The microbiological quality of honey obtained from different processing points and the environmental quality within honey houses were assessed in the Pampas Region (Argentina). Mold and yeast (MY), culturable heterotrophic mesophilic bacteria (CHMB), the number of spore-forming bacteria as well as the presence of Shigella spp., Salmonella spp. and fecal coliforms were evaluated in 163 samples. These samples were taken from eight honey houses. Results showed that 89 samples had ≤10CFU of MY/g honey, 69 ranged from 10 to 50CFU/g and two reached 65.5CFU/g. Eighty one percent of the samples showed ≤30CFU of CHMB/g honey and only seven samples had between 50 and 54.25CFU/g. Thirty six honey samples were obtained from drums: in 25 samples (69.4%) CHMB counts were less than ≤30CFU/g of honey; in 20 samples (55.5%) the values of MY were between 10 and 50CFU/g honey and total coliforms were only detected in 20 samples. Fecal coliforms, spores of clostridia as well as Salmonella spp. and Shigella spp were not detected and less than 50 spores of Bacillus spp. per g were observed in the honey from drums. Therefore, the microbiological honey quality within the honey houses did not show any sanitary risks. Our results were reported to honey house owners to help them understand the need to reinforce proper honey handling and sanitation practices.
Este estudio evaluó la calidad microbiológica de la miel dentro de varias plantas de extracción de miel y la calidad del medio ambiente de las mismas en la Región Pampeana (Argentina). Se trabajó con 163 muestras de miel provenientes de 8 plantas de extracción. Se cuantificaron hongos y levaduras, bacterias aeróbicas mesófilas, bacterias esporuladas y esporas de clostridios. Asimismo, se determinó la presencia de Salmonella spp., Shigella spp. y coliformes fecales. Los resultados mostraron que por g de miel, 89 muestras tuvieron menos de 10UFC de hongos y levaduras, 69 tuvieron entre 10 y 50UFC y 2 alcanzaron 65,5UFC. Ochenta y uno por ciento de las muestras presentaron menos de 30UFC de bacterias aeróbicas mesófilas por g de miel mientras que solo 7 tuvieron entre 50 y 54,25UFC. Se obtuvieron 36 muestras de miel directamente de tambor: los conteos de bacterias aeróbicas mesófilas fueron≤30UFC/g de miel en 25 muestras (69,4%); los valores de hongos y levaduras estuvieron entre 10 y 50UFC en 20 muestras (55,5%) y solo se detectaron coliformes totales. No se observaron coliformes fecales, esporas de clostridios así como tampoco Salmonella spp. y Shigella spp. y se obtuvieron menos de 50 esporas de Bacillus spp./g en miel de los tambores. Se concluye que la calidad microbiológica de la miel en las plantas de extracción no presentó riesgo sanitario. Los resultados fueron entregados a los dueños de las mismas como aporte para que valoren la importancia de reforzar la aplicación de buenas prácticas de manejo y saneamiento.
A honey house is a room or place within a building used for extracting, processing and/or handling honey. It is the center of activities for beekeepers. It represents an important portion of their investment, and may contribute greatly to the overall efficiency of their entire operation6. Due to the importance of Argentina's honey exports, SENASA33 passed resolution 870/2006, which deals with the hygienic and sanitary aspects of honey houses. Thus, commercial honeys are ruled by the Mercado Común del Sur (MERCOSUR) legislation and the Código Alimentario Argentino (CAA)3. The maximum level allowed by this legislation for molds and yeasts (MY) with trading purposes is 100CFU/g of honey. Likewise, the legislation does not allow the presence of Salmonella and Shigella bacteria or of total coliforms in honeys.
Although honey has high osmolarity, low water activity and nutrients, it holds microorganisms which could be present in pollen, dust, air, soil, phyllosphere and nectar11,27,35. It is well known that microbial contamination may be originated from food handlers, equipment and cross-contamination during harvest1, and processing in honey houses25,35. Consequently, appropriate standards of hygiene must be applied5,37 in all operations involving honey handling.
The microorganisms of concern in honey are some fungi and spore-forming bacteria such as Bacillus cereus and Clostridium spp. which under certain conditions might cause illness in humans35. Some of the most recognized potential sources of Clostridium botulinum spores are the soil, dust, honey and medicinal herbs11. Sagua et al.31 observed that 456 cases of infant botulism in Argentina were reported between 1982 and 2007, suggesting that some of these cases may be explained by the presence of C. botulinum spores in honey. Martins et al.19 found a low contamination percentage with Bacillus cereus and fungi in honeys from Portugal. Nevertheless, they suggested that these potentially pathogenic species could be harmful to predisposed patients. Pirttijarvi et al.29 reported that the potential toxigenic effects of Bacillus were achieved with 104 spores per g of honey.
Argentina is one of the major honey exporters to countries like the United States; Germany and Japan. There are currently about 25000 beekeepers working with three million hives in Argentina. This is the country with the largest number of hives in the Southern Hemisphere8. Several works have been published about the microbiological characteristics of honeys ready to be sold in the retail market13,14,16,17. However, little scientific research on the microbiological quality of honey obtained at different processing points in honey houses has been published. In Argentina, Mouteira et al.20–23, Basso et al.2 and Malacalza et al.18 have studied possible sources of microbiological, physical and chemical contamination as well as the effect of beekeeping equipment on honey production within the honey houses.
In 200910, we started an evaluation of the sanitary risks during honey processing within the honey houses. Mold and yeast, total coliform number as well as the presence of Salmonella spp. were determined in 50 samples10. In this work, we assessed the microbiological quality of honey obtained from different processing points, and the environmental quality within honey houses, to enlarge and complete that research. Mold and yeast, culturable heterotrophic mesophilic bacteria, spore-forming bacteria and sulfite-reducing clostridia numbers, and the presence of Shigella spp., Salmonella spp. and fecal coliform were determined in 163 samples of honey obtained from eight honey houses in the southeastern Pampas Region, Argentina.
Materials and methodsSampling sitesEight honey houses were sampled in the southeast of the Pampas Region, Argentina. This is the same region of the honey house sampled in 2009 by Gallez and Fernández10.
Honey was sampled at different points in each of the honey houses. The first point was at the super storage place, where supers are filled with the honeycombs. The second one was at the uncapping machine, which in all cases had thermostatically controlled heated blades. The next point was at the honey-beeswax separator, allowing to recover honey from the cappings. Different uncapping systems, with and without heating the cappings, were sampled. The fourth point was at the honey extractor which extracts the honey from the combs by centrifugal force. The fifth point was at the honey sump, which is a tank or chamber into which the honey drains. In the Pampas Region, it is usually below ground level, and it is not water jacketed. The last sampled point was at the 300kg honey drums.
Honey samplesOne hundred and sixty three honey samples were collected during February and March 2014 and 2015 from eight honey houses. All samples were aseptically collected in sterile 100ml vials and were grouped according to their origin. These samples were stored at room temperature and were processed within two months from collection30.
Microbial countsMicrobial analyses were carried out in all of the samples in triplicate.
Mold and yeast (MY) determination was carried out by plating appropriate dilutions of the honey samples. For this procedure, a 10g sample was homogenized in 90ml of 0.85% w/v NaCl (initial suspension) for 15min at 180rpm at room temperature. Decimal serial dilutions were plated onto fungi and yeast agar supplemented with chloramphenicol (Britania, Argentina) to inhibit bacteria. MY were counted after three – five days from plate incubation at 22–24°C.
For assessment of culturable heterotrophic mesophilic bacteria (CHMB), decimal serial dilutions from the initial suspension that was previously described, were plated onto nutrient agar (Britania, Argentina). Plates were counted 72h after the incubation at 30°C.
Total coliforms: aliquots of 1ml of the initial suspension were added to empty plastic Petri dishes. Violet Red Bile Lactose (VRBL, Merck) medium was poured over them. The plates were incubated at 35–37°C for three days.
Results from all determinations were expressed as colony forming units (CFU)/g of honey.
Complementary microbiological determinationsThe following microbial analyses were carried out in the samples coming from the honey drums.
Evaluation of Bacillus spp.: the second dilution was heat activated at 70/80°C for 10min, and cooled immediately in iced water for another 10min. Aerobic spore-forming bacteria were plated on nutrient agar (Britania, Argentina). Plates were incubated three days at 35°C.
Search for fecal coliforms: one ml of the initial suspension (10:90) for basic microbiological determinations was added to test tubes with brilliant green bile broth (2%) with Durham tubes inverted inside and were incubated at 37°C for two days. Positive samples (growth and gas production) in this medium were selected to streak in Mac Conkey solid medium. Suspicious colonies were isolated and placed in new tubes containing brilliant green bile broth (2%) and inverted Durham tubes and were incubated at 44°C for two days. Positive samples in this medium were reported as containing thermotolerant coliforms in 0.1g of honey. To confirm the presence of Escherichia coli, the positive tubes were tested by growth in EMB agar (Britania). Typical colony growth on EMB agar was confirmed by traditional assays including indole, methyl red, VP and citrate. Results expressed as presence or absence of E. coli.
Isolation of spores of sulfite-reducing clostridia: aliquots of 25ml of the initial suspension for basic microbiological determinations were added to empty tubes which were centrifuged for 15min at 5000rpm. The pellet was thermally treated at 80°C for 5min. Then, 100μl of this suspension was spread on plates with SPS (sulfite–polymixin–sulfadiazine) agar (Bioclar, Argentina), and they were incubated anaerobically with the AnaeroPack–Anaero culture system (Mitsubishi Gas Chemical, Japan) in a vacuum desiccator at 37°C for 5 days.
Isolation of Salmonella spp. and Shigella spp.: these bacteria were investigated according to a modification of the standard method suggested by the International Commission on Microbiological Specifications for Foods (ICSMF)12. For pre-enrichment, 25g of honey was added to 225ml of peptone water (Britania, Argentina) and cultures were incubated at 35°C for 24h. One ml of the pre-enrichment step was added to glass tubes containing selenite cystine broth (42°C for 24h) and another one ml to glass tubes containing tetrathionate broth (35°C for 24h). These enrichment steps were followed by the inoculation of selective solid media: EMB, salmonella shigella agar (SSA) and brilliant green agar (BGA). All media used were from Britania (Argentina). Petri dishes were incubated at 35°C for 48h and suspected colonies of Salmonella were tested in triple sugar iron (TSI, Britania) and lysine iron (LIA, Britania) agar. Colonies exhibiting typical reactions on TSI and LIA were purified and further characterized by traditional assays: urease, oxidase, phenylalanine decarboxylase, VP, indole, citrate and gelatin. Results were expressed as presence or absence of Salmonella spp.
Environmental microbiological evaluationThe honey house may contain various other facilities in addition to the extracting plant, such as storage space for hive equipment and honey, workshops, office space, and possibly a packing or salesroom or both6. The research was conducted in the main extraction zone as well as in the other facilities.
The culture settling plate technique, also known as sedimentation technique, was used for conducting a qualitative environmental assessment32. For this purpose, open Petri dishes filled with 20ml of a microbiological culture medium suitable for bacteria and fungi were used as the sampling surface. Fungi and yeast agar medium supplemented with chloramphenicol (Britania, Argentina) was used in order to determine the number of MY while nutrient agar medium (Britania, Argentina) was used for CHMB. Petri dishes were distributed at the processing areas previously mentioned and exposed for about one hour within the honey house. Once in the laboratory, plates were incubated at 26–28°C for 5 days for MY and 30–35°C for 3 days for CHMB. Results were expressed as CFU/min.
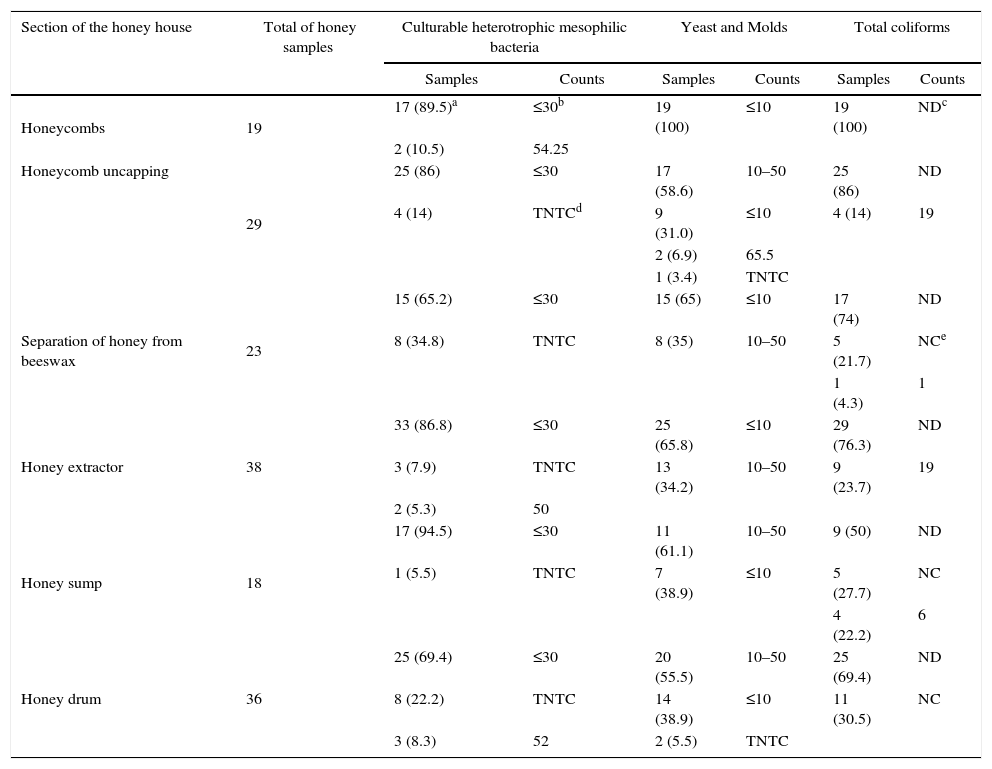
ResultsMicrobial counts at different points of honey processing within the honey houseSimilar results were obtained for all microbiological determinations within all the studied honey houses during 2014 and 2015 (Table 1).
Basic microbiological determinations in 163 samples of honey obtained from different processing points in eight honey houses in 2014 and 2015
| Section of the honey house | Total of honey samples | Culturable heterotrophic mesophilic bacteria | Yeast and Molds | Total coliforms | |||
|---|---|---|---|---|---|---|---|
| Samples | Counts | Samples | Counts | Samples | Counts | ||
| Honeycombs | 19 | 17 (89.5)a | ≤30b | 19 (100) | ≤10 | 19 (100) | NDc |
| 2 (10.5) | 54.25 | ||||||
| Honeycomb uncapping | 29 | 25 (86) | ≤30 | 17 (58.6) | 10–50 | 25 (86) | ND |
| 4 (14) | TNTCd | 9 (31.0) | ≤10 | 4 (14) | 19 | ||
| 2 (6.9) | 65.5 | ||||||
| 1 (3.4) | TNTC | ||||||
| Separation of honey from beeswax | 23 | 15 (65.2) | ≤30 | 15 (65) | ≤10 | 17 (74) | ND |
| 8 (34.8) | TNTC | 8 (35) | 10–50 | 5 (21.7) | NCe | ||
| 1 (4.3) | 1 | ||||||
| Honey extractor | 38 | 33 (86.8) | ≤30 | 25 (65.8) | ≤10 | 29 (76.3) | ND |
| 3 (7.9) | TNTC | 13 (34.2) | 10–50 | 9 (23.7) | 19 | ||
| 2 (5.3) | 50 | ||||||
| Honey sump | 18 | 17 (94.5) | ≤30 | 11 (61.1) | 10–50 | 9 (50) | ND |
| 1 (5.5) | TNTC | 7 (38.9) | ≤10 | 5 (27.7) | NC | ||
| 4 (22.2) | 6 | ||||||
| Honey drum | 36 | 25 (69.4) | ≤30 | 20 (55.5) | 10–50 | 25 (69.4) | ND |
| 8 (22.2) | TNTC | 14 (38.9) | ≤10 | 11 (30.5) | NC | ||
| 3 (8.3) | 52 | 2 (5.5) | TNTC | ||||
Eighty nine samples out of 163 had ≤10CFU per g of honey of MY, 69 samples ranged from 10 to 50CFU/g of honey, and two reached 65.5CFU/g of honey. Eighty one percent of the samples showed ≤30CFU of CHMB/g of honey, while only seven samples had between 50 and 54.25CFU/g. Total coliforms were different between the two harvesting periods. In 2014, 20 (35%) out of 57 samples had total coliforms, in which the count was less than 20CFU/g of honey. In 2015, total coliforms were not detected in samples taken from different points of the honey house.
Microbiological quality of the honey from drumsThirty-six samples of honey were obtained from drums in the different honey houses. In 25 samples (69.4%), the counts of CHMB were less than ≤30CFU per g of honey. In addition, 20 samples (55.5%) showed between 10 and 50CFU of MY per g of honey (Table 1). Total coliforms were not detected in 25 samples, while colonies different from the typical morphology of coliforms were only observed in 11 samples.
Fecal coliforms were not detected in samples from 2014 or 2015. All samples were contaminated with less than 50spores/g of Bacillus spp., except two from 2014 which showed between 50 and 100 spores. Typical black colonies from spores of sulfite-reducing clostridia were not detected in any sample when working under a total anaerobic atmosphere. In addition, bacteria from the genera Salmonella spp. Shigella spp. were not found in any of the honey drum samples.
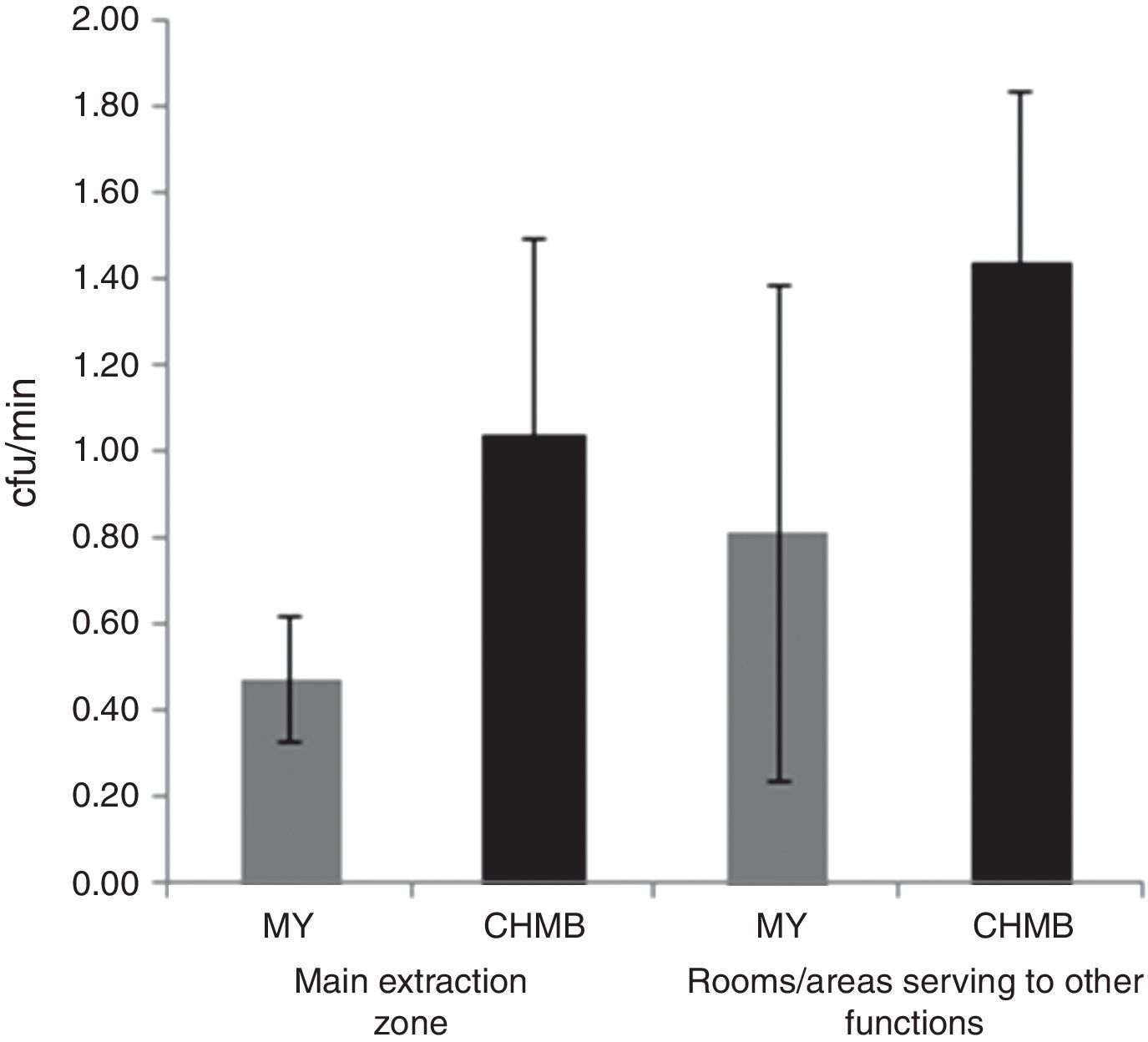
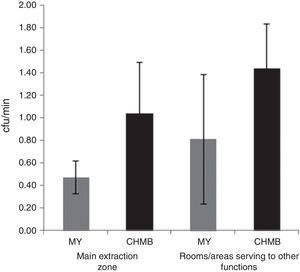
Microbiological quality of the honey house environmentThere were no statistical differences in MY and CHMB counts between the rooms/areas of the main extraction zone and other rooms/areas of the honey houses (Figure 1).
DiscussionMicroorganisms in honey might come from several and different sources. Primary sources include pollen, digestive tracts of honey bees, dust, air, soil and nectar. Secondary sources are due to honey handlers and processing, and are easy to control by the application of good manufacturing practices (GMP)3,11,15. In this work, we report the analysis of 163 honey samples which were obtained from different points of the honey processing within eight honey houses located in the southeast of the Pampas Region, Argentina. Our studies started in 2009 with 50 samples from one honey house10.
We did not find any differences in microbial counts at the different points of honey processing among the eight honey houses sampled. Therefore, the results are discussed according to the six points we sampled within the honey house (Table 1). In 2009, all samples from honeycombs and honey extractor samples showed low levels of MY (≤10CFU/g of honey). Low levels of MY and CHMB were also observed at those processing points and in the honey obtained from the uncapping machines (Table 1). Ten to 50CFU of MY/g honey were observed in four out of 30 samples in 200910 (13.3%), whereas 69 out of 163 samples were obtained with those counts in 2014/2015 (42.3%). These samples belonged to the following processing points: honeycomb uncapping, honey sump and honey drum. The higher MY counts in 2014/2015 than in 2009 might be due to higher rainfall, thus favoring microorganism development. In addition, total coliforms were present only in 11.65% of the samples in 2014, while there were no total coliforms in honey samples during 2009 and 2015. However, it is important to note that the food codes refer to honey for retail sale, at the end point of the food chain. Drums were the last sampling point in this study, previous to bottling, and total coliforms were absent in all the drum samples.
Little information is available in Argentina about microbiological contamination within the honey houses. Mouteira and Basso (2014)24 studied four points in a honey house of Ranchos in Buenos Aires Province. They reported counts of MY of 97CFU/g honey in honeycombs, 75CFU/g honey in honeycomb uncapping, 35CFU/g honey in honey extractor and 34.7CFU/g honey in drums. They also observed the absence of total coliforms. In another work, Mouteira et al.21 compared the physicochemical and microbiological quality of honeys from two honey house buildings with different technology in Argentina. They found that although MY counts were below the maximum established limit by the CAA3 (≤100CFU/g of honey), their number increased in all the studied processing points of the honey house which did not comply with GMP regulations. They also observed 19.87coliforms/g of honey in the drums in this honey house. Furthermore, Sereia et al.34 compared and verified the main contamination sources and the hygienic/sanitary conditions of organic honey from Parana River islands (Brasil). These authors conclusively demonstrated that secondary sources of contamination were responsible for the reduction in the quality of organic honey.
The maximum MY level allowed for trading by the MERCOSUR and CAA3 legislations is 100CFU/g of honey: all drum samples showed cell counts below this stipulated value (Table 1). Likewise, the legislation neither allows the presence of Salmonella and Shigella bacteria nor of total coliforms. All the honey drum samples in our work showed these characteristics. Our results of the microbiological quality of honey at the end of the process in the honey house were similar to those reported by other authors in Argentina9,13,20. In fact, our results are also similar to those published by other researchers who analyzed honey from different parts of the world7,26,36.
In the present study, we assessed the indoor air quality at the honey houses by the settling plate technique. It is interesting to note, that although there were no statistical differences of MY and CHMB counts between both studied areas, there was a tendency in both groups of microorganisms to be lower in the main extraction zone than in other rooms/areas. Similarly, Oliveira et al.28 found that the installation of honey houses in untidy environments, and the environmental variables, could have been responsible for the presence of MY in quantities above those permitted by the standards. Furthermore, Grabowski and Klein11 supported this idea explaining that like any other foodstuff, the hygienic status of honey is the result of the environmental conditions along the food chain.
The “unheated” honey-wax separation process might be better to preserve the chemical quality of the honey. However and in accordance with our previous data, it implied a higher microbiological hazard than the heated process. This highlights the need to perform more studies in the honey house in this regard10. In this research, we characterized honey samples from eight honey houses; the different points of honey processing within these honey houses in the Pampas Region did not show any sanitary risks.
Some simple good practices are not always applied, and their implementation could improve the microbiological quality of the honey. For instance, workers should wear clean outer clothing and adequate hair covering at all times during honey extraction and processing. Sanitary curtains and insect nets should be fitted over openings to reduce environmental contamination and to avoid bees from entering. Sumps must be adequately covered. While not in use, the equipment must be stored protecting it from dust, dirt, rodents, insects or other contamination sources. The application and/or reinforcement of proper honey handling and sanitation practices would allow to improve the microbiological quality of honeys.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingWe thank the Comisión de Investigaciones Científicas de la Provincia de Buenos Aires (CIC) Argentina, for financial support.
Conflicts of interestThe authors declare that they have no conflicts of interest.
The authors are thankful to CIC (Comisión de Investigaciones Científicas, Argentina) for financial support of the research work. We would like to thank the owners of the honey houses and all the beekeepers for their great collaboration. We also want to thank Juan Manuel Ringhetti for the donation of many culture media and Claudio Valverde from UNQ for his valuable comments on the manuscript.Microbiological quality of honey from the Pampas Region (Argentina) throughout the extraction process