The free-living ciliate Tetrahymena thermophila is a unicellular model organism in which landmark biological processes have been discovered, such as the first description of telomerase activity and the molecular structure of telomeres, the mechanism of self-splicing RNA and ribozymes, the function of histone acetylation in transcription regulation and a number of pioneer experiments on the interference (RNAi) mechanism for programmed genome rearrangements, among others5.
In contrast to most eukaryotic cells that require sterols in their membranes, Tetrahymena spp. satisfies its vegetative growth synthesizing tetrahymanol (a surrogate sterol similar to the hopanoids found in bacteria). Nevertheless, if a sterol is present, the biosynthesis of tetrahymanol is immediately repressed and the sterol is incorporated into the membrane through an unknown pathway3. Tetrahymena spp. has potential biotechnology applications in biocatalysis converting cholesterol to pro-vitamin D3 for the use in the foodstuff and synthesis industry7,8. In this regard, the study of cholesterol uptake could contribute to optimizing the biotransformation process as well as to understand pathways of sterol endocytosis which fulfill important functions in higher eukaryotes and share similarity with this unicellular organism.
To date, at least four distinct pathways of endocytic uptake have been identified: endocytosis of small vesicles from parasomal sacs, uptake of larger vesicles at the base of the oral apparatus (phagosomes), endocytosis coupled with the exocytosis of dense-core secretory vesicles (membrane turnover) and endocytic membrane recovery upon phagosome fusion, at a cortical site (cytoproct)3.
We performed an assay to identify the endocytic pathway involved in cholesterol uptake. For this purpose, a wild-type T. thermophila CU399 strain (WT) and a mutant obtained thereof, defective only in phagocytosis (II8G)6, were grown in rich culture medium containing the fluorescent cholesterol analog (BODIPY cholesterol).
BODIPY-cholesterol has been shown to mimic membrane partitioning and trafficking of cholesterol, enabling to perform this kind of experiments2. Here, BODIPY was complexed with methyl-β-cyclodextrin at a molar ratio of sterol/cyclodextrin 1/10, probe-sonicated for 3×15min on ice and centrifuged for 2×30min. Simultaneously, Escherichia coli expressing a red fluorescent protein (Ds Red) was added into the culture media and used as phagocytic marker1.
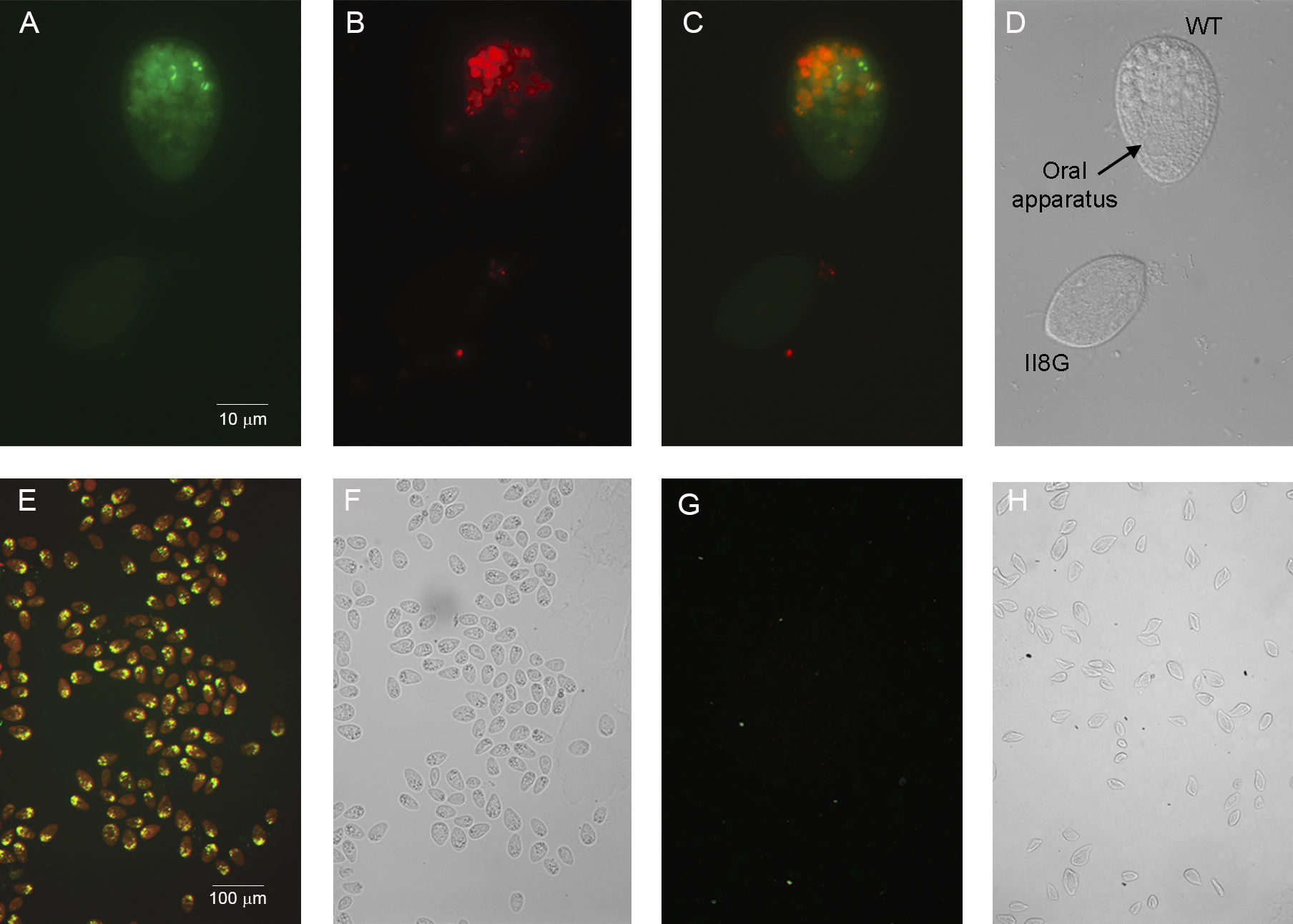
The upper panels of Figure 1 show the WT strain with large food vacuoles containing cholesterol and E. coli fluorescent cells, whereas the mutant II8G does not display any fluorescent signal. The lower panels show both strains analyzed separately, to test absolute phenotype in each population.
Fluorescence microscopy of T. thermophila WT and II8G strains feed with 23-(dipyrrometheneboron difluoride)-24-norcholesterol (green) and E. coli (red), fixed in PFA 2%. (A–D) Tetrahymena WT mixed with mutant II8G strain. 60× objective lens. Green fluorescence observed only in WT cell (A), red fluorescence to evidence E. coli is only present as large vesicles in the WT strain but not in II8G mutant (B). Merge of two filters (C). Images acquired in DIC to indicate WT and mutant cell (D). Images in a double fluorescence with FITC and TRITC filters of WT and II8G (E, G respectively) and acquired in phase contrast of WT and II8G (F, H respectively) 10× objective lens (E–H). Digital images were collected using Carl Zeiss Axio imager M2 fluorescence microscopy.
The above result indicates that cholesterol, and probably all sterols, are incorporated via phagocytosis and not by other endocytic mechanisms. Conversely, in the ciliate Paramecium tetraurelia, uptake assays have indicated that cholesterol is incorporated not only by phagocytosis but also through the plasma membrane4, suggesting different endocytic preferences in these two evolutionary close organisms.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
This work was funded by CONICET and MINCyT through grant PIP No. 0213 and PICT No. 2013-0701.