Several pathogens have been suspected of playing a role in the pathogenesis of schizophrenia. Chronic inflammation has been proposed to occur as a result of persistent infection caused by Chlamydophila pneumoniae cells that reside in brain endothelial cells for many years. It was recently hypothesized that brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) may play prominent roles in the development of schizophrenia. NT-3 and BDNF levels have been suggested to change in response to various manifestations of infection. Therefore, we aimed to elucidate the roles of BDNF and NT3 in the schizophrenia–C. pneumoniae infection relationship. RT-PCR, immunofluorescence and ELISA methods were used. Fifty patients suffering from schizophrenia and 35 healthy individuals were included as the patient group (PG) and the healthy control group (HCG), respectively. We detected persistent infection in 14 of the 50 individuals in the PG and in 1 of the 35 individuals in the HCG. A significant difference was found between the two groups (p<0.05). Twenty-two individuals in the PG and 13 in the HCG showed seropositivity for past C. pneumoniae infection, and no difference was observed between the groups (p>0.05). C. pneumoniae DNA was not detected in any group. A significant difference in NT-3 levels was observed between the groups, with very low levels in the PG (p<0.001). A significant difference in BDNF levels was also found, with lower levels in the PG (p<0.05). The mean serum NT-3 level was higher in the PG cases with C. pneumoniae seropositivity than in seronegative cases; however, this difference was not statistically significant (p>0.05). In conclusion, we suggest that NT-3 levels during persistent C. pneumoniae infection may play a role in this relationship.
Existe la sospecha de que algunos patógenos pueden desempeñar un papel en la patogénesis de la esquizofrenia; en ese contexto, se ha propuesto que la infección persistente causada por células de Chlamydophila pneumoniae presentes en las células endoteliales cerebrales durante muchos años lleva a la inflamación crónica. Recientemente se ha planteado la hipótesis de que el factor neurotrófico de origen cerebral (BDNF, por sus siglas en inglés) y la neurotropina-3 (NT-3) podrían estar implicados en el desarrollo de la esquizofrenia, y se ha sugerido que sus niveles se modifican en respuesta a diversas manifestaciones de la infección. En esta investigación intentamos esclarecer el papel que desempeñan el BDNF y la NT3 en la relación entre la esquizofrenia y la infección por C. pneumoniae. Se utilizaron métodos de RT-PCR, inmunofluorescencia y ELISA. Se incluyeron 50 pacientes con esquizofrenia y 35 individuos sanos como grupo de pacientes (GP) y grupo de controles sanos (GCS), respectivamente. Detectamos una infección persistente en 14 sujetos del GP y en 1 de los del GCS, lo que constituyó una diferencia significativa (p<0,05). Veinte participantes del GP y 13 del GCS fueron seropositivos para una infección pasada por C. pneumoniae, diferencia no significativa (p>0,05). No se detectó ADN de C. pneumoniae en ninguno de los dos grupos. Se observó una diferencia significativa entre los grupos en los niveles de NT-3, que fueron muy bajos en el GP (p<0,001), y de BDNF, inferiores en el GP (p<0,05). La concentración sérica media de NT-3 fue mayor en los individuos seropositivos para C. pneumoniae en comparación con los seronegativos, pero esta diferencia no alcanzó significación estadística (p>0,05). Sugerimos que los niveles de NT-3 durante una infección persistente por C. pneumoniae pueden estar implicados en la relación de Chlamydophila pneumoniae con la esquizofrenia.
Schizophrenia is a multi-factorial and complex neuropsychiatric disorder that affects a large number of people worldwide. In addition to genetic risk factors33, winter-spring birth52, rural or urban life42, a crowded household1, birth complications25, cat contact during the early stages of life53 and migration14 have been implicated in the development of schizophrenia. In studies on the etiopathogenesis of schizophrenia, some researchers have investigated the associations of this disease with Toxoplasma gondii12,54, Treponema pallidum32, Cytomegalovirus (CMV)34, Herpes simplex viruses I and II (HSV-I, HSC-II)43,46, West Nile virus (WNV)6, and Borna Disease virus (BDV)29, and important data have been obtained. In a meta-analysis that included 42 studies performed in 17 countries over 5 decades, Torrey et al. suggested that individuals suffering from schizophrenia have an increased prevalence of antibodies against T. gondii55. A combined odds ratio (OR) of 2.73 was reported by the authors. In another meta-analysis that included 56 studies that assessed the possible association between the detection of different infectious agents and schizophrenia, Arias et al. reported statistically significant associations between schizophrenia and infection with Human herpes virus 2 (OR=1.34), BDV (OR=2.03), Human endogenous retrovirus W family (OR=19.31), Chlamydophila pneumoniae (OR=6.3499, Chlamydophila psittaci (OR=29.05) and T. gondii (OR=2.70)4. The principal reason for focusing on these infectious agents is that these agents have the ability to settle persistently in the central nervous system (CNS); in addition, neurological and psychiatric symptoms have been observed in some genetically predisposed people39. However, in recent years, important results related to the relationship between neurotrophic and intracellular C. pneumoniae and schizophrenia have only been reported in a few studies20–22.
C. pneumoniae is a gram negative bacterium that undergoes biphasic growth within the host. The ability of C. pneumoniae elementary bodies to infect various human cells (e.g., macrophages, monocytes and lymphocytes, as well as endothelial and smooth muscle cells)15 suggests that C. pneumoniae infection may result in systemic dissemination10. In addition, the detection of C. pneumoniae DNA in peripheral blood mononuclear cells (PBMCs) using molecular methods suggests that this dissemination occurs in various tissues10,41. C. pneumoniae is primarily responsible for atypical pneumonia30; however, several recent studies have asserted that C. pneumoniae might be related to neuropsychiatric (e.g., schizophrenia) and neurodegenerative diseases (e.g., multiplesclerosis)21,22,49, as well as other diseases, such as coronary heart disease26,47, lung cancer31, and arthritis5. The cause of the association between Chlamydiaceae and many different clinical manifestations, especially schizophrenia, may be the ability of the organism to interact with its host by remaining in body tissues, causing chronic infection and relapse.
C. pneumoniae infection has been reported to stimulate the transendothelial entry of monocytes through human brain endothelial cells (HBMECs)9, and it has been asserted that chronic inflammatory pathogenesis might arise as a result of persistent infection due to the ability of C. pneumoniae to reside in brain endothelial cells for many years20,21. It was also proposed that proliferating immune system cells might favor the synthesis of different neurotrophins21. Brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) are responsible for prenatal neuronal differentiation and development, as well as postnatal neuronal plasticity51. These factors are essential for the regulation of synaptic activity, affect glutamatergic neurotransmission and dopamine synthesis, and are effective in transmitter synthesis in adults36. Hippocampal damage was reported to cause the upregulation of BDNF in related regions of schizophrenic patients7,48. The influence of this factor on dopaminergic neurons may be relevant to the pathogenesis of schizophrenia, which is suggested to be related to dopaminergic dysfunction48. NT-3 enhances the survival of dopaminergic neurons suggesting a possible role of this factor in the pathophysiology of dopaminergic-related neuropsychiatric disorders (e.g., Parkinson's disease, schizophrenia, and Tourette's syndrome)13,24. Neurotrophins may exert their effects via multiple mechanisms: decreased or increased levels of neurotrophins, imbalances between different neurotrophins, and abnormalities in the control and timing of neurotrophin production, secretion and signaling pathways48. There is a growing interest in neurotrophins and their capacity to regulate central neurotransmission and promote neuroplasticity. Recent findings are consistent with our current understanding of neurobiology and the course of schizophrenia, so that there may be a prominent role of these agents in the development of schizophrenia. NT-3 and BDNF levels have been proposed to change in response to various manifestations of infection11,27.
Here we aimed to detect the presence of C. pneumoniae infections using serological and molecular methods and to investigate the schizophrenia–C. pneumoniae relationship. We also assessed the levels of BDNF and NT-3 to evaluate the role of BDNF and NT-3 in this relationship.
Materials and methodsThis study was planned as a cross-sectional and randomized case–control study between March 2013 and August 2013. The patients who were admitted to the Bakirkoy Mental Health Hospital in the Psychiatry Clinic and Department of Psychiatry of the Cerrahpasa Medical Faculty at Istanbul University were included as the patient group (PG), and healthy blood donors (who donated blood to the blood center of Sisli Etfal Training and Research Hospital) as well as healthy volunteer hospital workers were included as the healthy control group (HCG).
Patient and control groupsPatient group (PG)Fifty patients who were diagnosed by two experienced psychiatrists in accordance with the Diagnostic and Statistical Manual of Mental Disorders IV-Text Revision (DSM IV-TR) criteria3 and who had a disease duration of 1–10 years (mean of 3.75±0.25 years) were included as the PG. Their ages ranged from 18 to 52 years [mean±s.d. (29.9±9.26)]. The PG included 21 males (42%) and 29 females (58%). Patients suffering from any other physical illness such as brain tumors, thyroid disease, severe hepatic disease, severe cardiac disease, any other psychiatric diagnosis, organic brain disorder, or serious neurological disease, alcohol and/or drug abuse and pregnancy were excluded from the study. The study was approved by the local Ethical Committee of the Cerrahpasa Medical Faculty. A written informed consent was obtained from all participants. Following the first whole blood-collection and serum samples from the patients, the second whole blood samples were collected after 4–8 weeks to observe seroconversion, in accordance with the CDC (Centers for Disease Control and Prevention, USA) recommendations16. All patients received antipsychotic treatment [atypical antipsychotics (clozapine, risperidone, quetiapine, olanzapine, etc.) and typical antipsychotics (haloperidol)]. An informed consent form was also verbally read to all blood donors and volunteers. We also recorded information related to the participants’ age, education, marital status, medical history, family history, family ownership of pets, use of alcohol and smoking.
Healthy control group (HCG)The HCG consisted of 35 healthy blood donors and volunteer hospital workers who were matched with the patient group with respect to gender and age distribution. The patients and the matched normal subjects had similar socioeconomic status and dietary patterns. The age of the participants in the HCG ranged from 19 to 57 years [mean±s.d. (32.3±10.27)], and that group included 13 males (37.1%) and 22 females (62.8%). All the participants in the HCG declared no history of schizophrenia, bipolar disorder, or major depression, had no previous antipsychotic drug use, drug use or antibiotic use in the previous two years, and had no history of alcohol consumption or smoking. An informed consent form was also verbally read to all blood donors and volunteers.
Collection of serum samplesThis study was performed between March and August 2013. Two whole blood samples were collected from each individual both in the patient and control groups with an interval of 4–8 weeks to observe seroconversion. The immunofluorescence assay (IFA) of the second whole blood samples collected were repeated after 4–8 weeks. Fasting blood samples (5ml and 4ml) from the PG and the HCG were collected into sterile vacuum collection tubes with no anticoagulants and sterile collection tubes with EDTA (ethylenediaminetetracetic acid), respectively.
Whole blood and serum samples from all PG and HCG cases were transferred to the Microbiology Department of the Cerrahpasa Medical Faculty under optimal conditions. The whole blood samples were divided into two portions. One portion was centrifuged at 1000rpm for 15min to obtain serum samples and was then used with a commercially available kit to determine BDNF and NT-3 concentrations. These serum samples were stored at −20°C. The remaining portion was centrifuged at 5000rpm for 5min for the detection of C. pneumoniae antibodies, and the serum samples were stored at −70°C. The 4ml blood samples with EDTA were treated with Ficoll to separate PBMCs, divided into sterile Eppendorf tubes containing 0.5ml of a saline solution and stored at −70°C.
MethodsSerological methodsDetection of C. pneumoniae antibodies using the immunofluorescence antibody (IFA) methodSerum samples from the PG and HCG participants were studied for the presence of IgM, IgG and IgA antibodies against C. pneumoniae by IFA (Euroimmun Labordiagnostica, Germany). The serum samples were tested after treatment with Gullsorb (Gull Laboratories, Salt Lake City, Utah) in accordance with the manufacturer's instructions to remove interfering IgG antibodies28. Antigens prepared from C. pneumoniae elementary bodies were used as antigens. Most of this antigen consists of major outer membrane proteins (MOMPs), lipopolysaccharide (LPS), and outer membrane proteins (OMPs). A doubling dilution method, which started from 1/16 for IgM, 1/40 for IgA and 1/16 to 1/512 for IgG, was employed for antibody detection. The manufacturer's recommendations were followed for the determination of C. pneumoniae IgM. All slides were evaluated using a fluorescence microscope (Zeiss-Axioskop40)2,16. The seropositivity criteria for the diagnosis of persistent C. pneumoniae infection were IgG ≥512 and IgA ≥40; for the study population, IgG titers of >16 were accepted as the criterion for previous infection. Our study results were evaluated in accordance with the CDC criteria2,16,31.
Determination of BDNF and NT-3 concentrations using the sandwich ELISA methodSerum BDNF and NT-3 levels were measured with the sandwich enzyme-linked immunosorbent assay (ELISA) using a commercially available kit (BOSTER Immunoleader, ABD) following the manufacturer's guidelines. In brief, in flat-bottom microtiter plates (96-well), the samples were diluted 1:2 in sample diluent, and the standard curves ranged from 31.2 to 2000pg for BDNF and from 15.6 to 1000pg for NT-3.
The standards and samples (0.1ml) were added to each well. The microtiter plates were then incubated for 90min at 37°C. A biotinylated anti-human BDNF antibody and a biotinylated anti-human NT-3 antibody (0.1ml) were added to the wells and incubated for 60min at 37°C. The plates were then washed three times with wash buffer. Avidin–Biotin Peroxidase solution was then added to each well and incubated for 30min at 37°C. After washing (five times with wash buffer), the substrate was added and incubated for 20–25min at 37°C. After the addition of the stop solution, the amounts of BDNF and NT-3 were determined (absorbance set at 450nm).
Molecular methodsC. pneumoniae DNA was detected in PBMCs using a quantitative real time-polymerase chain reaction (qRT-PCR) method with commercially available kits (LightMix® Kit Chlamydophila pneumoniae and LightCycler® FastStart DNA MasterHybProbe, Roche, Berlin, Germany) following the manufacturer's guidelines.
Isolation of peripheral blood mononuclear cells (PBMCs)As C. pneumoniae is an obligate intracellular human pathogen, we isolated PBMCs from whole blood. The whole blood samples that were collected from each patient into EDTA-treated tubes were diluted 1:1 in PBS. The diluted blood was carefully layered over Ficoll-Paque (Wisent, St-Bruno) at a blood:Ficoll ratio of 3:1. Centrifugation (1600g, 35min) was performed to obtain a mononuclear cell fraction, which was aspirated, diluted 1:1 in PBS and centrifuged at 1600g for 10min. The PBMCs were stored at −70°C until the time of qRT-PCR analysis.
DNA extraction from PBMCs and detection of C. pneumoniae DNA in PBMCs via RT- PCRA qRT-PCR method was used for the detection of C. pneumoniae in PBMCs. Nucleic acid extraction was performed using a commercial nucleic acid purification kit (High Pure PCR Template Preparation Kit, Roche Germany) according to the manufacturer's instructions. We used a LightMix®C. pneumoniae (TIB MOLBIOL GmbH, Germany) real-time PCR kit for the detection of C. pneumoniae DNA. The kit included primers that were designed to amplify a 140bp fragment of the J38 genomic region of C. pneumoniae and a hybridization probe.
The mix includes an internal control system to exclude PCR amplification errors. We included a positive control, calibrators (ranging from 10 to 1000000copies), and negative controls in each test batch.
Statistical analysisThe data obtained in the trial were analyzed using the SPSS (version 17.00) statistics program. The data were evaluated using a multivariate logistic regression test (forward conditional model) and the Chi-square and Fisher exact tests. The Chi-square tests were used to evaluate the relationships between chlamydial infection and the presence of schizophrenia. The Chi-square test and the Fisher's exact test were used to compare frequencies among the groups. The distribution patterns of BDNF and NT-3 were analyzed using the Chi-square test. Most of the BDNF and NT-3 values did not fit a standard distribution curve, and the data were therefore subjected to non-parametric analyses. Thus, the Mann–Whitney U test was employed to compare groups. All statistical tests were two-sided, and p values lower than 0.05 were considered to be statistically significant.
ResultsWhen the PG and HCG participants were compared, primary school graduation and the presence of relatives with neuropsychiatric disorders were found to be significantly more common in the PG than in the HCG (p<0.05). However, graduation from a university was found to be significantly less common in the PG than in the HCG (p<0.05). The rate of smoking was significantly higher in the PG than in the HCG (p<0.001) (Table 1).
Demographic parameters, analyzed in the patient group and healthy control group cases
| Demographic parameters | Patient group | Healthy control group | p | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Marital status | |||||
| Married | 19 | 38 | 17 | 48.57 | >0.05 |
| Single | 31 | 62 | 18 | 51.45 | >0.05 |
| Educational status | |||||
| Primary school | 29 | 58 | 9 | 25.7 | <0.001 |
| High school | 15 | 30 | 9 | 25.7 | >0.05 |
| University | 6 | 12 | 17 | 48.5 | <0.05 |
| To have relatives with neuropsychiatric | |||||
| Present | 12 | 24 | 0 | 0 | <0.05 |
| Absence | 38 | 76 | 35 | 100 | |
| Smoking habit | |||||
| Present | 17 | 34 | 0 | 0 | <0.001 |
| Absence | 33 | 66 | 35 | 100 | |
| Alcohol use | |||||
| Present | 3 | 6 | 0 | 0 | >0.05 |
| Absence | 47 | 94 | 35 | 100 | |
Fourteen (28%) and one (2.85%) of the PG and HCG participants, respectively, were determined to have persistent C. pneumoniae infection (the seropositivity criteria for the diagnosis of persistent C. pneumoniae infection were IgG ≥512 and IgA ≥40), based on microimmunofluorescence (MIF) IgA and IgG results of two whole blood samples tested with an interval of 4–8 weeks (Table 2). A significant difference in persistent C. pneumoniae infection was observed between the PG and the HCG (OR=13.222, CI 95%: 1.648–106.07, χ2; 8.95; p<0.05); however, no significant difference in past C. pneumoniae infection was found between the groups (OR=1.330, CI 95%: 0.549–3.219, χ2; 0.400; p>0.05).
The IgG and IgA values of patient and control groups with the mean serum BDNF and NT-3 levels
| PGa | HCGb | pc | |
|---|---|---|---|
| Past C. pneumoniae infection (n: 35) | |||
| IgG≥16-IgG<512 | 22 (44%) | 13 (37.1%) | >0.05 |
| Mean BDNFd (pg/ml) | 1689.1 | 2140.1 | <0.05 |
| Mean NT-3e (pg/ml) | 594 | 903.8 | <0.05 |
| Persistent C. pneumoniae infection (n: 15) | |||
| IgG≥1/512+IgA≥1/40 | 14 (28%) | 1 (2.8%) | <0.05 |
| Mean BDNFd (pg/ml) | 1560.8 | 2061.4 | –f |
| Mean NT-3e (pg/ml) | 480.1 | 1034.7 | |
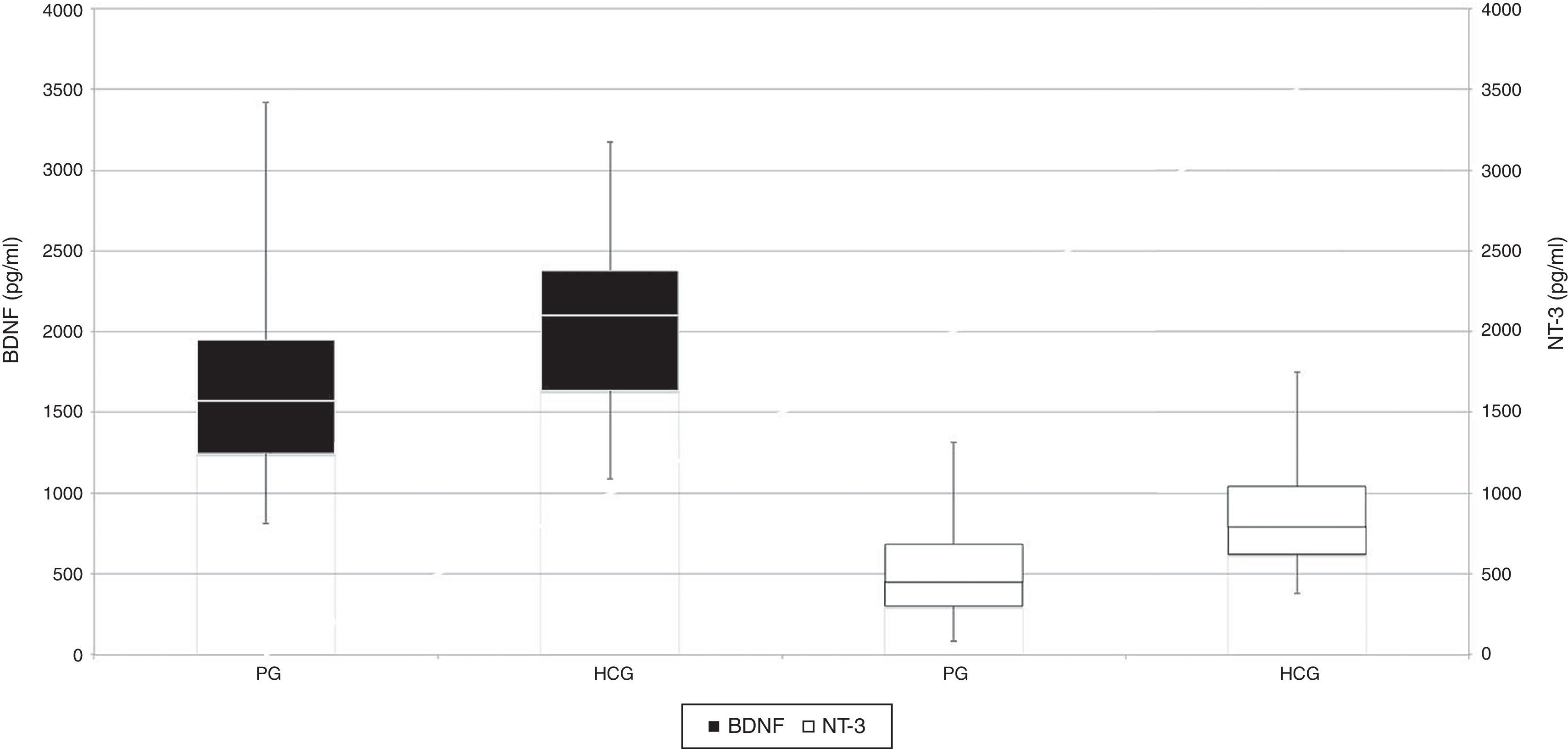
When the PG and HCG participants were compared with respect to gender and age for past and persistent C. pneumoniae infection seropositivity, PG females had significantly greater rates of persistent C. pneumoniae infection than HCG females. Additionally, a significant difference in persistent C. pneumoniae infection was found between PG and HCG participants, with infection being more frequent among females in the PG (p<0.05). Persistent C. pneumoniae infection was also found more frequently in 18–29 years and 30–40 years age of both groups. However, no significant differences in past C. pneumoniae infection were found among gender- and age-matched groups from the PG and the HCG (p>0.05). When the mean serum BDNF and NT-3 levels of the PG and HCG participants were compared, serum BDNF levels were significantly lower in the PG participants than in the HCG participants (p<0.05), and serum NT-3 levels were significantly lower in the PG than in the HCG (p<0.001) (Table 2, Fig. 1).
When the PG and HCG participants were compared with respect to the mean serum BDNF and NT-3 levels based on seropositivity for past C. pneumoniae infection, the serum BDNF and NT-3 levels were found to be significantly lower in the PG than in the HCG (p<0.05). Statistical analyses were not performed to compare the mean serum BDNF and NT-3 levels between the PG and the HCG based on persistent C. pneumoniae seropositivity because only one persistent C. pneumoniae case was detected in the HCG; therefore, a standard deviation could not be determined (Table 2).
When considering PG participants with past (NT-3:594pg/ml) or persistent (NT-3:480.1pg/ml) C. pneumoniae infection seropositivity, the mean serum NT-3 levels were higher in PG patients with C. pneumoniae seropositivity than in seronegative patients (NT-3:461pg/ml); however, this difference was not statistically significant (p>0.05). In contrast, when considering serum BDNF levels in schizophrenic patients with past (BDNF: 1689.1pg/ml) or persistent (BDNF: 1560.8pg/ml) C. pneumoniae infection, the mean serum BDNF levels were lower in PG patients with C. pneumoniae seropositivity than in seronegative patients (BDNF: 1962pg/ml) (p>0.05) (Fig. 2).
Side-by-side box-whisker plots of BDNF and NT3 levels of patients with past (PaCP, past C. pneumoniae), persistent (PeCP, persistent C. pneumoniae) C. pneumoniae infection and C. pneumoniae seronegativity (CPS). PaCP, past C. pneumoniae infection; PeCP, persistent C. pneumoniae infection; CPS, C. pneumoniae seronegativity.
We detected no C. pneumoniae DNA in the PBMCs from 85 participants by qRT-PCR. Although we detected C. pneumoniae seropositivity in schizophrenic patients (n:10) with persistent infection, we failed to detect C. pneumoniae DNA.
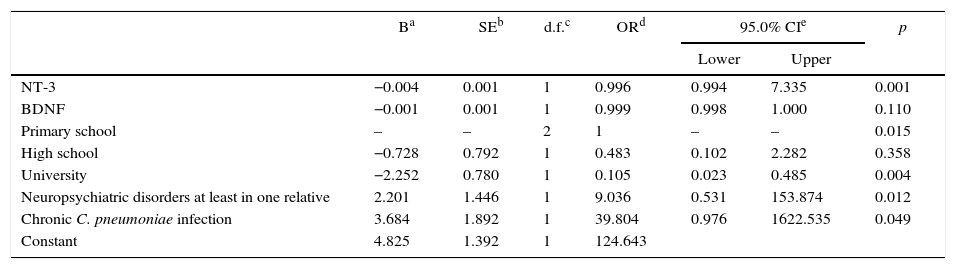
The results of a multivariate logistic regression analysis (following a forward conditional model) of demographic parameters (e.g., education and neuropsychiatric disorders in at least one relative), serological tests (C. pneumoniae IgG≥512+IgA≥40) and mean serum neurotrophic factor levels (BDNF/NT-3) are shown in Table 3.
Multivariate logistic regression analysis of demographical parameters, serologic tests and mean serum BDNF and NT-3 levels
| Ba | SEb | d.f.c | ORd | 95.0% CIe | p | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| NT-3 | −0.004 | 0.001 | 1 | 0.996 | 0.994 | 7.335 | 0.001 |
| BDNF | −0.001 | 0.001 | 1 | 0.999 | 0.998 | 1.000 | 0.110 |
| Primary school | – | – | 2 | 1 | – | – | 0.015 |
| High school | −0.728 | 0.792 | 1 | 0.483 | 0.102 | 2.282 | 0.358 |
| University | −2.252 | 0.780 | 1 | 0.105 | 0.023 | 0.485 | 0.004 |
| Neuropsychiatric disorders at least in one relative | 2.201 | 1.446 | 1 | 9.036 | 0.531 | 153.874 | 0.012 |
| Chronic C. pneumoniae infection | 3.684 | 1.892 | 1 | 39.804 | 0.976 | 1622.535 | 0.049 |
| Constant | 4.825 | 1.392 | 1 | 124.643 | |||
Based on the results of our analysis, primary school graduation (OR:1, p<0.05), the presence of seropositivity for persistent C. pneumoniae infection (OR:39.8, p<0.05) and the presence of neuropsychiatric disorders in at least one relative (OR:9.03, p<0.05) were identified as independent risk factors for schizophrenia. In addition, the rate of university graduation (p<0.05) and the mean serum neurotrophin-3 level (p<0.001) were found to be significantly lower in the PG than in the HCG.
DiscussionImportant data were recently obtained in studies that addressed the role of infectious agents in the etiopathogenesis of schizophrenia. The most interesting finding of these studies has been the presence of chronic infection caused by C. pneumoniae. Although persistently elevated IgA (or IgG) antibody levels have been proposed and used by some researchers to indicate chronic infection, there is no validated serological marker of persistent infection. In addition, it has been suggested that high IgA titers may be a better marker of chronic C. pneumoniae infection than IgG titers because serum IgA has a half-life of 5–7 days, but IgG has a half-life of weeks to months. First, we attempted to identify persistent C. pneumoniae infection in our study groups. The detection of persistent C. pneumoniae infection based on the presence of elevated titers of IgA-type C. pneumoniae antibodies for 6 months and the persistent production of IgG is generally indicative of persistent infection with C. pneumoniae rather than re-infection16.
The presence of C. pneumoniae DNA in PBMCs suggests that the systemic dissemination of C. pneumoniae occurs after exposure to a respiratory infection10. To establish a link between Chlamydia and schizophrenia, we performed a search for related studies. In a study performed by Xaiver et al., a female patient with pharyngitis caused by C. pneumoniae showed excessive manifestations related to acute psychosis during a 3-day period58. Those authors suggested that this acute psychosis may have been triggered by meningoencephalitis. C. pneumoniae infection in human brain microvascular endothelial cells was demonstrated to alter the permeability of the blood–brain barrier37,50.
C. pneumoniae infection may facilitate the transmigration of monocytes through human brain endothelial cells; this observation may represent one mechanism by which the organism enters the CNS. Consequently, the organism may enter the CNS inside infected monocytes, resulting in chronic injury15,38. The pro-inflammatory chemokines and cytokines that are produced by Chlamydia-infected cells may be involved in inflammation and tissue injury35,50.
BDNF and NT-3, which are produced by neurons, peripheral and CNS tissues, microglia, and Th1 and Th2 cells, might be altered in psychiatric disorders, such as schizophrenia. These factors were good candidates in our efforts to establish a link between Chlamydia infections and schizophrenia8,17. Considering the above mechanisms, we evaluated the role of C. pneumoniae in the development of schizophrenia. Some researchers reported that NGF, BDNF and NT-3 are produced via in vitro and in vivo microglial expression18. Additionally, it was suggested that Th1 cells may produce NT-3 and Th2 cells may produce BDNF. Two neurotrophins may be produced via the stimulation of aTh1-orTh2-based immune response8,17,18,40. The expression levels of neurotrophins that influence synaptic activity in the CNS by binding to the TrkA, TrkB and TrkC tropomyosin receptors may change based on the developing immune response (i.e., Th1 or Th2). This interaction between the immune system and the CNS might be triggered by infections with organisms like C. pneumoniae20,21.
Fellerhoff et al. detected 40% and 6.7% chlamydial DNA in the PBMCs of patients with schizophrenia and control subjects, respectively21. In another study performed by Fellerhoff et al., 4-times more chlamydial DNA was detected in the frontal cortex of postmortem schizophrenic and bipolar cases22. This finding led the authors to propose that neural development is likely to be impaired by the inflammation that is caused by Chlamydiaceae infections with neural and microglial involvement and might consequently cause neuropsychiatric disorders.
In this study, we detected a statistically significant difference in chronic C. pneumoniae infection between PG and HCG participants (p<0.05). We also determined that chronic C. pneumoniae infection causes a 13-fold greater risk of developing schizophrenia. Moreover, persistent C. pneumoniae infection was detected as an independent risk factor in our multivariate regression analysis. Based on our results, we suggested that C. pneumoniae infections may be related to schizophrenia. Despite our positive serological results, we were unable to detect C. pneumoniae DNA in PBMCs using qRT-PCR, which has an analytic sensitivity of 10 DNA copies. This finding contrast with the results reported in the above studies; in our opinion, this difference could be related to the absence of infected monocytes in the peripheral blood or the presence of C. pneumoniae DNA at a frequency below 10 copies in peripheral blood cells. Additionally, Murray et al. reported that the treatment of schizophrenic patients with neuroleptic drugs (e.g., haloperidol and pimozide) inhibited the calmodulin-dependent process that Chlamydiaceae use to enter cells44. Thus, these drugs may prevent the uptake of Chlamydiaceae by the cell and thus prevent the detection of Chlamydiaceae DNA in those cells. Our PG individuals were diagnosed with schizophrenia and treated for approximately 3–5 years, suggesting the possibility that these drugs might affect the uptake of Chlamydiaceae by the cells.
In a meta-analysis related to the epidemiology of schizophrenia, the frequency of schizophrenia was reported to be equal in both sexes, but the mean age at the onset of illness was higher for females than for males (25–35 vs. 18-25 years)19. We suggested that the detection of significantly different rates of chronic C. pneumoniae infection in female schizophrenic patients than in HCG patients and the detection of significantly more chronic C. pneumoniae infections in young and middle-aged schizophrenic patients are important demographic findings and are consistent with the available literature.
Although we expected to detect high levels of neurotrophin expression because chronic infection triggers inflammatory processes during early episodic schizophrenic psychosis, we actually detected low neurotrophin serum levels in schizophrenic patients. The lower serum BDNF and NT-3 levels observed in schizophrenic patients who had been receiving long-term treatment with antipsychotic drugs were consistent with the results of Fourney et al., who reported that antipsychotic drugs (especially haloperidol) down-regulated neurotrophins and decreased hippocampal BDNF mRNA expression23. Similar findings have been reported by other groups56,57. Most remarkably, we found that antipsychotic drug users with past or chronic C. pneumoniae infection had higher mean serum NT-3 levels than C. pneumoniae-seronegative patients with schizophrenia. We propose that this result may be related to continued NT-3 expression, which is caused by microglial or T cell activation in the presence of persistent C. pneumoniae infection, together with specific host immune responses to microglial C. pneumoniae infection, even with potential antipsychotic drug pressure in the hippocampal region.
In our study, having graduated from a primary school and not having graduated from a university were identified as independent risk factors. Park et al. reported that “to have a neuropsychiatric disease in the family” was not a significant factor in the Chlamydiaceae–schizophrenia relationship45. This factor has been reported to be a significant risk factor in two other Turkish studies that addressed the T. gondii-schizophrenia and BDV–schizophrenia relationships29,59. To have a neuropsychiatric disease “in the family” showed a significant difference between two groups in accordance with the univariate analysis in our study (p<0.05) and was also detected as an independent risk factor according to the multivariate analysis.
Serologically, persistent C. pneumoniae infection was identified as an independent risk factor according to the multivariate regression analysis. The serological detection of C. pneumoniae was also found to differ significantly between patients and controls in accordance with the univariate analysis, suggesting that persistent C. pneumoniae infections may play a role in the development of schizophrenia, as has been proposed by numerous other studies21,22. In addition, serum neurotrophin levels (especially NT-3) were lower in the PG than in the HCG, likely due to antipsychotic treatment. However, the serum NT- 3 levels of the schizophrenic patients with past or persistent C. pneumoniae seropositivity were higher than those of the schizophrenic patients with no C. pneumoniae seropositivity, although this difference was not statistically significant. We suggest that this result may be related to the expression of certain amounts of NT-3 by microglia and T cells that are activated by chronic and persistent C. pneumoniae infection and have migrated to the brain via monocytes (despite drug pressure).
This study has several limitations. First, during chronic C. pneumoniae infection, the expression of the organism's own 60kDa heat shock proteins (HsP60) increases, especially when the proteins are elevated persistently. The host immune response to microbial HsP60 may cause autoimmunity to human HsP60 and the subsequent development of some chronic diseases, such as asthma, atherosclerosis, or coronary heart disease15. We did not investigate HsP60 of C. pneumoniae. Secondly, our sample size was limited. Moreover, the schizophrenic patients were in different stages of schizophrenia and received different antipsychotic treatments. Antipsychotic medication is known to influence the immune system; thus, the infectivity of the organism may be affected. Moreover, the potential confounding variables were smoking, domestic pet contact, differences in education level between the patients and the controls and the effects of different drug doses on the C. pneumoniae–schizophrenia–neurotrophin (BDNF and NT-3) relationship.
In conclusion, the presence of serological C. pneumoniae positivity in the PG suggested the possible role of persistent C. pneumoniae infections in the development of schizophrenia. The presence of lower levels of serum neurotrophins (especially NT-3) in the PG than in the HCG suggested that prior antipsychotic treatment may have an effect. However, the high serum NT-3 levels of the schizophrenic patients with past or persistent C. pneumoniae seropositivity suggested that NT-3 is expressed by microglia and T cells that are activated by chronic and persistent C. pneumoniae infection and have migrated to the brain via monocytes (despite drug pressure). To define this relationship more clearly, similar, matched, broad-spectrum case–control studies should be conducted.
Ethical disclosureProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of DataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and /or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestThe authors declare that there are no conflicts of interest.
This study was approved by the Clinical Research Ethics Board of Istanbul University, Cerrahpasa Faculty of Medicine. Ethical approval: 83045809/25483 03 September 2013. This work was supported by Research Fund of the IstanbulUniversity, project number 34170. This research was presented as a poster in the 16th International Congress on Infectious Diseases (ICID), Cape Town, South Africa, 2014. Abstract no.: 58.017.