A molecular survey was conducted in Cochabamba, Bolivia, to characterize the mechanism involved in the resistance to clinically relevant antibiotics. Extended Spectrum β-lactamase encoding genes and plasmid-mediated quinolone resistance (PMQR) markers were investigated in a total of 101 oxyimino-cephalosporin-resistant enterobacteria recovered from different health centers during four months (2012–2013). CTX-M enzymes were detected in all isolates, being the CTX-M-1 group the most prevalent (88.1%). The presence of blaOXA-1 was detected in 76.4% of these isolates. A high quinolone resistance rate was observed among the included isolates. The aac(6′)-Ib-cr gene was the most frequent PMQR identified (83.0%). Furthermore, 6 isolates harbored the qnrB gene. Interestingly, qepA1 (6) and oqxAB (1), were detected in 7 Escherichia coli, being the latter the first to be reported in Bolivia. This study constitutes the first molecular survey on resistance markers in clinical enterobacterial isolates in Cochabamba, Bolivia, contributing to the regional knowledge of the epidemiological situation. The molecular epidemiology observed herein resembles the scene reported in South America.
Se llevó a cabo un relevamiento molecular de la resistencia a antibióticos de importancia clínica en aislamientos recuperados en Cochabamba, Bolivia. Se estudiaron los genes codificantes de β-lactamasas de espectro extendido y de resistencia a quinolonas de localización plasmídica (PMQR) en un total de 101 aislamientos de enterobacterias resistentes a oximinocefalosporinas recuperados en distintos centros de salud, durante 4 meses (2012-2013). En todos ellos se detectó la presencia de cefotaximasas, las CTX-M grupo 1 fueron las más prevalentes (88,1%). La presencia de blaOXA-1 se detectó en el 76,4% de estos aislamientos. Se observó una elevada proporción de aislamientos resistentes a quinolonas. El gen aac(6′)-Ib-cr fue el determinante PMQR más frecuentemente identificado (83%). Además, 6 aislamientos resultaron ser portadores de qnrB. Por otro lado, cabe remarcar que 7 Escherichia coli presentaron qepA1 (6) y oqxAB (1); se documenta así por primera vez la presencia de oqxAB en Bolivia. Este estudio constituye el primer relevamiento de marcadores de resistencia en aislamientos clínicos de enterobacterias en Cochabamba, Bolivia; de este modo se contribuye al conocimiento regional de la situación epidemiológica, la cual presenta un escenario similar al observado en el resto de Latinoamérica.
Antibiotic resistance is one of the most challenging problems in modern medicine worldwide. High resistance levels are observed in Latin America, probably due to the widespread, and often inappropriate, use of antibiotics in our region, resulting in ineffective treatments which enhance morbidity and mortality rates.
Third generation cephalosporins (TGC) and quinolones constitute first line antibiotics in enterobacterial infections; however increasing resistance levels are continuously reported. The production of extended spectrum β-lactamases (ESBL) is the most relevant TGC resistance mechanism, being CTX-M-type enzymes prevalent globally (more than 160 different enzymes have already been reported at www.lahey.org/studies/). CTX-M-enzymes are clustered in different groups, CTX-M-1 group, CTX-M-2 group, CTX-M-8 group, CTX-M-9 group and CTX-M-25 group; many of these groups include different allelic variants. CTX-M-15, corresponding to the CTX-M-1 group and CTX-M-14, belonging to the CTX-M-9 group, are by far the most clinically relevant ESBL worlwide3.
Spontaneous chromosomal mutations in gyrA and parC, within the quinolone resistance-determining region (QRDR), constitute the main mechanism conferring high level quinolone resistance. Plasmid-mediated quinolone resistance (PMQR) determinants, such as Qnr proteins (A, B, C, D and S), Aac(6′)-Ib-cr enzyme, QepA and OqxAB efflux pumps are increasingly reported12. Even though these markers only provide low level quinolone resistance by themselves, it has been shown that they facilitate the selection of chromosomal mutations in QRDR, in vitro12.
No surveillance studies on resistance markers in clinical isolates have been previously conducted in Cochabamba, Bolivia. The aim of this study was to characterize β-lactam and quinolone resistance mechanisms in TGC resistant Enterobacteriaceae. Therefore, a prospective, observational, descriptive and transversal study was carried out.
All cefotaxime and/or ceftazidime-resistant Enterobacteriaceae recovered from 5 health centers in Cochabamba, Bolivia, from December 2012 to March 2013 were included. Identification was performed by conventional biochemical tests. Antimicrobial susceptibility profiles were determined by disk diffusion and agar dilution methods in accordance with CLSI, 2014.
Total DNA was obtained by boiling bacterial suspensions, and plasmid DNA was extracted by alkaline lysis. Screening of ESBL coding genes, blaSHV, blaCTX-M-1 group, blaCTX-M-2 group, blaCTX-M-8 group, blaCTX-M-9 group, blaCTX-M-25 group, blaOXA-1, blaOXA-2, blaOXA-10, blaOXA-48, blaPER-2, blaKPC and blaGES, was performed by simple PCR amplifications. Primers used and the expected amplicon sizes are shown in a supplementary table (Table S1). Plasmid encoded ampC genes8 and Escherichia coli phylogenetic groups4 were determined by multiplex PCR as previously reported.
PMQR genes (qepA, oqxAB, aac(6’)-Ib-cr, qnr (A, B, C, D, S)) were investigated by PCR amplification using specific primers9. Amplicons identity was assessed by digestion and/or sequencing. Nucleotide sequences were compared with NCBI-BLAST databases. Statistical analysis was performed using the Fisher's exact test (SPSS version 22).
Clonal relatedness was analyzed by REP/ERIC-PCR and dendrograms were constructed using the Treecon 1.3b program. Isolates displaying more than 90% identity were considered to be clonally related.
A total of 101 TGC-resistant enterobacteria, corresponding to E. coli (87), K. pneumoniae (11), C. freundii (1), M. morganii (1) and E. cloacae (1), were recovered. A high proportion of the samples (71.3%) were recovered from women aged between 50 to 80 years old. Eighty five enterobacteria (84.0%) were isolated from urine, 7 from wound secretions, 4 from blood, 4 from tracheal secretions and the remaining from ear secretions. A similar distribution was observed between outpatients and inpatients (49.0% and 51.0%, respectively).
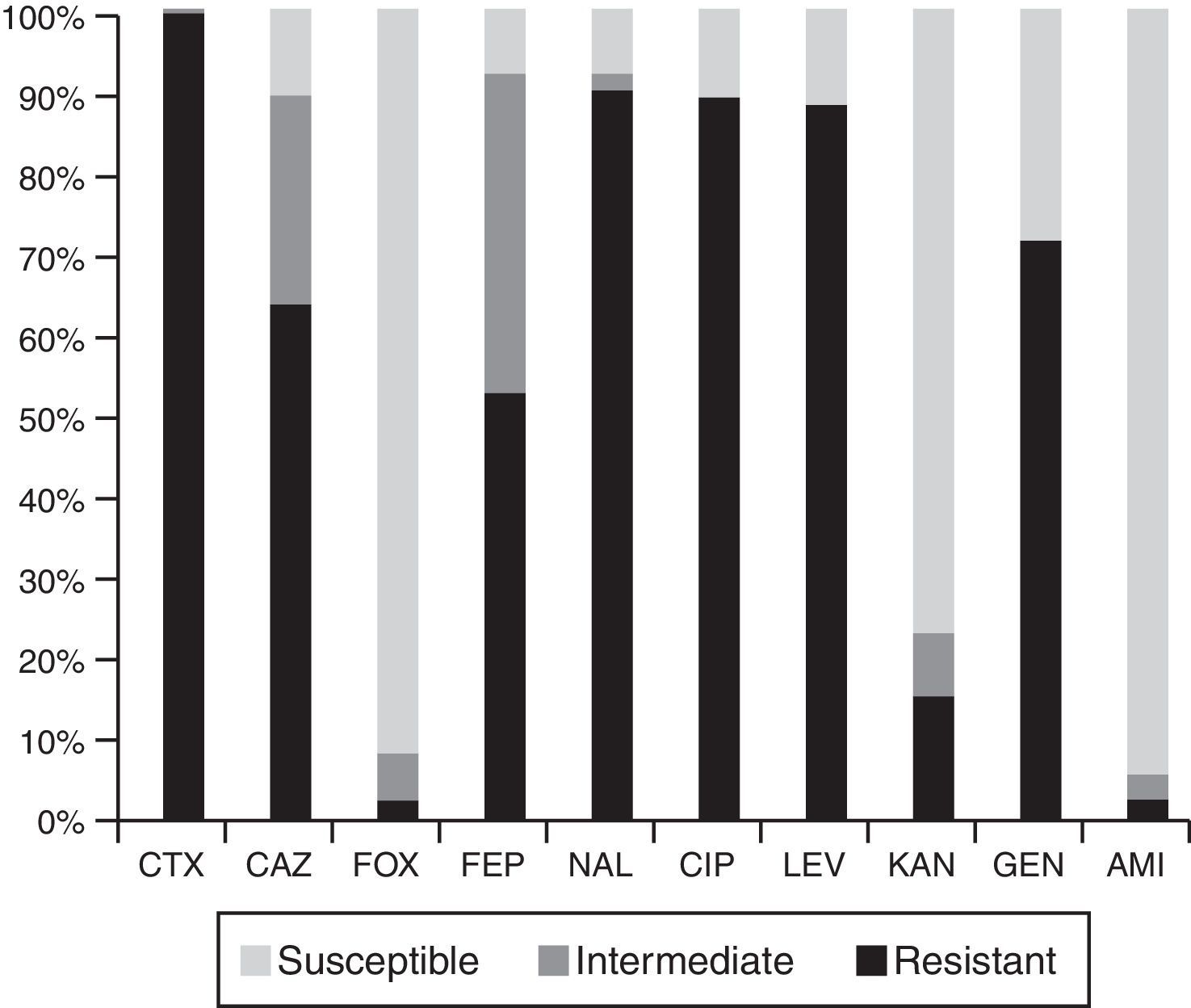
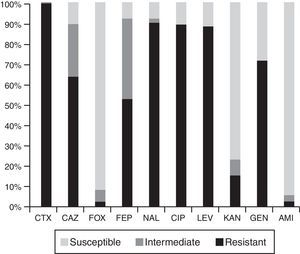
High resistance rates to nalidixic acid, ciprofloxacin, levofloxacin and gentamicin were observed. Eighty five percent of the isolates were not susceptible to ceftazidime and 90% to cefepime. Resistance to cefoxitin, kanamycin and amikacin was less frequent (Fig. 1).
MIC50 and MIC90 values were as follows: cefotaxime 128μg/ml and >128μg/ml, ceftazidime 16μg/ml and 128μg/ml, cefepime 8μg/ml and 32μg/ml, nalidixic acid >64μg/ml and ciprofloxacin >32μg/ml.
All isolates were CTX-M producers, 89/101 (88.1%) harbored blaCTX-M-1 group markers corresponding to: E. coli (78), K. pneumoniae (9), E. cloacae (1) and M. morganii (1). Ten isolates carried blaCTX-M-9 group markers belonging to: E. coli (9) and K. pneumoniae (1). The only C. freundii displayed a blaCTX-M-2 group gene, showing a very low prevalence of this marker. Finally, one K. pneumoniae isolate was positive for both blaCTX-M-1 and blaCTX-M-9 group markers. In good agreement with the South American scene, a radical change of the CTX-M-2 group to CTX-M-1 and CTX-M-9 groups was noted3,13,16.
Ten randomly selected PCR products were sequenced, those positive for the blaCTX-M-1 group were identified as blaCTX-M-15, while those positive for the blaCTX-M-9 group corresponded to blaCTX-M-14. Neither the blaCTX-M-8 group nor the blaCTX-M-25 group were detected. In accordance with CLSI breakpoints, from 89 blaCTX-M-1 group-harboring isolates, 62 (69.7%) were resistant to ceftazidime, 24 (27.0%) were categorized as intermediate and only 3 (3.3%) were categorized as susceptible. Among the blaCTX-M-9 group positive isolates, 8/10 were categorized as susceptible to ceftazidime, 1 was categorized as intermediate and the remaining as resistant. C. freundii harboring the blaCTX-M-2 group was categorized as intermediate to this agent.
A blaOXA-1like marker was detected in 70/101 (69.3%) ESBL-producing isolates, mainly in those carrying blaCTX-M-1 group markers (68/89, 76.4%). Sequenced amplicons corresponded 100% to blaOXA-1. None of the blaCTX-M-9 group positive isolates rendered positive amplification for blaOXA-1. The association of blaCTX-M-1 group markers with blaOXA-1 was confirmed in this study (p<0.001). Neither the presence of plasmidic AmpC coding genes nor blaOXA-2, blaOXA-10, blaOXA-48, blaPER-2, blaKPC and blaGES were detected.
Fifty eight from 87 E. coli isolates (67.0%) belonged to phylogenetic group B2, 17.0% to group A and 16.0% to group D. The CTX-M-1 group producers were mostly associated with the phylogenetic group B2 (p<0.01), followed by A and D. CTX-M-9 group producers were mainly associated with phylogenetic group D, followed by A and B2.
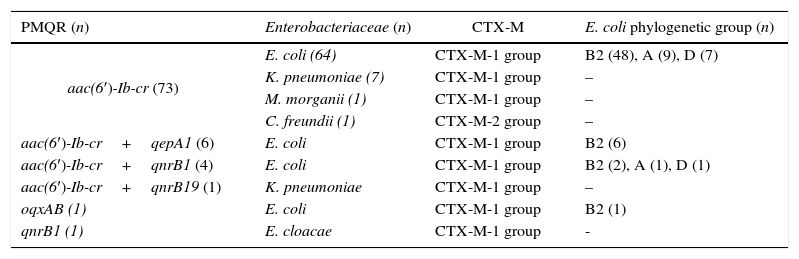
Table 1 describes the different PMQR detected in this study. The aac(6′)-Ib-cr gene was present in 83.0% of the isolates, being the most prevalent PMQR determinant among the studied enterobacteria. Other less common markers were also identified. The CTX-M-9 group producers did not harbor any of the analyzed PMQR.
Main features of the PMQR producing enterobacterial isolates.
| PMQR (n) | Enterobacteriaceae (n) | CTX-M | E. coli phylogenetic group (n) |
|---|---|---|---|
| aac(6′)-Ib-cr (73) | E. coli (64) | CTX-M-1 group | B2 (48), A (9), D (7) |
| K. pneumoniae (7) | CTX-M-1 group | – | |
| M. morganii (1) | CTX-M-1 group | – | |
| C. freundii (1) | CTX-M-2 group | – | |
| aac(6′)-Ib-cr+qepA1 (6) | E. coli | CTX-M-1 group | B2 (6) |
| aac(6′)-Ib-cr+qnrB1 (4) | E. coli | CTX-M-1 group | B2 (2), A (1), D (1) |
| aac(6′)-Ib-cr+qnrB19 (1) | K. pneumoniae | CTX-M-1 group | – |
| oqxAB (1) | E. coli | CTX-M-1 group | B2 (1) |
| qnrB1 (1) | E. cloacae | CTX-M-1 group | - |
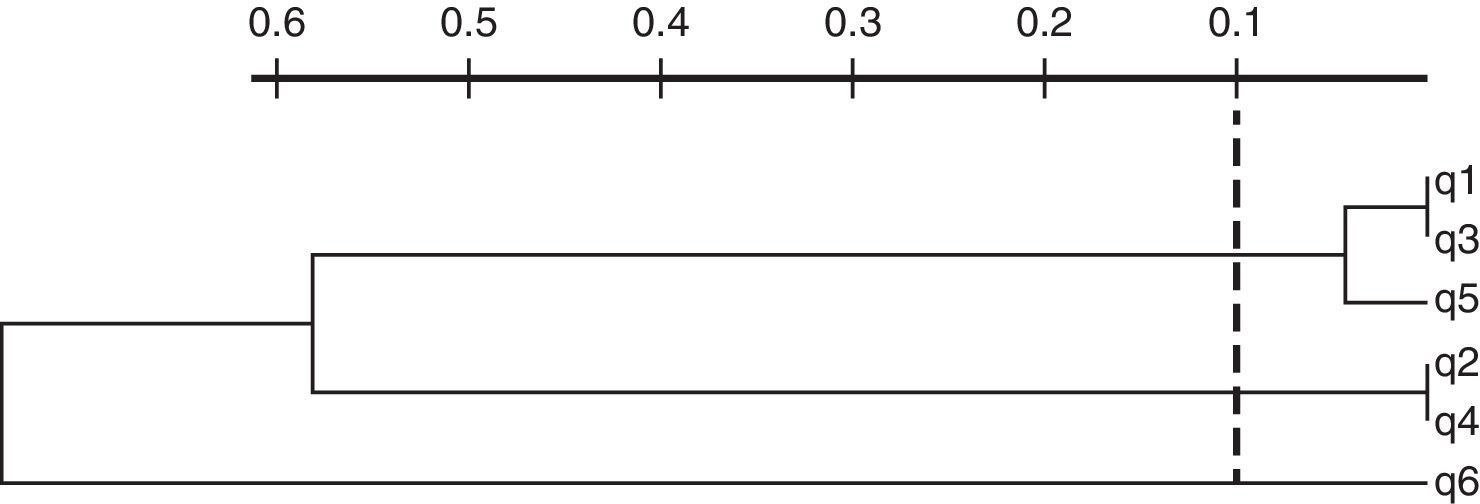
Among Qnr proteins, only qnrB determinants were detected, corresponding to qnrB1 (5) and qnrB19 (1). Plasmid-mediated efflux pumps were identified in 7 E. coli isolates corresponding to phylogenetic group B2. A single isolate carried oqxAB while the other 6 harbored qepA1. The latter isolates also harbored aac(6′)-Ib-cr and blaCTX-M-15, however they clustered in three different groups in accordance with REP-PCR profiles (Fig. 2). Each cluster contains isolates collected from different health centers.
Dendrogram of REP-PCR profiles of qepA-positive isolates. Sample origin—q1: inpatient, health center A, urine sample; q2: outpatient, health center A, urine sample; q3: outpatient, health center B, urine sample; q4: outpatient, health center B, urine sample; q5: inpatient, health center B, secretion; q6: inpatient, health center C, urine sample.
All enterobacteria included in this study were ESBL producers, corresponding to different CTX-M groups. In this work, CTX-M-1 group enzymes were prevalent in good agreement with different studies conducted by Bartoloni et al. in the Bolivian Chaco (in healthy children and urinary tract infection samples), and also with other recent studies carried in other South American countries2,3,13,16.
Although all isolates were cefotaximase producers, 10.9% and 9.9% were categorized as susceptible to ceftazidime in accordance with the current breakpoints of the CLSI and EUCAST, respectively. Ceftazidime susceptible isolates were strongly associated with the production of CTX-M-9 group ESBLs (p<0.001).
Most CTX-M-15-producing E. coli belong to phylogenetic group B2. These strains display a high virulence potential and have been mainly associated with ST 131 constituting a major public health problem worldwide2,4,13,16. Accordingly, in this study, CTX-M-1 group enzymes were strongly associated with phylogroup B2 (p<0.001). However, in Venezuela it was reported that phylogenetic group A was prevalent among CTX-M-15-producing uropathogenic E. coli isolates6.
Different PMQR markers were identified, with aac(6′)-Ib-cr by large as the most predominant. This variant has been reported in previous studies performed in different Latin American countries such as Argentina, Brazil, Chile, Mexico, Peru and Uruguay5,9,14,16. A higher aac(6′)-Ib-cr rate was observed in this study with respect to previous reports in Bolivia; nevertheless comparisons between these studies should be performed carefully due to the different bacterial selection criteria used2.
Among the qnrB genes detected, qnrB1 was dominant, in agreement with studies conducted in Mexico and Brazil14,15. Moreover, in previous studies conducted in Bolivia and Argentina qnrB19, qnrB10 and qnrB2 genes7,9 were reported. To the best of our knowledge, this constitutes the first report of qnrB1 in Bolivia, and even the description of its association with aac(6′)-Ib-cr and blaCTX-M-1 group resistance determinants in E. coli isolated from clinical samples.
There are few reports of plasmid-mediated fluoroquinolone efflux pumps in Latin America. In good agreement with studies performed in Bolivia and Mexico, qepA1 was detected in CTX-M-15-producing E. coli isolates2,14. The qepA gene was also described in CTX-M-14 producing E. coli clinical isolates in Peru, and in CTX-M-2 producing E. coli in Colombia (Rincón Cruz G., PhD. Thesis, 2015); however, in Argentina qepA1 was found in non-ESBL producing E. coli10.
Finally, regarding efflux pump OqxAB, which is uncommon in enterobacteria other than K. pneumoniae, 1 isolate of E. coli carrying oqxAB was identified, being the first report in Bolivia and one of the first descriptions in Latin America. Recently 3oqxAB- positive E. coli were detected in Peru and 1 in Argentina1,11.
This study constitutes the first molecular survey on β-lactam and quinolone resistance markers in clinical isolates of Enterobactericeae, in Cochabamba, Bolivia. These results contribute to the regional knowledge of the epidemiological situation regarding the resistance to frequently used antibiotics. The molecular epidemiology of CTX-M-enzymes and its association with PMQR, mainly aac(6′)-Ib-cr; observed herein resembles the scene reported recently in South America.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors claim that they have no conflicts of interest to declare.
This work was partly supported by UBACyT to GG and MR; by CAI+D-UNL to JDC; by PIP-CONICET to GG, MR y JDC and PICT-ANPCyT to GG and MR. GG, MR and JDC are members of CONICET.
We would like to thank Mirtha Villarroel García, Virginia Aguilar, Tatiana Pimentel, Leovegildo Álvarez, Norah Balderrama for their help in collecting the samples and Giovanna Rincón for her technical assistance and collaboration.