Infections, including zoonoses, constitute a threat to human health due to the spread of resistant pathogens. These diseases generate an inflammatory response controlled by a resolving mechanism involving specialized membrane lipid-derived molecules called lipoxins, resolvins, maresins, and protectins. The production of some of these molecules can be triggered by aspirin or statins. Thus, it is proposed that modulation of the host response could be a useful therapeutic strategy, contributing to the management of resistance to antiparasitic agents or preventing drift to chronic, host-damaging courses. Therefore, the present work presents the state of the art on the use of statins or aspirin for the experimental management of parasitic infections such as Chagas disease, leishmaniasis, toxoplasmosis or malaria. The methodology used was a narrative review covering original articles from the last seven years, 38 of which met the inclusion criteria. Based on the publications consulted, modulation of the resolution of inflammation using statins may be feasible as an adjuvant in the therapy of parasitic diseases. However, there was no strong experimental evidence on the use of aspirin; therefore, further studies are needed to evaluate its role inflammation resolution process in infectious diseases.
Las infecciones, incluyendo las zoonosis, constituyen una amenaza a la salud humana debido a la diseminación de patógenos resistentes. Estas enfermedades generan una respuesta inflamatoria controlada por un mecanismo de resolución, en el que participan moléculas especializadas derivadas de lípidos de membrana llamadas lipoxinas, resolvinas, maresinas y protectinas. La producción de algunas de estas moléculas puede ser gatillada por aspirina o estatinas. Así, se propone que la modulación de la respuesta del hospedero podría ser una estrategia terapéutica útil, que contribuye al manejo de la resistencia a agentes antiparasitarios o que puede prevenir la derivación hacia cursos crónicos, dañinos para el hospedero. En esta revisión se presenta una puesta al día sobre el uso de estatinas o aspirina para el manejo experimental de infecciones parasitarias, como enfermedad de Chagas, leishmaniasis, toxoplasmosis y malaria. Se hizo una revisión narrativa, buscando artículos originales de los últimos siete años, se encontraron 38 que cumplieron con los criterios de inclusión. De acuerdo con las publicaciones consultadas, la resolución de la inflamación modulada mediante estatinas podría ser un adyuvante en la terapia de enfermedades parasitarias. Por otro lado, no se observó una evidencia experimental fuerte con respecto al uso de aspirina, por lo que se recomiendan más estudios para evaluar su rol en el proceso de resolución de la inflamación en enfermedades infecciosas.
The treatment and prevention of infections focus on eradicating the microorganisms that cause them using a broad pharmacological arsenal of highly effective antibiotics and vaccines generating a sense of safety and confidence in reaching the end of infectious diseases43. The COVID-19 pandemic and the spread of resistant pathogens capable of evading the immune system demonstrated that this goal is still far from being achieved. Moreover, the lack of safer and more effective drugs for the treatment of parasitic diseases, especially those causing chronic conditions, underscores this situation. Furthermore, the transformation of microbiological ecosystems and the global interconnection of societies have accelerated the evolution of microorganisms and infections43,106, including the emergence of new threats. Moreover, the epidemiological behavior of traditional zoonotic diseases (as seen, for example, in the increase in congenital and transfusion transmission of Chagas disease in non-endemic countries) is changing. Thus, new therapeutic approaches are required for the treatment of infectious diseases.
The inflammatory response against pathogens is a self-limiting process. In general, tissue damage or invasion by pathogens triggers local activation of macrophages through recognizing pathogen-associated molecular patterns (PAMPs) by specialized Toll-like receptors (TLRs)99. Thus, the secretion of several interleukins (Ils), prostaglandins and leukotrienes, tumor necrosis factor-alpha (TNF-α), and gamma interferon (IFN-γ), among others, is promoted, depending on the type of pathogen involved. Free radicals are also generated, facilitating phagocytosis10,97, triggering the inflammatory process. Although essential for eliminating pathogens, the prompt re-establishment of homeostasis is required through the resolution of inflammation. When this resolution is inadequate, inflammation gets out of control, causing further tissue damage or installing chronic processes, with or without active infection97,102.
The resolution of inflammation is a highly regulated process orchestrated by multiple mediators, the so-called specialized pro-resolving mediators (SPMs)46, a superfamily of lipid molecules derived from the ω-3 and ω-6 polyunsaturated fatty acids of the plasma membrane, grouped into four classes: lipoxins A4 and B4 (LXA4, LXB4), derived from arachidonic acid; resolvins (Rv) D1-6, protectins (PD) and maresins (MaR), derived from docosahexaenoic acid (DHA); and Rv E1-4, derived from eicosapentaenoic acid (EPA)18,48. SPMs are described as potent anti-inflammatory molecules with an immunoregulatory role48.
SPMs are synthesized by several lipooxygenases in different cell types, including neutrophils and macrophages, and act on various G protein-coupled receptors, such as Formyl Peptide Receptor-2 (FPR2) or G protein-coupled receptor 32 (GPR32)87. The process is initiated during the exudate formation in the active phase of inflammation. The classic initiators, prostaglandins and leukotrienes, are key in the subsequent switch of lipid mediators90, activating the expression of the enzymes necessary for producing SPMs59. During this process, LXs87 appear first, followed by Rvs and, finally, PDs and MaRs88; however, the resolution is more a result of the concerted action of these mediators than of the time course of their secretion; although it is clear that their early appearance guarantees the self-limiting nature of the inflammation92.
The resolution of inflammation is aimed, in part, at clearing the pathogenic load through the SPMs. These are also responsible for promoting local neutrophil apoptosis, reducing the systemic inflammatory response by decreasing the production of cytokines and other Nuclear Factor κB (NFκB)-associated products, and increasing the production of IL-10 and nitric oxide in macrophages47. In addition, they increase phagocytosis of apoptotic leukocytes (efferocytosis) and pathogen clearance by tissue macrophages47,102. For these reasons, SPMs have high therapeutic potential in managing inflammation89, including the possibility of circumventing the adverse reactions and immunosuppression associated with the treatment of hyperinflammatory states triggered by severe infections, such as sepsis or COVID-1974.
Interestingly, the synthesis of SPMs can be triggered by the prostaglandin production inhibitor aspirin since cyclooxygenase 2 (COX2) acetylation blocks prostaglandin synthesis and, at the same time, confers the ability to produce the epimer intermediates 15R-HETE from arachidonic acid, 18R-HEPE from EPA and 17R-HDHA from DHA. Neutrophils transform these intermediates into lipoxins, resolvins, and protectins triggered by aspirin91. Likewise, statins, inhibitors of 3-hydroxy-methyl-glutaryl coenzyme A reductase, lower blood cholesterol levels and have other effects, called pleiotropic, by decreasing the synthesis of isoprenoids, intermediates in the mevalonate pathway86. These effects include regulation of endothelial function, coagulation, and anti-inflammatory actions such as reduced leukocyte migration and proinflammatory cytokine generation55. Statins induce COX2 nitrosylation, generating the same epimer intermediates of SPMs as aspirin but causing S-nitrosylation49, explaining, in part, the anti-inflammatory effects of statins21.
SPMs are relevant in acute infections because they shorten the inflammatory interval and increase the elimination of bacteria and other microorganisms; eventually, allowing the decrease in antibiotic doses17,90. Therefore, the relationship between infection and resolution is attractive due to the immunosuppressive potential of some anti-inflammatory agents30. For example, in self-limited Escherichia coli infections, resolution programs are activated, and PD1 and RvD1, and D5 levels are elevated21,22. In addition, LXA4, Rv D1, and MaR1 increase the survival of mice with experimental sepsis16,50,107; moreover, LXA4 decreases biofilm formation by Pseudomonas aeruginosa while increasing the ability of ciprofloxacin and imipenem to kill this bacterium101. Interestingly, a recent report links the protective effect of the antimalarial artesunate to the activation of GPR32, another SPM receptor, mimicking the effects of PD1 on macrophages in the context of murine Plasmodium infections and Listeria sepsis6. Thus, given the importance of antimicrobial resistance, a pharmacological intervention of resolution could provide a different approach to decreasing exposure to antibiotics17.
Modulating the host response with statins or aspirin may be helpful in the therapeutics of infections with significant inflammatory components. It could contribute to managing antimicrobial resistance or prevent referral to potentially harmful chronic courses. Thus, it is interesting to ask whether the modulation of relevant host factors with aspirin or a statin could be beneficial for the adjuvant treatment of parasitic infections caused by systemic protozoa such as the causative agents of malaria, leishmaniasis, toxoplasmosis or Chagas disease. Therefore, we performed a systematic search and a narrative review of the state-of-of-the-art in the use of statins or aspirin as potential therapies to modify host factors in treating parasitic diseases, the most neglected of all infectious diseases, to identify the potential benefits of aspirin or statins in the treatment of these parasitic diseases, based on their properties for modulating the inflammatory response.
MethodologyThe present work was structured as a narrative review based on a systematic search for articles on the use of aspirin or statins in experimental models or clinical studies of parasitic infections caused by Plasmodium, Leishmania, Toxoplasma or Trypanosoma.
The database used for the search was PUBMED (https://pubmed.ncbi.nlm.nih.gov/). The parasitic diseases included in this study were Chagas disease, leishmaniasis, malaria, and toxoplasmosis. These infectious diseases were included because of their epidemiological importance or the difficulties in finding an effective therapy.
The search strategy was designed using terms included in “all fields” or as a medical subject heading (MeSH). The subheading “pharmacological action” was used as an additional descriptor.
For the aspirin search, the terms “aspirin” or “acetylsalicylic acid” were used. For the statin search, “statin” OR “lovastatin”, “simvastatin”, “pravastatin”, “atorvastatin”, “fluvastatin”, “rosuvastatin”, and “pitavastatin”.
For the parasitic diseases, the keywords used were “Trypanosoma cruzi”, “Chagas disease”, “Trypanosomabrucei”, “African trypanosomiasis”, “Toxoplasma gondii”, “toxoplasmosis”, “malaria”, “Plasmodium falciparum”, “Plasmodium vivax”, “Plasmodium malariae”, “Plasmodium ovale”, “leishmaniasis”, “Leishmania tropica”, “Leishmania donovani”, “Leishmania major”, “Leishmania Mexicana”, “Leishmania amazonensis” and “Leishmania infantum”.
Inclusion criteria were (1) English language of publication, (2) articles published between 2015 and 2022, (3) original article, (4) in vitro or in vivo study design. The in vivo studies included animal or human models (prospective or retrospective clinical trials).
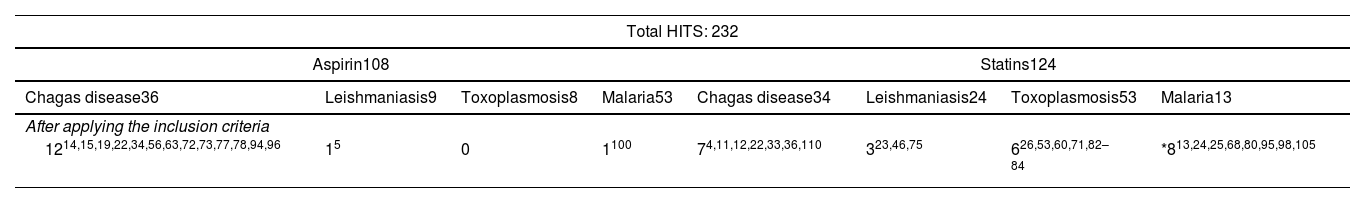
Two hundred thirty-two (232) hits were retrieved, and after applying the inclusion criteria, 38 studies were obtained and included in this narrative review (Table 1).
Selection of articles after applying the search strategy and inclusion criteria.
| Total HITS: 232 | |||||||
|---|---|---|---|---|---|---|---|
| Aspirin108 | Statins124 | ||||||
| Chagas disease36 | Leishmaniasis9 | Toxoplasmosis8 | Malaria53 | Chagas disease34 | Leishmaniasis24 | Toxoplasmosis53 | Malaria13 |
| After applying the inclusion criteria | |||||||
| 1214,15,19,22,34,56,63,72,73,77,78,94,96 | 15 | 0 | 1100 | 74,11,12,22,33,36,110 | 323,46,75 | 626,53,60,71,82–84 | *813,24,25,68,80,95,98,105 |
When necessary, works before 2011 were mentioned to contextualize the concepts discussed.
Role of aspirin and statins in the therapy of some parasitic diseases of medical interestMalaria infectionsMalaria, caused by protozoa of the Plasmodium genus, is the most important parasitic disease in the world, with a high burden of disease and mortality79. P. falciparum is responsible for 95% of morbidity and mortality, including severe manifestations derived from brain involvement, reaching a mortality rate of 10–20%79. Although its incidence has been decreasing20, one of the main problems of this disease is the emergence of resistance to pharmacological treatment and insecticides used for vector control; therefore, its control has not been easy104,109. Moreover, it is necessary to create new treatment strategies with consistent efficiency and new drug combinations to protect and reduce severe and resistant forms of malaria109. Thus, statins or aspirin could play a role in modulating the inflammatory response to infection by the Plasmodium parasite.
From 2015 to date, only two articles were found reporting the effectiveness of lovastatin or atorvastatin in reducing the cerebral inflammatory response to P. berghei infection in a cerebral malaria model13,68. For this reason, the search was extended to cover a longer period of 10 years (2011–2022). This strategy yielded 20 articles, only eight of which studied the modulation of host factors with statins. The remaining articles fell beyond the focus of the present review because they report the activity of statins or their analogs directly on Plasmodium in vitro models.
The concept of the use of statins in malaria is not without controversy. On the one hand, in 2009, Helmers et al. reported that statins do not protect against cerebral malaria in an experimental murine model40; moreover, in a preclinical trial, the administration of atorvastatin did not alter the course of infection with P. bergei, although it acted synergistically with methylene blue24. On the other hand, preliminary in vitro studies suggest a direct effect of statins on the parasite76. However, importantly, there is preclinical evidence pointing to the control of neuroinflammation by decreasing endothelial damage13,68,98 and even improving cognitive alterations caused by cerebral malaria80. Moreover, the combined therapy of atorvastatin with artemeter105, dihydroartemisinin25 or mefloquine95 can alter the course of cerebral infection, reducing mortality from this cause in murine models infected with P.bergei25,95.
Only one article met inclusion criteria for aspirin in malaria. Although it sounds logical to think that prostaglandin synthesis inhibition could influence the host response in malaria, considering the endothelial and platelet alterations observed during plasmodial infection, studies in this regard are scarce and controversial. Indeed, it has been suggested that prostaglandins may protect against endothelial damage108; moreover, following a prospective, randomized study in 97 patients with P. falciparum infection, aspirin did not alter the course of the disease41. However, in a recent study in a murine model of P. yoelii-induced renal failure, aspirin prevented renal cell damage, especially in mice subjected to monocytic depletion100. Therefore, further preclinical studies are needed to support aspirin as an adjuvant in malaria therapy.
Toxoplasma gondii infectionsToxoplasma gondii is an apicomplexan intracellular parasite that can infect humans, producing a generally asymptomatic infection, but with the persistence of the parasite in tissues in the form of cysts53, which can be activated by immunosuppression42. The most severe consequences of congenital infection in the fetus are observed during pregnancy64, in addition to severe ocular sequelae in immunosuppressed patients27. It is a public health concern whose pharmacological treatment is ineffective due to the persistence of cysts in infected tissues. However, in symptomatic and congenital cases, a combination of pyrimethamine and sulfadiazine is used, which blocks folate synthesis, with the consequent risk of causing hematological toxicity in the host due to the high doses required and the prolonged duration of treatments37. However, trimethoprim–sulfamethoxazole, which is less toxic, is an affordable alternative for some patients103. Therefore, the search for alternative treatments is necessary1. Modifying host factors has been proposed owing to the manipulation performed by the parasite for its continuous replication and survival in the host cell54. However, few studies analyze the effect of aspirin and statins in toxoplasmosis.
The use of statins as an experimental pharmacological strategy for the modification of host factors in models of toxoplasmosis is reported in six articles published in the last seven years. The availability of isoprenoids in both parasite and host had been previously suggested54, being essential for establishing the pathogen-host relationship, which can be altered using atorvastatin. However, it has been recently reported that only high doses of atorvastatin inhibited enteric cell invasion, suggesting that de novo synthesis of isoprenoids is not essential for replication60. Moreover, when atorvastatin is combined with bisphosphonates, there is a synergistic effect on isoprenoid production and, therefore, on decreasing the parasite load by reducing its replicative capacity53,54. Furthermore, rosuvastatin inhibited intracellular replication of T. gondii in HeLa cells84. Still, in addition, pravastatin and simvastatin, combined with low doses of pyrimethamine-sulfadiazine, synergistically decreased tachyzoite infection in HeLa cells83, reducing the host levels of cytokines IL-6 and IL-7, responsible, in part, for the spread of the disease82. These findings are supported by a recent study in which rosuvastatin decreased brain parasite load and local inflammation in a murine model of toxoplasmosis71 and improved the neurological alterations produced by the infection, including memory disorders26. Those findings suggest that rosuvastatin could be helpful in the treatment of chronic toxoplasmosis.
The search strategy for the use of aspirin did not yield results in the last seven years. However, preliminary reports link PGE2 production and IL-10 secretion with a neuroprotective effect against T. gondii infection81. In addition, this same effect could contribute to regulating the innate immune response since when mice were treated with T. gondii extracts, a significant increase in the production of lipoxin A4 was reported. Lipoxin A4-epimer can be induced by aspirin, related to the suppression of cytokine signaling mediated by SOCS261.
Leishmania spp. infectionsLeishmaniasis is a group of endemic diseases caused by different species of parasites of the genus Leishmania. They are obligate intracellular parasites infecting host macrophages, causing significant morbidity and mortality worldwide46. They are transmitted to humans by the bite of female insects of the Phlebotomus and Lutzomyia genus, generating a cutaneous infection in most cases and, less frequently, affecting the liver and spleen, which can be fatal if left untreated2,43. To date, no effective vaccines exist, and the pharmacological treatment includes pentavalent antimonial agents43. However, the antifungal amphotericin B, the aminoglycoside paromomycin, and the phospholipid metabolism inhibitor miltefosine have also shown efficacy. They are directed against the parasite but require monitoring and evaluation of serious adverse effects or the emergence of resistance43. Therefore, it is of utmost importance for searching drugs with antiparasitic activity, as well as adjuvants or those that control the inflammatory process of the host and could intervene in the parasite invasion in macrophages38.
In this regard, only three studies were identified using the search strategy described above in the context of Leishmania infections. An in vitro study with L. donovani highlights the importance of an adequate cholesterol level in host cells for optimal parasite entry because chronic cholesterol depletion caused by lovastatin reduced promastigote binding to the macrophage surface, with a consequently lower intracellular amastigote load46. This finding suggests that lowering cholesterol content with statins in the host cell membrane could be effective for infection control46. Moreover, Haughan et al. previously reported that inhibition of sterol synthesis with lovastatin and miconazole is synergistic39. However, Ghosh et al. found that an atherogenic diet in mice could be protective against L. donovani infection since the parasite extracts cholesterol from the membrane preventing T-cell activation32; thus, statin-induced cholesterol depletion could increase susceptibility to infection. However, the intracellular parasite load did not improve, suggesting a role of statin in controlling parasite growth32, as sterol synthesis by the parasite can be inhibited by mevastatin, the first statin of its kind, affecting cell replication23. The use of simvastatin in a model of cutaneous leishmaniasis caused by L. major decreased the local parasite load and inflammation by accelerating phagosome maturation and enhancing the oxidative burst in macrophages75; which had been previously described with pravastatin in a murine model with L.amazoniensis44,45,70.
Only one recent report suggests that aspirin decreases the parasite load in macrophages, improves the phagocytic activity of these cells and modulates cytokine secretion5. However, more research on this drug is needed for leishmaniasis.
Trypanosoma cruzi infectionT. cruzi is the protozoan responsible for Chagas disease, an endemic illness in Latin America, which is treated with benznidazole and nifurtimox28. Both agents have high toxicity and limited efficacy, especially in the chronic phase of the disease78. In 30% of cases, without adequate treatment, the disease may progress to a chronic inflammatory stage, causing heart failure, arrhythmias, and death3. Different therapeutic strategies have been sought, where statins and aspirin could be candidates for repositioning8.
Seven experimental studies had been identified since 2015 to date, in which a statin was tested in murine models. Considering that T. cruzi has an affinity for cholesterol and that the parasite uses the LDL receptor as part of the mechanism to infect cells, the role of atorvastatin in lipid metabolism is studied, reporting a deleterious effect on the course of the disease, especially in subjects fed a high-fat diet110. However, Soares de Souza et al. observed that simvastatin can mitigate disease progression in a similar model22. Notwithstanding the role that statins may have on the mechanics of T. cruzi infection, it is more likely that their actions in the chronic phase are related to the modulation of inflammation induced by the persistence of the parasite. Thus, it has been suggested that simvastatin could have anti-inflammatory potential in models of acute Chagas disease93. Moreover, Campos-Estrada et al. proposed that simvastatin triggers the production of 15-epi-LXA4 in endothelial cell models12, although without causing a synergistic effect with benznidazole. However this synergism could exist, according to Araujo-Lima et al., who also highlights the low cardiotoxigenic potential of atorvastatin4, probably facilitating parasite clearance in cardiac tissue induced by the resolution of inflammation33. Moreover, treating T. cruzi-infected human umbilical vein endothelial cells (HUVEC) with simvastatin promotes the differential expression of inflammation-related genes. Furthermore, simvastatin treatment of human umbilical vein endothelial cells infected with T. cruzi promotes the differential expression of inflammation-related genes. It also influences the Notch111 pathway, which is related to cardiac development in the embryo and may also participate in the cardiac protective effect of simvastatin during the chronic phase of the disease35.
Cyclooxygenase (COX) is a relevant player in the pathophysiology of T. cruzi infection34, and much has been studied since the publication of the relationship of prostaglandins and the phagocytosis of apoptotic bodies29. Since 2015, 12 studies have been published linking aspirin as a potential modulator of the course of T. cruzi infection. During the acute phase and as an evasive measure, the parasite modifies the macrophage response, generating an anti-inflammatory environment resulting from the production of prostaglandin E2 and the activation of TGF-β, especially in the presence of apoptotic T lymphocyte apoptotic bodies29. Additionally, COX inhibition has been reported to facilitate parasite survival in the host67,69. Furthermore, COX involvement in the invasion process has been recently demonstrated in a process inhibited by aspirin15,56,69.
In an in vivo model of chronic Chagas disease, it was observed that aspirin treatment during the acute phase was able to prevent damage to esophageal nitrergic myenteric neurons63. Those findings were corroborated in another study comparing aspirin administration during the acute or chronic phase, which showed a significant decrease in colonic inflammatory foci associated with a neuroprotective effect on myenteric neurons63,72,96. Moreover, it has been reported that aspirin could have some beneficial impact on the neurological manifestations of Chagas disease, as it prevents behavioral alterations in mice acutely infected with T.cruzi94. Moreover, the early use of aspirin in combination with benznidazole proved effective in preventing chronic heart disease78. Its use during the chronic phase of the disease can prevent the progression of cardiomyopathy, decreasing endothelial activation, cardiac inflammatory infiltrate, and fibrosis, probably by generating pro-resolving lipid mediators of inflammation such as 15-epi-LXA4 and AT-RvD1, among others14,58,65,66,73. In a pilot study in humans, aspirin showed efficacy in reducing the symptoms associated with microvascular abnormalities caused by Chagas heart disease77.
Despite the controversy about the role of COX inhibitors in T. cruzi infection19, there is abundant evidence supporting the immunomodulatory role of aspirin in the context of Chagas disease57,62. However, there are still no clinical studies to corroborate this. On the other hand, there are no studies evaluating the use of statins or aspirin in the context of T. brucei infections during the period analyzed.
ConclusionsThe relationship between a pathogen and its host determines the course of infection and defines whether the infection progresses or is effectively contained. Progression of infection can have deleterious consequences for the host because it can lead to disability or death. Immunity is one of these major determinants in the host–pathogen relationship. Parasitosis is not excluded from this interaction. However, therapeutic development to expand the antiparasitic pharmacological arsenal is insufficient, slow, or non-existent. Programs such as the Drugs for Neglected Diseases initiative seek to correct this scenario. The active search for compounds capable of modifying key aspects of the inflammatory process that are already approved for use in humans, have proven to be safe and reasonably priced, and a sound strategy9. In the present review, we present state-of-the-art agents such as aspirin and statins, which induce the production of inflammation-resolving agents and, therefore, could eventually change the course of infections, not only parasitic, as we have seen, but also bacterial or fungal infections. The uncontrolled nature of the inflammatory response in severe SARS-CoV2 infections, leading to patient death in many cases74, boosted the field for exploring the use of agents to modulate the host factors85. The prevention of organ damage induced by the persistence of the pathogen and the functional recovery of the innate immune response that prevents chronic inflammation are elements that can help to improve the efficacy of specific anti-infective treatments.
Undoubtedly other strategies to modify the host response would make more sense because they are more direct and probably broader in the spectrum, such as the direct blockade of TNF-α action or interferon-γ administration111. However, these agents are not without adverse events, producing increased susceptibility to infections or severe arthropathy, respectively. Moreover, in many cases, they are also expensive. On the other hand, the use of vaccines for managing and preventing parasitic infections is far from optimal, and little progress has been made in this field, except for the RTS S/AS01 malaria vaccine20.
Unfortunately, the evidence supporting the utility of aspirin in inducing inflammation resolution is not strong. Moreover, the potential for severe reactions such as, for example, bleeding or gastric intolerance considerably diminishes the attractiveness of its use in humans, especially in parasitic diseases, mainly if they are chronic such as in Chagas disease or leishmaniasis. However, this drug may be helpful for further study of the phenomena of inflammation resolution in parasitic infections, as well as other immunopathogenic mechanisms that can be further modified with immunomodulatory drugs.
Furthermore, although our literature review mainly focused on in vitro and in vivo experimental models, a beneficial effect is glimpsed with statins as adjuvant therapy through their cholesterol lowering-independent effects, also called pleiotropic. Although the isoprenoid metabolism in parasites can be directly inhibited by statins, halting their growth, there is no doubt that modulating the host response to the pro-inflammatory effects of infection is an attractive alternative. This effect must necessarily be proven in human studies. The study of modulating the resolution of inflammation with statins is a very active field in which they have also been tested for viral51 and bacterial infections7, including tuberculosis52, COVID-1985, and septicemia31.
Thus, the modulation of host factors is an attractive tool to help combat these diseases in a context of emerging treatment resistance and spread to non-endemic areas, with the consequent risk of severe epidemic outbreaks. Because it is still controversial30, it is necessary to improve the strength of evidence, determine the most effective statin, and, most importantly, analyze the clinical outcomes to further understand statin use in parasitic diseases.
FundingThis work was funded by Agencia Nacional de Investigacion y Desarrollo (ANID) programa FONDECYT, grant number 1210359.
Conflict of interestThe authors declare that they have no conflicts of interest.