The most used and reliable indicator of equine infectious anemia virus (EIAV) infection is the detection of its specific antibodies in horse serum. In the present study, the performance of two commercial ELISA tests for the detection of EIAV antibodies as well as the potential advantages of their use as an EIAV infection screening tool were evaluated in 302 horse serum samples. Both ELISA assays showed 100% diagnostic sensitivity, and 92.3–94.3% diagnostic specificity. Discordant results were analyzed by immunoblot. The results showed that both ELISA tests are very efficient at detecting EIAV infected animals, allowing to identify a higher number of positive horse cases. Thus, ELISA assays can be useful tools in EIA control and eradication.
El mejor indicador de la infección por el virus de la anemia infecciosa equina (Equine infectious anemia virus, EIAV) es la detección de anticuerpos específicos en el suero del caballo. En el presente trabajo se evaluó la capacidad de detección de anticuerpos contra EIAV de dos equipos de ELISA comerciales utilizando 302 muestras de suero equino, así como las ventajas potenciales de su uso como herramientas de screening. Ambos ensayos de ELISA presentaron 100% de sensibilidad diagnóstica y una especificidad diagnóstica del orden de 92,3 a 94,3%. Las muestras discordantes fueron analizadas por inmunoblot. Los resultados mostraron que las dos pruebas ELISA son muy eficientes para detectar animales infectados por EIAV, al permitir identificar un mayor número de animales positivos que la prueba de inmunodifusión en gel de agar, oficialmente aprobada en la República Argentina para la certificación de los animales. Las pruebas de ELISA constituyen herramientas muy útiles en los programas de control y de erradicación de la infección por EIAV.
Equine infectious anemia (EIA) is a blood-borne infection that affects horses and other equids, which is caused by the Equine infectious anemia virus (EIAV), genus Lentivirus of the family Retroviridae. The disease has a severe economic impact on the equine industry, and thus falls under a regulatory control program in many countries, including Argentina9.
One of the key measures to prevent the transmission of EIAV infection is the identification and segregation of infected horses3. So far, the only reliable indicator of EIAV infection has been the presence of specific antibodies against the envelope glycoproteins (gp90 and gp45) and/or the major core protein (p26) of EIAV. The agar gel immunodiffusion (AGID) test, designed by Coggins in 19722, identifies antibodies against the major core protein p26 of EIAV, and constitutes the gold standard test, approved for the diagnosis of EIA by both national9 and international6 authorities. This test is inexpensive, easy to perform and highly specific; yet, the turnover time is more than 48h. There are several reports describing the enzyme-linked immunosorbent assay (ELISA) tests using purified viral proteins, recombinant proteins and/or synthetic peptides as antigen; all have shown an excellent correlation with the AGID assay, and some of them, a higher sensitivity3.
The purpose of this study was to evaluate the performance of two commercial ELISA tests for the detection of EIAV antibodies, as well as the potential advantages of their use as an EIA screening tool.
In this study, 302 individual horse serum samples were used. The horses were from 14 premises located in the provinces of Corrientes, Chaco and Santa Fe, in the north-eastern and central region of Argentina, an EIA endemic area4. Blood for serum was obtained from horses by jugular venipuncture according to the guidelines described in the Manual for Use and Care of Experimental Animals, issued and approved institutionally by INTA. All samples were tested by two ELISA methods [ELISA-A (Ingezim anemia equina, Ingenasa, Spain), and ELISA-B (Eradikit EIAV, In3diagnostic, Italy] and by the AGID test [INCUINTA (rp26 IDGA Incuinta AIE, Incuinta, Argentina). The AGID test was considered as the standard for comparison. All assays were performed according to the manufacturers’ instructions. The degree of agreement between each pair of tests was determined by a κ test (http://graphpad.com/quickcalcs/kappa2/).
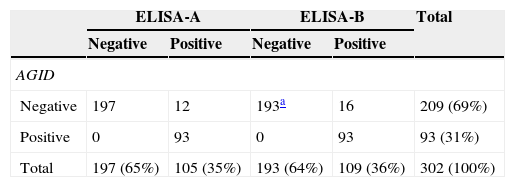
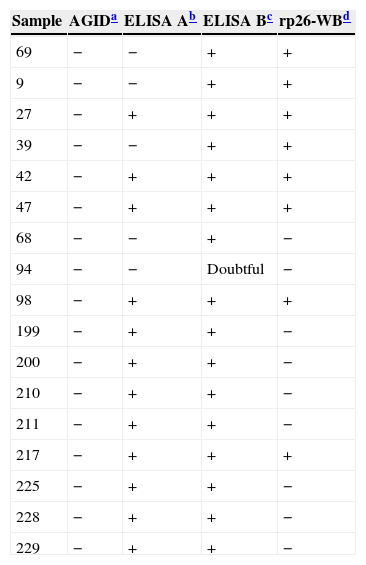
Both ELISA assays showed higher sensitivity than the AGID test. The proportion of positive animals detected was 35%, 36% and 31% for the ELISA-A, the ELISA-B and the AGID tests, respectively. Both ELISAs had a high degree of agreement with the AGID test, and, in addition, were even able to detect antibodies in samples that would have been recorded as negative when tested by AGID (Table 1). The Kappa coefficients were: AGID test versus ELISA-A test, 0.910; AGID test versus ELISA-B test, 0.881; between both ELISAs tests, 0.971. In all cases, they indicated a high concordance level. Diagnostic specificity was 94.3% for ELISA-A, and 92.3% for ELISA-B. In order to identify the true positive samples, both ELISA (A and/or B) positive and AGID negative samples were analyzed by immunoblot (rp26-WB)1 (Table 2). Eight out of 17 (47%) AGID negative samples were scored as positive according to their reactivity to the rp26-WB test. Five out of 12 “negative AGID/positive ELISA-A” samples, 8 out of 16 “negative AGID/positive ELISA-B”, and 3 out of 4 “negative ELISA-A/positive ELISA-B” samples, tested positive by the rp26-WB test. One sample (ID: 94), which showed an inconclusive result by ELISA-B, tested negative by the rp26-WB test. These results show a higher sensitivity of the ELISA-A and ELISA-B tests than of the AGID test. ELISA-B detected more positive samples than ELISA-A, probably due to the fact that ELISA-B targets antibodies to both the envelope and core EIAV antigens whereas ELISA-A targets antibodies to the p26 core protein of EIAV only.
Equine infectious anemia virus antibody detection by AGID and ELISA tests in 302 horse serum samples.
| ELISA-A | ELISA-B | Total | |||
|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | ||
| AGID | |||||
| Negative | 197 | 12 | 193a | 16 | 209 (69%) |
| Positive | 0 | 93 | 0 | 93 | 93 (31%) |
| Total | 197 (65%) | 105 (35%) | 193 (64%) | 109 (36%) | 302 (100%) |
rp26-western blot (rp26-WB) analysis of samples showing AGID and ELISA A and B discordant results.
Currently, there is no vaccine or treatment for EIA, therefore, the control strategy consists in the detection and segregation of infected animals; thus, a reliable diagnostic test is a crucial issue. The AGID test is still the only one to be internationally recognized as the gold standard for the diagnosis of EIA-infected animals, and is the prescribed test for international trade and movement of horses6. Yet, even though the specificity of the AGID test is very high, its sensitivity is lower than that of other tests, especially to detect low levels of specific antibodies3. The ELISA test has the advantage of allowing efficient and accurate testing of large numbers of serum samples within a relatively short time, and of results admitting an objective calculation if assay color development is measured with a spectrophotometer. In concordance with previous reports recently reviewed by Issel and others6, both ELISA tests used in our study have a higher sensitivity than the AGID test, and, in addition, 47% (8/17) of ELISA positive-AGID negative samples were confirmed as EIA positive by WB. However, false positive results were also found: 6% (7/105) by ELISA-A and 7% (8/109) by ELISA-B; therefore, a sample detected as positive by ELISA must be confirmed by AGID, the “gold standard” test5.
The use of a combination of tests to more accurately diagnose EIAV infections has been already proposed5,7,8. The diagnostic algorithm consists in using the advantage of the higher sensitivity of ELISA tests to identify the EIA negative population, the increased power (specificity) of the AGID test to take actions (segregate/sacrifice of AGID positive horses), and the use of immunoblot tests in the uncommon samples yielding ELISA positive/AGID negative results. Most samples that are positive by ELISA tests and negative by the AGID test, react with both the envelope glycoproteins and the major core protein (p26) of EIAV, when tested by immunoblot. Fortunately, equids only presenting the p26 antigen of EIAV by immunoblot (and judged as not specific for EIA) are but rarely encountered in equid populations. False positive results with ELISA tests might be explained as a cross-reaction to a related antigen5.
So far, testing for EIAV infection is mandatory for the national and international movement of horses. Reliable data on the true prevalence of the infection in Argentina is not yet available. Anecdotal data from diagnostic laboratories indicates that Argentina has an endemic area in its north-eastern region, a low and very low prevalence area in the center, and is free of infection in the south4. The obtained data (31%, 93/302) contributes to the knowledge of the EIA infection in Argentina, confirming the high prevalence of EIA in the horse population of the north-eastern and central regions of our country.
Although the results in this work are preliminary, the ELISA tests evaluated seem to be very effective to detect EIA infected animals, as both tests allowed to identify more “positive” animals than the AGID test, enabling the early identification of potential “Trojan horses” capable of transmitting the EIAV to susceptible animals.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare that they have no conflicts of interest.
This work was funded by INTA HARAS Agreement (CVT 023) and by INTA project INTA AEGR 2412. The authors would like to thank Dr. Gian Lucca Autorino, Head of the National Reference Center for Equine Infectious Diseases of the Istituto Zooprofilattico Sperimentale delle Regioni Lazio e Toscana, Roma, Italia, for kindly providing the In3diagnostic kit. I would also like to thank Dr. Ángel Venteo and Belén Rebollo from INGENASA, for kindly providing the Ingezim anemia equina kit. IA and AW are members of CONICET Research Career Program.