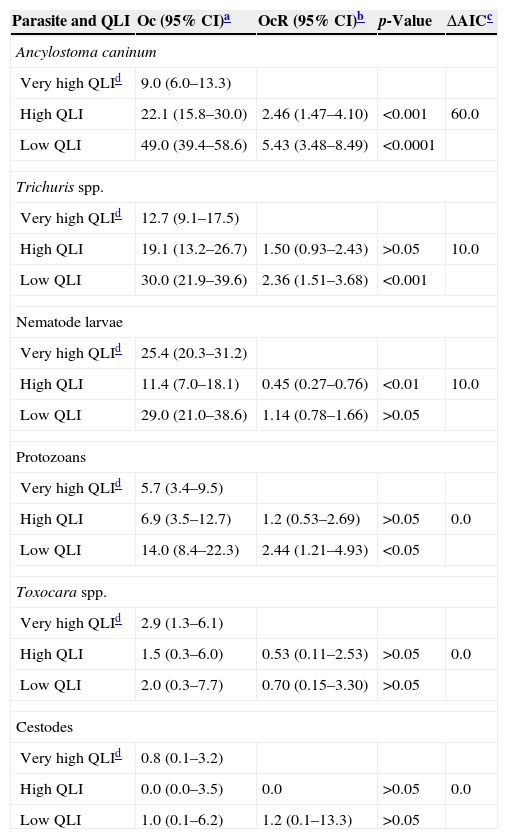
Logistic regression models describing the association between areas with different quality of life and occurrence (expressed as percentage) of enteroparasites in dog feces from Bahía Blanca, Buenos Aires, Argentina.
| Parasite and QLI | Oc (95% CI)a | OcR (95% CI)b | p-Value | ΔAICc |
|---|---|---|---|---|
| Ancylostoma caninum | ||||
| Very high QLId | 9.0 (6.0–13.3) | |||
| High QLI | 22.1 (15.8–30.0) | 2.46 (1.47–4.10) | <0.001 | 60.0 |
| Low QLI | 49.0 (39.4–58.6) | 5.43 (3.48–8.49) | <0.0001 | |
| Trichuris spp. | ||||
| Very high QLId | 12.7 (9.1–17.5) | |||
| High QLI | 19.1 (13.2–26.7) | 1.50 (0.93–2.43) | >0.05 | 10.0 |
| Low QLI | 30.0 (21.9–39.6) | 2.36 (1.51–3.68) | <0.001 | |
| Nematode larvae | ||||
| Very high QLId | 25.4 (20.3–31.2) | |||
| High QLI | 11.4 (7.0–18.1) | 0.45 (0.27–0.76) | <0.01 | 10.0 |
| Low QLI | 29.0 (21.0–38.6) | 1.14 (0.78–1.66) | >0.05 | |
| Protozoans | ||||
| Very high QLId | 5.7 (3.4–9.5) | |||
| High QLI | 6.9 (3.5–12.7) | 1.2 (0.53–2.69) | >0.05 | 0.0 |
| Low QLI | 14.0 (8.4–22.3) | 2.44 (1.21–4.93) | <0.05 | |
| Toxocara spp. | ||||
| Very high QLId | 2.9 (1.3–6.1) | |||
| High QLI | 1.5 (0.3–6.0) | 0.53 (0.11–2.53) | >0.05 | 0.0 |
| Low QLI | 2.0 (0.3–7.7) | 0.70 (0.15–3.30) | >0.05 | |
| Cestodes | ||||
| Very high QLId | 0.8 (0.1–3.2) | |||
| High QLI | 0.0 (0.0–3.5) | 0.0 | >0.05 | 0.0 |
| Low QLI | 1.0 (0.1–6.2) | 1.2 (0.1–13.3) | >0.05 | |
VACs were considerably more abundant in neighborhoods with very high and high QLI, compared with areas with low QLI. The number of VACs was strongly correlated (R=0.89) with the QLI of different areas as defined by Prieto (2008) (very high=36; high=34; low=3). These results justified the exclusion of the VACs variable from the logistic regression model.
Parasite competitionThe frequency distribution of the number of species per stool sample (all neighborhoods combined) was described by a Poisson distribution (mean=0.69; variance=1.2; χ2=152.3; p<0.0001), indicating that the presence of a parasite genus in individual samples was neither facilitated nor impeded by the presence of other parasite genera. The analyses showed that when A. caninum co-occurred with another parasite, 80% of those samples also had Trichuris spp. No co-occurrence associations were detected in samples with more than two parasitic genera.
The number of parasite genera in positive stools varied between one and six, and the neighborhood with low QLI had the widest range (Table 1).
DiscussionThis work describes, both qualitatively and quantitatively, the occurrence of canine zoonotic enteroparasites in Bahía Blanca, Argentina, and, for the first time, the association between parasite occurrence and QLI in the studied area.
The overall occurrence found (36.6%) was near the lowest range of prevalence (37.9–77.4%) reported by other authors in urban and rural areas of Argentina35,47,49,50, but fell within the range reported in other countries; e.g., 17.6% in Prague21, 28.7% in Australia9, 35.5% in Venezuela43, 34.8% in the United States31, 18–42% in Japan5, 53% in Hungary25, 68% in Nigeria4, 71.3% in Spain36, and 85% in Mexico22. Here, we identified eight species of parasites, which fall within the range of 6–12 species documented in dogs worldwide26.
In the present work, we characterized the spatial distribution of canine zoonotic enteroparasites in Bahía Blanca according to different QLI values. Although we found no significant association between parasite overall occurrence and QLI, the specific occurrence level for Ancylostoma caninum, Trichuris spp., nematode larvae, and protozoa was spatially structured following a heterogeneous pattern with higher values in neighborhoods with lower QLI (Table 2).
A. caninum showed the largest and most significant differences in occurrence between areas with different QLI. When considering all the neighborhoods, occurrence was not particularly high (21.1%), being 3.5 times lower than the 73% value reported for an urban area in Zambia11 and very similar to the 21.6% value reported in southern Buenos Aires province26.
In Bahía Blanca there are no reported cases of cutaneous larva migrans, which could be attributable to environmental characteristics (e.g. low average ambient temperature) and cultural factors such as shoe wearing. In our study, the highest occurrence of A. caninum eggs in fecal samples was in Villa Nocito, which is considered one of the longest neglected areas in the city. This neighborhood has the lowest QLI among those included in the study, and has no VACs. Although no official data are available, the stray dog population in this area is suspected to be larger, and pet owners are more prone to letting their dogs roam free. This scenario, coupled with the presence of A. caninum in stools from this area, would be expected to predispose the human population, and mainly children, to cutaneous larva migrans.
Trichuriasis is a disease associated with infection by nematode Trichuris spp. with an estimated 1 billion infected people worldwide in 200220. This disease poses a huge burden on some areas of the world such as sub-Saharan Africa, where estimations suggest that 24% of people are infected29. In the present study, occurrence of Trichuris spp. was higher as the QLI decreased, and overall occurrence (18.1%) was almost twice as high as that reported in other research works conducted in urban areas of Argentina (10.1%)26. Similarly, occurrence in Villa Nocito (30.0%) was almost two-fold higher than the maximum level (17.9%) reported by Fontanarrosa et al26.
Moreover, nematode larvae found here were not identified, which precludes drawing any definite conclusions about their zoonotic importance.
In our research, overall occurrence of Toxocara spp. was low (2.3%) and was not higher in the area with lowest QLI, where only 2% of samples were positive. Previous research in people from Villa Nocito reported the presence of antibodies against Toxocara spp. in 18% of the studied subjects18, whereas another work found that 83% of water samples from a natural stream that crosses the city were contaminated with Toxocara spp. eggs16. Occurrence of Toxocara spp. in the present study was remarkably low compared with other research works conducted in Argentina, which ranged between 3.4% and 35.1% in soil samples from a subtropical city2 and a dry steppe habitat in Patagonia24, respectively. This finding is in agreement with a hypothesis that people living in urban areas are at lower risk for infection than those dwelling in rural regions27,28. We suggest that different awareness levels regarding health practices and responsible pet ownership in rural regions and urban areas might account for the differences observed.
Regarding zoonotic protozoa, the excretion of their cysts/oocysts in the host's feces is intermittent Therefore, the reported occurrence levels should be considered with some caution48. Occurrence of Giardia spp. in the area with low QLI (14.0%) was 2.4 times higher than in the area with very high QLI (5.7%). This difference is in line with other research works15 conducted in Bahía Blanca, where prevalence of Giardia spp. was 9% in children stools (under 4 yr. old) dwelling in areas with very high QLI, and 33.3% in older children (4–10 yr. old) from areas with low QLI. Furthermore, the level of overall occurrence (1.4%) found here was very similar to the 1.3% reported by other authors in Argentina49.
On the contrary, Cryptosporidium spp. occurred 73.5 times more frequently (14.7% vs. 0.2%) in the present study compared with related research in southern Buenos Aires province26. The occurrence of Cryptosporidium spp. in dog feces contaminating public places raises concern about the potential transmission of this protozoan to immunosuppressed people45,55.
Blastocystis spp. was found in 2.9% (4/139) of the samples from areas with very high and high QLI combined. Recent research conducted in people from Bahía Blanca reported Blastocystis spp. fecal prevalence between 19.0 and 30.6%23. However, a paucity of knowledge regarding the zoonotic potential of this pathogen precludes any conclusions about the role of dog defecation as a relevant source of infection in humans. Despite this fact, recent research proposes domestic dogs as a potential source of Blastocystis infection to humans39. However, considering the low prevalence of this intestinal protozoan in dogs and a lack of evidence supporting a dog-specific or predominant genetic subtype, dogs are unlikely to act as natural hosts of Blastocystis, only becoming infected opportunistically via coprophagia of other host feces or by drinking contaminated water53.
In our study, the absolute number of VACs in the neighborhoods correlated positively with the QLI. However, as mentioned before, an unknown proportion of owners take their dogs to VACs outside their corresponding neighborhood. This fact could bias any conclusions regarding the role of unequal access to VACs as a predisposing factor for enteric parasites in the studied population. Additionally, the variety of helminth species found here warrants a critical revision of deworming protocols currently used in dogs from the study area. It is worth noting that the neighborhood with lowest QLI studied here showed the largest number of parasite genera per stool sample (Table 1). This could be attributable to the markedly lower number of VACs and, consequently, of deworming practices in that area51.
The distribution of parasite genera per stool sample cannot be assumed to be significantly different from a random distribution, suggesting that in the samples assayed, the presence of a parasite genus was neither facilitated nor impeded by the presence of other parasite genera. One possible explanation for the high frequency (80%) of co-occurrence between A. caninum and Trichuris spp. is the high occurrence of these genera in the dog population from Bahía Blanca.
In conclusion, several factors associated with decreased quality of life (e.g. income, housing conditions, etc.) would seem to play an important role in the occurrence of zoonotic enteroparasites in the dog population of Bahía Blanca. Our findings will facilitate the implementation of preventive public health measures toward the control of these parasites.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors declare that they have no conflicts of interest.
We are grateful to Secretaría de Ciencia y Tecnología de la Universidad Nacional del Sur, to Fundación. Alberto J. Roemmers for financial support, and to the Municipal Government of Bahía Blanca for declaring this project of national interest (Ordinance No. 14177).