At present, consumers are looking for more natural foods so as to improve health through their active compounds. Within this context, soybean is an excellent substrate due to its beneficial effects on consumers’ health. Moreover, lactic cultures are widely used in the food industry to improve the technological, nutritional and functional characteristics of fermented foods. It is interesting to find new matrices in which to transport these starter cultures (potentially probiotic microorganisms). The aim of this research was to obtain a solid state fermentation system from soybean to analyze the behavior of selected lactobacilli and bifidobacteria, with the potential to develop a functional vegetarian food to serve as carrier for the microorganisms. A soybean solid substrate system was optimized by selecting the relationship of the main processing parameters. Homogeneous soybean pastes with different moisture content (60–80%) were obtained and used as substrate and support for solid substrate fermentation. Moisture, inoculum size and temperature were optimized: 80%, 4%, 37°C, respectively. L. rhamnosus CRL 981 was chosen as the best starter to use in this kind of fermentation, showing high acidification and cell counts at 24h of fermentation and increased specific growth rate in tested soybean pastes. It was demonstrated that the selected soybean paste could be used as a carrier of these microorganisms having probiotic potential for the production of vegetarian foods. Moreover, these microorganisms are able to modify the substrate to enhance their nutritional and functional characteristics, which would change the soybean into a more attractive product for consumers.
Actualmente los consumidores están en la búsqueda de alimentos naturales, a fin de mejorar la salud a través de sus compuestos activos. En este contexto, la soja es un excelente sustrato debido a sus efectos beneficiosos sobre la salud del consumidor. En la industria alimentaria se emplean cultivos lácticos para mejorar las características tecnológicas, nutricionales y funcionales de los alimentos fermentados. Es interesante encontrar nuevas matrices para transportar estos cultivos iniciadores, que potencialmente son microorganismos probióticos. El objetivo de este estudio fue obtener un sistema de fermentación en estado sólido a partir de soja para analizar el comportamiento de lactobacilos y bifidobacterias seleccionadas, con potencial para desarrollar un alimento vegetariano funcional que sirva de portador de los microorganismos. El sistema de sustrato sólido de soja se optimizó mediante la selección de la relación de parámetros principales de procesamiento. Se obtuvieron pastas de soja homogéneas con diferente contenido humedad (60-80%) y se utilizaron como sustrato y soporte para la fermentación en sustrato sólido. Las variables humedad, tamaño del inóculo y temperatura fueron optimizadas en 80%, 4% y 37°C, respectivamente. Lactobacillus rhamnosus CRL 981 fue elegido como el mejor cultivo iniciador para utilizar en este tipo de fermentación; este mostró acidificación y recuentos celulares altos en 24 horas de fermentación, y mayor velocidad específica de crecimiento en las condiciones evaluadas. Se demostró que la pasta de soja seleccionada podría ser utilizada como portadora de estos microorganismos con potencial probiótico para la elaboración de alimentos vegetarianos. Además, estos microorganismos son capaces de modificar el sustrato y mejorar sus características nutritivas y funcionales, lo que convertiría a la soja en un producto más atractivo para los consumidores.
Solid state fermentation (SSF) is an alternative tool to obtain new products for human consumption. In the last years SSF has been appreciated due to the fact that its process uses industrial and household waste, byproducts of industries and raw materials (such as legumes and cereals) as substrate. Moreover, the major advantages of the SSF process are the higher yield of products and less water need in up-stream processing resulting in lesser wastewater generation in downstream processing4,18. In SSF the microorganisms grow on moist solid substrates in the absence of free flowing water. Many microorganisms can be used in SSF5,12,18,20; it was previously thought that only fungi and yeasts were able to grow in this type of fermentation because the bacteria require higher water activities. Therefore, research was oriented to the use of fungi and yeasts in SSF and there are few publications using bacteria9,18.
Furthermore, lactic acid bacteria (LAB) and bifidobacteria are microorganisms that play a key role in fermented food and beverages, contributing not only to the development of the desired sensory properties in the end product but also to their microbiological safety. LAB and bifidobacterias have GRAS (Generally Recognized As Safe) status and their action as probiotic microorganisms with their effect in the host was recognized in the last years16,24.
Most of the research work about LAB was done using submerged fermentation. Several studies using SSF for the production of lactic acid from agro-industrial waste have been developed10,11,17. Moreover, the effect of lactic acid bacteria in solid state fermentation using different kind of substrates has recently been studied3,19,23. However few studies based on the optimization of kinetic and technological parameters of lactic bacteria in FSS were developed for the production of food.
Soybean is an excellent substrate for the production of functional foods due to its low cost and high nutritional value (high content of proteins, presence of carbohydrates such as sucrose, raffinose, and stachyose, lipids and other components). In our country there is great availability of this legume, being the third largest producer and exporter6. However, consumption of soybeans in Argentina is low mainly because of our different food culture and due to the characteristic beany flavor and the presence of certain anti-nutritional factors. Some anti-nutritional factors of soybeans can be reduced with thermal treatments or lactic fermentation7.
The aim of this research was to obtain a solid state fermentation system from soybean to analyze the behavior of selected strains of lactobacilli and bifidobacteria in order to increase the knowledge to develop a functional vegetarian food as carrier for the microorganisms. These systems could improve the nutritional and functional properties of the soybean substrate. In addition, the effects of the inoculum size and temperature on the behavior of a selected strain were evaluated.
Materials and methodsMicroorganisms and growth conditionsLactobacillus (L.) paracasei subsp. paracasei CRL 207, L. rhamnosus CRL 981, L. fermentum CRL 251 and Bifidobacterium (B.) longum CRL 849 were obtained from the culture collection (CRL) of the Centro de Referencia para Lactobacilos (CERELA). These organisms were selected for their ability to grow on soy substrate using available carbohydrates (sucrose, raffinose, and/or stachyose), to produce enzymes (α-galactosidase and/or β-glucosidase) or to hydrolyze proteins. Before experimental use, the cultures were propagated (2%, v/v) twice in MRS medium (Laboratorios Britania S.A., Argentina) for Lactobacillus and incubated at 37°C for 18h without agitation. Bifidobacterium was grown in MRS supplemented with 1% sucrose, 0.00005% vitamin K and 0.0005% hemin, and incubated at 37°C in microaerophilic conditions without agitation. All solutions were sterilized separately (0.22μm filtration), and then added to the MRS base. In order to obtain the inocula for solid substrate fermentation, cells at the end of the exponential phase of growth in MRS medium were collected by centrifugation, washed twice and resuspended in sterile physiological solution (around to 109CFU/ml).
Solid state fermentationFor each assay, SSF used 150g of soy paste (wet weight) which was prepared from soybeans and distilled water to obtain the different moisture contents. The soybeans were processed as follows: First, a known weight of soybeans was washed with tap water and then the soybeans were left to soak for 12h. The soybean hull was removed and the remaining part of the soybeans was washed with distilled water. The wet weight of soaked soybeans was recorded. The ratio of grams of soaked soybeans/ml of distilled water was varied in order to obtain soy pastes with different moisture levels (60–80%) (Table 1). Finally, the soaked soybeans with the corresponding amount of added distilled water were ground in a blender, using different times and processing speeds to obtain a homogenized texture (Table 1). The grinding processes A and B were applied with low ratios of solids (1:1, 1.5:1 and 2:1g/ml, moisture expected 80%), being: A: Five minutes with intermittences every minute at medium speed (S3) and an additional minute at high speed (S6); B: Five minutes with no intermittences at medium speed (S3) and an additional minute at high speed (S6). The differences between grinding processes A and B were in the intermittences. The former had flashes every minute and the latter did not. The grinding processes C and D were used to prepare a paste with a higher ratio of solids (2.5:1 and 3:1g/ml, moisture expected 60%), being: C: Five minutes with intermittences every minute at medium-low speed (S2) and an additional minute at medium-high speed (S4); D: Five minutes with intermittences every minute at low speed (S1). In order to obtain pastes with a higher ratio of solids it was necessary to reduce the final speed processing.
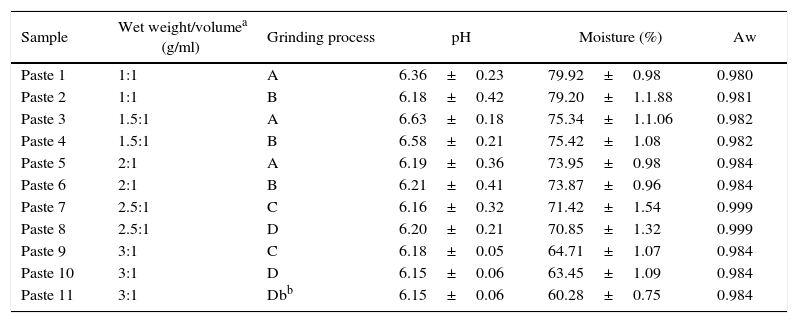
Moisture, pH and water activity of different kinds of soybean pastes according to the ratio solid/water and grinding process.
| Sample | Wet weight/volumea (g/ml) | Grinding process | pH | Moisture (%) | Aw |
|---|---|---|---|---|---|
| Paste 1 | 1:1 | A | 6.36±0.23 | 79.92±0.98 | 0.980 |
| Paste 2 | 1:1 | B | 6.18±0.42 | 79.20±1.1.88 | 0.981 |
| Paste 3 | 1.5:1 | A | 6.63±0.18 | 75.34±1.1.06 | 0.982 |
| Paste 4 | 1.5:1 | B | 6.58±0.21 | 75.42±1.08 | 0.982 |
| Paste 5 | 2:1 | A | 6.19±0.36 | 73.95±0.98 | 0.984 |
| Paste 6 | 2:1 | B | 6.21±0.41 | 73.87±0.96 | 0.984 |
| Paste 7 | 2.5:1 | C | 6.16±0.32 | 71.42±1.54 | 0.999 |
| Paste 8 | 2.5:1 | D | 6.20±0.21 | 70.85±1.32 | 0.999 |
| Paste 9 | 3:1 | C | 6.18±0.05 | 64.71±1.07 | 0.984 |
| Paste 10 | 3:1 | D | 6.15±0.06 | 63.45±1.09 | 0.984 |
| Paste 11 | 3:1 | Dbb | 6.15±0.06 | 60.28±0.75 | 0.984 |
Db, heat treated sample.
The obtained soy pastes were homogenized and aliquots of approximately 150g were placed in a Schott flask and sterilized by autoclaving at 115°C for 15min. When the pastes reached room temperature, a control (paste without inoculum) was taken and then the pastes were inoculated with 4% (v/w) of each culture (the amount of cells after this inoculation was around 7.90×107CFU/g). Then the pastes were homogenized and uniformly distributed into Petri plates and incubated at the established temperatures for 24h. Samples were taken at different times (0, 3, 6, 9, 12 and 24h).
Moisture determinationThe initial moisture of the soy pastes (after sterilization) was expressed as wet basis moisture content, experimentally determined by method 950.46.B AOAC2.
pH measurementsChanges in pH were monitored during fermentation of the soy paste at 0, 3, 9, 12 and 24h using a pH meter (SARTORIUS PT-10, Germany).
Microbial countsCell viability was determined by the plate dilution method using MRS agar for lactobacillus and Reinforced Clostridial Agar (RCA, Biokar diagnostics, France) in microareophilic conditions for B. longum CRL 849. Serial dilutions of each fermented soy-paste sample were plated in duplicate and the plates were incubated at 37°C for 48–72h. The results were expressed as colony forming units per gram (CFU/g). In order to compare the growth of the microorganisms in the soy paste, some results were expressed as lnX/X0 versus time, where X is the number of CFU/g at a given time (t) and X0 the initial CFU/g at zero time (t=0).
Study of the effect of inoculum amount and temperature on growth of selected strain in the best moisture condition of soybean pasteFor the following experiments, selected soybean pastes were prepared from soybeans as detailed in previous protocols. In order to evaluate the effect of inoculum, the soybean paste was inoculated at 1%, 4% and 8% (individually with the selected strain) and incubated at 37°C for 24h. Furthermore, the effect of temperature was studied. The chosen soybean paste was inoculated at 4% with the selected culture, which was divided into three portions where each one was incubated at different temperatures: 30°C, 37°C and 44°C for 24h. For both studies, samples were taken at different times (0, 3, 6, 8, 12, 24h) and growth was assessed by measuring pH and colony count.
Statistical analysisAll assays were carried out in triplicate, and the results were expressed as mean values with standard deviations. Data were compared by one-way analysis of variance (ANOVA) followed by the Dunnett t-test. The statistical analyses were performed with the Minitab-15 software (Minitab Inc., State College, PA, USA) and differences were considered significant at p<0.05.
ResultsSelection of matrix ratio soy/water as support for solid substrate fermentationThe soybean paste was prepared from 100 to 300g of dried beans, which were processed according to the protocol detailed in the Materials and Methods section. Table 1 shows the different kinds of prepared pastes (paste 1–10), which vary in the ratio of grams of soaked beans/ml of distilled water, processing type and physical characteristics (pH, percent moisture and water activity (Aw)). Although the moisture was variable, no changes were observed in the Aw.
The ratio of grams of wet beans/ml of distilled water was varied in order to obtain different final moisture contents. Speed and grinding time were varied to obtain a homogeneous paste in texture. Because the consistency of the soybean pastes was similar in the case of pastes 1–6, the chosen grinding process for preparing them was process B. Ratio 1:1 (solid/water) was chosen to obtain values close to 80% moisture (paste 2). The best homogeneity in the soybean pastes with lower water content (pastes 7–10) were obtained by applying grinding process D with intermittences at a lower speed because the grinding was more difficult. Due to the achieved final moisture, which was higher than expected (60%), an additional heat treatment was necessary to achieve the evaporation of residual water. A different heating exposure time of the paste was tested. Paste 10 made in accordance with process D (ratio solid/water, 3:1) was placed in a rectangular aluminum board covered with foil, looking as a thin and homogeneous layer with a large surface exposure. (a) It was placed in an oven at 105°C 1h; stirred with a spatula twice (at 30 and 45min). (b) The same previous methodology was used, varying the exposure time and removal frequency (105°C, 1h 15min, mixing every 15min during the heat exposition). Table 1 only showed the heat process (b) for tested paste 11. The other process was less efficient. Paste 11 made with a ratio 3:1 (solid/water), applying process D and additional drying (heat process b) showed a final moisture of about 60% (Table 1).
Pastes number 2 and 11 were selected for subsequent assays. The procedures to obtain the pastes with the desired moisture were:
- -
For 80% moisture: solid/water 1:1 – Grinding process: B
- -
For 60% moisture: solid/water 3:1 – Grinding process: D plus additional heat process b.
The other soybean pastes were discarded because their achieved final moisture values were far from the 80% or 60% moisture sought in this work.
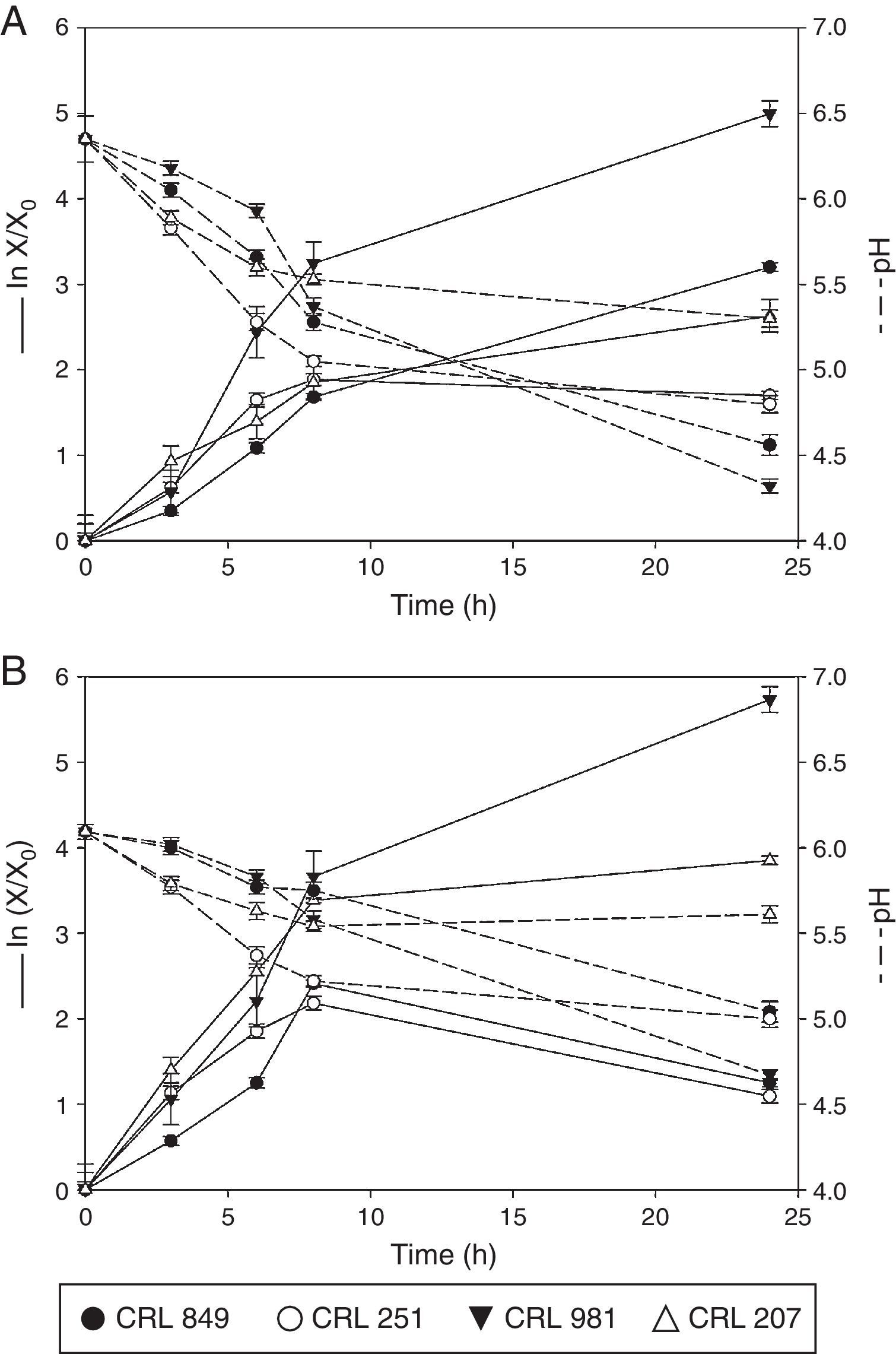
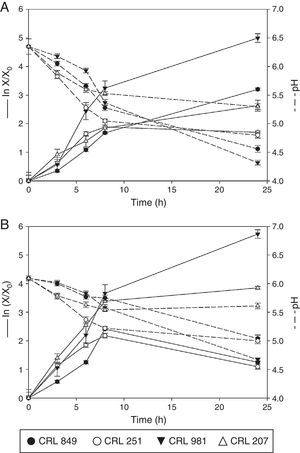
Analysis of the behavior of lactobacilli and bifidobacterium on solid state fermentationPreviously selected soybean pastes (80% and 60% moisture) were inoculated individually with 4% of each lactic culture. They were incubated at 37°C for 24h and samples were taken at different times to study growth. Figure 1A and B shows the behavior of the four lactic cultures assayed during this time. The pH of the soy paste without inoculum remained unchanged throughout the fermentation time (24h).
Growth and pH of L. rhamnosus CRL 981, L. paracasei subsp. paracasei CRL 207, L. fermentum CRL 251, and B. longum CRL 849 on a soybean paste with 80% (A) and 60% moisture (B) incubated for 24h at 37°C. Growth was expressed as lnX/X0, where X is the number of CFU/g at a given time (t) and X0 the initial CFU/g at zero time (t=0).
L. rhamnosus CRL 981 on the soybean paste with 80% moisture (Fig. 1A) showed great adaptation, increasing its population to 2.18 log units after 24h of fermentation, which corresponds to the sharp drop in pH between 0 and 24h (ΔpH=−2.20) (Table 2). Figure 1B shows the growth of this microorganism in the soybean paste with 60% moisture. The microbial population increased to 2.48 log units and ΔpH was −1.45 after 24h (Table 2). This microorganism showed ability to grow on soybean pastes even with lower moisture content. The growth kinetics was very similar in both pastes with growth rates of 0.441/h and 0.521/h respectively. The pH of slurry at 80% moisture was 4.32 at the end of the fermentation, while in the paste with 60% of moisture, the pH reached 4.67 at 24h of fermentation. The number of viable cells at the end of fermentation had a very similar value in the pastes with 80% and 60% of moisture, 10.07 and 9.98logCFU/g respectively.
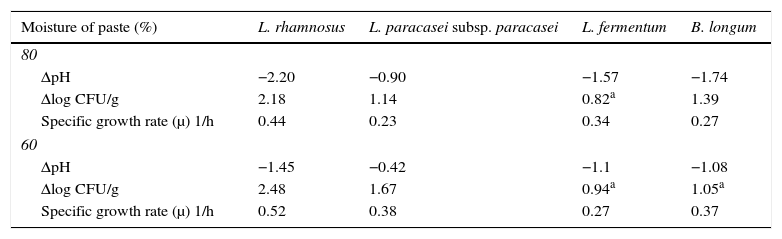
Growth differences (ΔpH and Δlog CFU/g) between 0 and 24h of fermentation and specific growth rate (μ) for L. rhamnosus CRL 981, L. paracasei subsp. paracasei CRL 207, L. fermentum CRL 251, B. longum CRL 849 on soybean pastes with 80 and 60% moisture.
| Moisture of paste (%) | L. rhamnosus | L. paracasei subsp. paracasei | L. fermentum | B. longum |
|---|---|---|---|---|
| 80 | ||||
| ΔpH | −2.20 | −0.90 | −1.57 | −1.74 |
| Δlog CFU/g | 2.18 | 1.14 | 0.82a | 1.39 |
| Specific growth rate (μ) 1/h | 0.44 | 0.23 | 0.34 | 0.27 |
| 60 | ||||
| ΔpH | −1.45 | −0.42 | −1.1 | −1.08 |
| Δlog CFU/g | 2.48 | 1.67 | 0.94a | 1.05a |
| Specific growth rate (μ) 1/h | 0.52 | 0.38 | 0.27 | 0.37 |
The growth kinetic of L. paracasei subsp. paracasei CRL 207 is shown in Figure 1A and B, in soybeans with 80% and 60% moisture, respectively. In the pastes with 80% moisture a marked decrease in pH was observed between 0 and 8h of fermentation (ΔpH=-0.67), which coincides with the exponential growth phase of the microorganism. At the end of the fermentation, the microbial population was 1.14 log units higher than the initial amount. Moreover, the exponential growth phase also occurred between 0 and 8h of fermentation, for the soybean paste with 60% moisture, in which ΔpH was -0.49. At 24h of fermentation the viable cell number increased 1.67 log units (Table 2). No significant differences were observed in the growth of this organism in both pastes. For the soybean paste with 80% moisture, the bacterial population reached 9.46logCFU/g at the end of the fermentation while for the soybean paste with 60% moisture it was 9.66logCFU/g. In addition, higher acidity at 24h of fermentation in the paste with 80% moisture was observed, with a final pH of 5.30. For the paste with 60% moisture, a small decrease of pH was observed, reaching 5.61 at the end of fermentation. In this case, a pH increase between 8h and 24h was observed.
Figure 1A and B shows the growth kinetic for L. fermentum CRL 251 in soy pastes with 80% and 60 moisture respectively. An excellent decrease in pH in both fermented pastes by this microorganism at 24h of fermentation was observed. The pH decreased to 4.80 and 5.00 at 80% and 60% moisture respectively at 24h of fermentation. Furthermore, a loss of viability was observed for both conditions at 24h. However, very similar values in the number of viable cells were obtained at 8h of fermentation: 8.94logCFU/g for 80% moisture and 8.93logCFU/g for 60% moisture. Loss of viability was more pronounced in the paste with 60% moisture, dropping the value from 8.93 to 8.46logCFU/g.
Figure 1A shows the growth of B. longum CRL 849 in the soybean paste with 80% moisture. The microbial population was increased to 1.39 log units after 24h of fermentation. A sharp drop in pH was observed between 3 and 8h of fermentation (ΔpH=-1.02). Figure 1B represents the growth of the same strain in the soybean paste with 60% moisture. The pH decrease was not pronounced during the exponential phase (ΔpH=-0.37) while a more pronounced drop in the pH was observed at the end of the fermentation (ΔpH=−1.08). The growth of this strain was similar in both pastes up to 8h of fermentation, 8.45logCFU/g for 80% moisture and 8.68logCFU/g for 60% moisture. However, in the latter condition a loss of viability was observed at 24h, whereas in the first case the biomass was increased to 9.11logCFU/g. The pH reached 4.56 in the soybean paste with 80% moisture and 5.04 in the soybean paste with 60% moisture.
In order to evaluate the best growth of these studied microorganisms, the maximum values of ΔpH and Δlog CFU/g during fermentation time were calculated. Furthermore, the specific growth rate for each strain was calculated (Table 2). L. rhamnosus CRL 981 and L. paracasei subsp. paracasei CRL 207 showed the highest Δlog CFU/g in both soybean pastes. L. fermentum CRL 251 and B. longum CRL 849 presented low growth in both soybean pastes. L. fermentum CRL 251 had loss of viability in both situations and B. longum CRL 849 only when the moisture of the soybean paste was 60%. Although, both strains showed low pH values in contrast with L. paracasei subsp. paracasei CRL 207, which did not show loss of viability. L. rhamnosus CRL 981 showed the best growth rate in both soybean pastes.
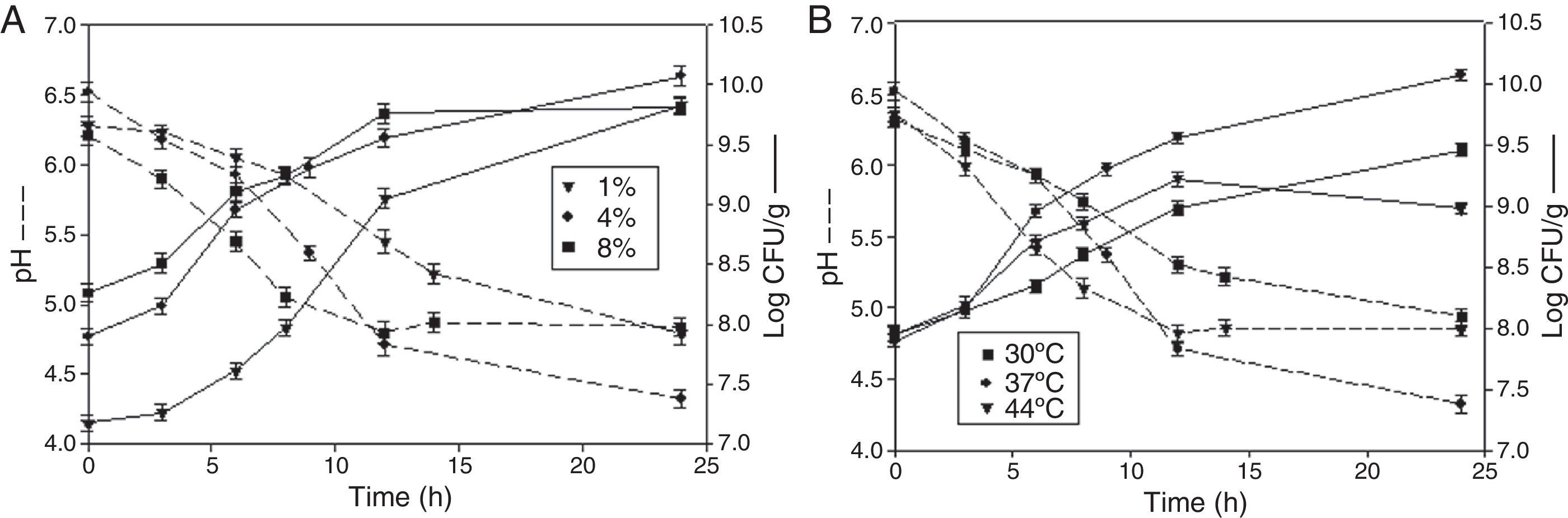
Effect of inoculum amount and temperature on growth of L. rhamnosus CRL 981 in the selected pasteL. rhamnosus CRL 981 was selected as starter strain and the soybean paste with 80% moisture as substrate to study other fermentation parameters.
Effect of inoculum sizeThe effect of the amount of initial inoculum was studied at three different concentrations corresponding to 1% (v/w) (equivalent to 1.50×107CFU/g), 4% (v/w) (equivalent to 7.90×107CFU/g) and 8% (v/w) (equivalent to 1.84×108CFU/g) (Fig. 2A). For 1% of inoculum, the exponential phase of the microorganism started at 6h of fermentation; however, for the other inoculum concentrations (4 and 8%), the exponential phase started at 3h of fermentation. For inoculum amounts 1 and 8%, the deceleration stage started at 12h of fermentation and the number of viable cells was very similar at 24h of fermentation (9.83 and 9.82logCFU/g, respectively). The specific growth rate was 0.551/h for 1% and 0.321/h for 8% of inoculum. In the case of the soybean pastes inoculated at 4%, the number of viable cells reached 10.07logCFU/g during 24h of fermentation. In this case the exponential phase was developed between 3 and 9h with a specific growth rate of 0.441/h. The number of cells reached 9.31logCFU/g at the end of this stage and it was very similar to the obtained value in the other two cases at 12h of fermentation.
Effect of temperatureFigure 2B shows the effects of temperature (30°C, 37°C and 44°C) on the behavior of L. rhamnosus CRL 981. The specific growth rate was similar at 30 and 44°C (0.21 and 0.261/h, respectively), as seen in the slopes of the lines between 3 and 12h of fermentation (exponential phase). The number of cells at 12h was similar for 44°C and 30°C (9.22 and 8.98logCFU/g respectively). However, a viability drop was observed at 44°C at 24h, while at 30°C the value of logCFU/g continued growing up to 9.46 at 24h. Even at this point the microorganism had not yet begun its stationary phase; an increase of the number of viable cells was observed at this time. The pH at the end of fermentation was very similar in two cases: 4.94 at 30°C and 4.85 at 44°C. In the case of 37°C the specific growth rate was approximately twice higher than at the other two tested temperatures (0.441/h). Furthermore, the number of cells reached 9.31logCFU/g at the end of exponential phase (8h) while the other conditions reached similar values at 12h of fermentation. The number of viable cells and the acidity of the soybean paste were greater at 37°C and 24h of fermentation: 10.07logCFU/g and pH 4.32, respectively.
DiscussionThe process of solid state fermentation for food applications is one of the oldest knowledges available to humans. For many communities, preparing fermented food is part of their traditional knowledge5.
The interest in soybean products has increased in the last years because of their biological properties. Soybean is an interesting substrate to use in food applications due to its nutritional value and economical aspect.
In this work we analyzed the behavior of several selected lactic cultures in soy pastes as substrate in order to obtain information about their behavior in this system. All lactic cultures used in this study were able to grow in soybean-SSF without the addition of carbohydrate or protein supplements. These strains have only used the available components in the complex soy-matrix: sucrose, raffinose, stachyose, proteins, aminoacids, and others. In contrast with the other strains L. rhamnosus CRL 981 and L. paracasei subsp. paracasei CRL 207, they showed the highest viable count at 24h of fermentation in both soybean pastes. However, the latter strain showed low acidification. This result is in concordance with Thi et al.22 who reported that L. paracasei subsp. paracasei LG3 was able to grow in a medium from soybean where low acidification was observed. L. paracasei subsp. paracasei has a heterofermentative facultative metabolism. When glucose is present in the medium a high production of lactic acid is observed with concomitant reduction of pH. However, when heterofermentative microorganisms ferment other kind of sugars, a production of lactic acid, acetic acid and other organic acids with a higher pH is observed. This metabolism could be responsible for the highest pH observed for this strain in soybean paste where the main sugar present is sucrose. Furthermore, L. paracasei subsp. paracasei CRL 207 is able to degrade soy protein1 and the compounds released from the hydrolysis of proteins are able to buffer the medium. More studies are necessary to understand this behavior.
A loss of viability was observed in L. fermentum CRL 251 at 24h for both pastes and in B. longum CRL 849 when it was grown on the soybean paste with 60% moisture. The reason for the loss of viability of the latter bacterium could be that this microorganism has strict nutritional requirements from the viewpoint of isolation and growth in the laboratory. In addition, the fact of working with lower moisture could be a difficulty for its development.
Moreover, the results obtained in this study were compared with previous studies in aqueous extract of soybean (EAS) using the same microorganisms7,8,13,14. The analysis of these studies indicated that L. rhamnosus CRL 981 showed improved development on soybean paste (80% -60% moisture) than EAS. Thereby, the growth in soybean pastes increased 2 time log units whereas only 1.4 times for EAS and the final pH was similar. However, for L. paracasei subsp. paracasei CRL 207 its growth was unfavorable on soybean paste with respect to EAS. In the latter condition the microorganism growth rate reached 0.741/h while the soybean paste 0.381/h. The ΔpH fermentation at 24h was about 3 in EAS for this strain. L. fermentum CRL 251 had similar behaviors in both assays. Furthermore, B. longum CRL 849 showed similar development in EAS and the soybean paste with 60% moisture, since in both cases their viability decreased after 8h. This strain did not show loss of viability in the soybean paste with 80% moisture. Rodríguez de Olmos et al.21 optimized the fermentation parameters of strains L. paracasei subsp. paracasei CRL 207 and B. longum CRL 849 using a solid substrate from soy flour. The chosen paste moisture was 65%. The growth rate for both strains was 0.23 and 0.321/h for L. paracasei subsp. paracasei CRL 207 and B. longum CRL 849 respectively. These results were similar to our finding for soybean pastes with low moisture (60%). The growth rate of these microorganisms was relatively low in both reports in contrast with the growth rate of L. rhamnosus CRL 981 in this present study.
Analyzing all the results in this work, strain L. rhamnosus CRL 981 was chosen for subsequent studies. With respect to paste moisture, it was observed that the growth of this strain was quite similar in both pastes. However, the soybean paste with 80% moisture was chosen due to the fact that the preparation of a paste with 60% moisture requires an extra drying process, which implies higher investment time and costs. The variation of inoculum amount and temperature was carried out to analyze their effects on the selected microorganism behavior. The optimal fermentation values were 4% for inoculum amount and 37°C of temperature for the selected strain. Furthermore, L. rhamnosus CRL 981 is of great interest since its probiotic effect on liquid fermentation using soy milk was demonstrated by the working group15. This strain ameliorates hyperglycemia, lipid profiles and increases antioxidant enzymes in fermented soy milk. Due to this fact, more studies in soy-SSF should be performed to understand this behavior in the semisolid matrix.
In contrast to Park et al., the lactic cultures used in the present work were able to develop as starters in a solid soy substrate (soybean paste) without additional nutrients19. These authors used Bacillus subtilis as main fermentation followed by lactic fermentation with milk addition.
In conclusion, this work allowed to obtain extensive information about the behavior of these starter cultures which could be used in solid state fermentation as another alternative to produce new soybean food. In this report we propose solid substrate from soybean as a vegetarian food carrier for selected lactic cultures. In this connection, a methodology to prepare soybean paste was obtained and the fermentation parameters for the selected strain were optimized.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data are included in this article.
Right to privacy and informed consentThe authors declare that no patient data are included in this article.
Conflict of interestThe authors declare that they have no conflicts of interest.
This study was partly supported by grants from Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET/PIP0006), Agencia Nacional de Promoción Científica y Tecnológica (ANPyCT-FONCYT/PICTs 1773/1949), and Consejo de Ciencia y Técnica de la Universidad Nacional de Tucumán (CIUNT), Argentina.