One third of the elderly population with symptomatic calcified aortic stenosis cannot undergo surgery due to their high operative risk. The transcatheter aortic-valve implantation (TAVI) has emerged as an alternative therapy for this group of patients.
MethodsAll patients submitted to TAVI from November 2008 to April 2012 were included in our study. We report the baseline clinical characteristics, procedural data, hospital outcomes and clinical follow-up of this population. Definitions were based on the Valve Academic Research Consortium criteria.
ResultsTAVI was performed in 23 patients, with 79 ± 6.7 years of age, and 56% were female. The EuroSCORE was 20.4 ± 11.1%. The CoreValve® prosthesis was used in 19 patients (82.6%) and the Edwards SAPIEN™ valve was used in the remaining ones. Procedure success rate was 96%. The mean follow-up was 22 ± 12.8 months, with 6 deaths (26.1%) in this period, 3 of which were observed in the first 30 days (13%) and other 2 (21.7%) by the end of the first year. One patient had a transient ischemic attack during hospitalization (4.3%), but there were no episodes of stroke or myocardial infarction in the periprocedural period or in the follow-up. The composite safety endpoint at 30 days was observed in 5 patients (21.7%) and the composite efficacy endpoint at 12 months was 78.3%.
ConclusionsThe results of this study demonstrate that TAVI is an attractive procedure for the treatment of patients with calcified aortic stenosis and high operative risk.
Resultados Imediatos e Seguimento Clínico dos Pacientes Submetidosa Implante Valvar Aórtico Transcateter
IntroduçãoUm terço da população idosa portadora de estenose aórtica calcificada sintomática não apresenta condições cirúrgicas em decorrência do elevado risco operatório. O implante valvar aórtico transcateter (IVAT) surgiu como uma alternativa terapêutica para esses pacientes.
MétodosIncluímos, no período de novembro de 2008 a abril de 2012, todos os pacientes submetidos a IVAT em nosso serviço. Relatamos as características clínicas basais, os dados dos procedimentos, os resultados hospitalares e o seguimento clínico dessa população. As definições utilizadas foram baseadas nos critérios do Valve Academic Research Consortium.
ResultadosO IVAT foi realizado em 23 pacientes, com 79+6,7 anos de idade, 56% do sexo feminino. O EuroSCORE foi de 20,4+11,1%. A prótese CoreValve® foi utilizada em 19 pacientes (82,6%) e a Edwards SAPIEN™ nos demais. A taxa de sucesso do procedimento foi de 96%. O tempo médio de seguimento clínico foi de 22+12,8 meses, observando-se 6 óbitos (26,1%) nesse período, 3 dos quais ocorreram nos primeiros 30 dias (13%) e outros 2 (21,7%), até o final do primeiro ano. Um paciente apresentou ataque isquêmico transitório na fase hospitalar (4,3%), mas não ocorreram episódios de acidente vascular encefálico ou de infarto do miocárdio no período periprocedimento ou no acompanhamento tardio. O desfecho combinado de segurança aos 30 dias ocorreu em 5 pacientes (21,7%) e o desfecho combinado de eficácia aos 12 meses foi de 78,3%.
ConclusõesOs resultados obtidos neste estudo demonstram o IVAT como procedimento atrativo para o tratamento de pacientes portadores de estenose aórtica calcificada de alto risco cirúrgico.
Calcified aortic stenosis is the most commonly diagnosed valve disease in the United States and Europe,1 and its severe form affects 2% of the population older than 65 years of age.2 Considering that this is the rate of prevalent severe aortic stenosis in the Brazilian population, 380,000 cases are expected to be diagnosed by 2020 and approximately 1,000,000 cases by 2050.3
Initially considered to be natural valve tissue degeneration, current data supports the hypothesis that calcified aortic stenosis is caused by an inflammatory process, similar to atherosclerosis. The accumulation of oxidised lipoproteins is observed in the intima, as well as in endothelial activation and the recruitment of T lymphocytes and monocytes.4 Several mechanisms have been proposed for valve tissue calcification, and the transdifferentiation of myofibroblasts into osteoblasts is one of them.5
In adult patients with aortic stenosis, the obstruction to blood flow increases gradually over the years. During this period, there is a gradual adaptation of the left ventricle to pressure overload through the development of concentric myocardial hypertrophy. This results in diastolic dysfunction, reduced coronary flow reserve, myocardial ischaemia, and eventually left ventricular systolic dysfunction.6
Typically, patients with aortic stenosis remain asymptomatic for a long period of time; however, after the onset of symptoms, the prognosis is poor, with an interval to death of two years for those with heart failure, three years for syncope, and five years for angina pectoris.7
The standard treatment for symptomatic patients is surgical replacement of the aortic valve.8 However, approximately one-third of the elderly population with this disease cannot benefit from treatment due to the presence of comorbidities or other conditions that are unfavourable to surgery.9,10 These patients have adverse outcomes, with a one to two year survival rate of 50%.11,12
Transcatheter aortic valve implantation (TAVI), introduced by Cribier et al.,13 has emerged as an attractive alternative treatment for these patients, and its use has rapidly increased. The initial results were favourable,14,15 and the use of TAVI in patients with contraindications to surgery was shown to be superior to medical therapy.12,16 As such, this technique has been adapted by several departments throughout the world, beginning with this department in 2008. This study aims to present the immediate and long-term outcomes in this group of patients, using the criteria proposed by the Valve Academic Research Consortium (VARC).17
METHODSPatientsIn November of 2008, a multidisciplinary team consisting of specialists in anesthesia, cardiology, echocardiography, haemodynamics, interventional radiology, and cardiac surgery at the Hospital Beneficência Portuguesa de São Paulo started the TAVI program. All patients at this hospital who underwent TAVI between November of 2008 and April of 2012 were included in this registry. This study was approved by the institution ethics and research committee under protocol number 669-11/CAAE: 0104.1.360.000-11.
Selection criteriaIndividuals were selected based on clinical criteria and the morphological parameters of the aortic valve/ aorta and the access route to determine the technical feasibility of percutaneous valve replacement.18 For that purpose, transthoracic echocardiography with colour Doppler, multi-detector CT angiotomography, and angiography of the ascending aorta, iliac-femoral territory, and coronary arteries were performed.
Patients in this registry included those with an aortic valve area < 1cm2 or with an indexed aortic valve area ≤ 0.6cm2/m2, symptomatic patients with an aortic valve annulus diameter ≥ 20mm and ≤ 27mm, an ascending aortic diameter ≤ 45mm, and a logistic EuroSCORE ≥ 15% or those at high surgical risk as established by a multidisciplinary assessment, and considering the following comorbidities: hepatic cirrhosis, pulmonary hypertension > 60mmHg, extensively calcified aorta (porcelain aorta), recurrent pulmonary embolism, right ventricular failure, or refusal of the patient to undergo surgery.
Device and procedure descriptionOne of two commercially available prostheses was used: the third generation CoreValve® prosthesis (Medtronic Inc. – Minneapolis, USA) or the Edwards SAPIEN™ XT prosthesis (Edwards Lifesciences Inc. – Irvine, California, USA).
The CoreValve® prosthesis (Figure 1) consists of three porcine pericardium leaflets mounted on a Nitinol self-expandable stent. The prosthesis diameters used were 26mm, for a valve annulus of 20mm to 23mm, and 29mm, for a valve annulus > 23mm to 27mm. The Edwards SAPIEN™ XT prosthesis (Figure 2) consists of three bovine pericardial leaflets mounted on a cobalt-chromium balloon-expandable stent. This device is available in Brazil with diameters of 23mm, for an annulus of 21mm to 23mm, and of 26mm, for a valve annulus > 23 to 24.5mm. Both systems use 18F diameter sheaths.
The procedures were performed under general anesthesia or sedation, depending on the patient’s clinical condition and the prosthesis chosen, because it is necessary to use transoesophageal echocardiography to guide prosthetic implantations with the Edwards SAPIENTM XT. All patients received 200mg of acetylsalicylic acid and 300mg of clopidogrel orally 24 hours before the procedure and were maintained for six months postprocedure with 100mg of acetylsalicylic acid and 75mg of clopidogrel daily. At the beginning of each procedure, unfractionated heparin was administered intravenously (100 IU/kg) with adequate corrections to achieve an activated clotting time between 300 and 350 seconds.
The implantation techniques for these two prostheses have been previously described in detail.14,19 Briefly, the retrograde access was used, preferably by puncture of the femoral artery. Following the selective catheterisation of the left ventricle, aortic valvuloplasty was performed with a balloon when using the Edwards SAPIEN™ XT prosthesis or, when necessary, when using the CoreValve® prosthesis. During balloon pre-dilation, a temporary pacemaker was used to raise the heart rate to between 180 beats to 220 beats per minute, thus preventing balloon displacement at the time of inflation. The stent was then implanted. At the end of the procedure, a control ascending aortography was performed to assess the presence of perivalvular regurgitation. Hemostasis was performed with the vascular occlusion device Perclose ProGlide (Abbott, Inc. – Abbott Park, USA). Patients were referred to the intensive care unit, where they remained for at least 48 hours. They also underwent preventive maintenance with a transvenous pacemaker during that period, as there was a possibility complete atrioventricular block.
Clinical follow-up was performed through medical consultation or by contacting patients by telephone.
Study definitions and endpointsProcedural success was defined as the implantation of a properly functioning single prosthesis in the correct position.
The primary study endpoint was defined as the number of all-cause mortality at 30 days and at one year. The combined safety endpoint at 30 days was defined as the number of occurrences of death from any cause, major stroke, periprocedural myocardial infarctions, life-threatening bleeding, need for dialyses, or severe vascular complications within 30 days of the procedure. The combined efficacy endpoint at one year was defined as the number of survivals, free of death by any cause, treatment failure for aortic stenosis, or prosthesis dysfunction.
Other assessed outcomes were cardiovascular mortality at 30 days and one year, the incidence of stroke and acute myocardial infarction, the occurrence of major vascular complications, the need for implantation of a permanent pacemaker, and new left bundle of His branch block.
RESULTSFrom November of 2008 to April of 2012, 23 patients were included in the registry, and their outcomes were followed for a mean period of 22 ± 12.8 months, ranging from three months to 44 months. The mean age was 79 ± 6.7 years, and 56% of the patients were female. All patients were white; 87% of individuals had hypertension, 65% had dyslipidemia, and 30% had diabetes mellitus.
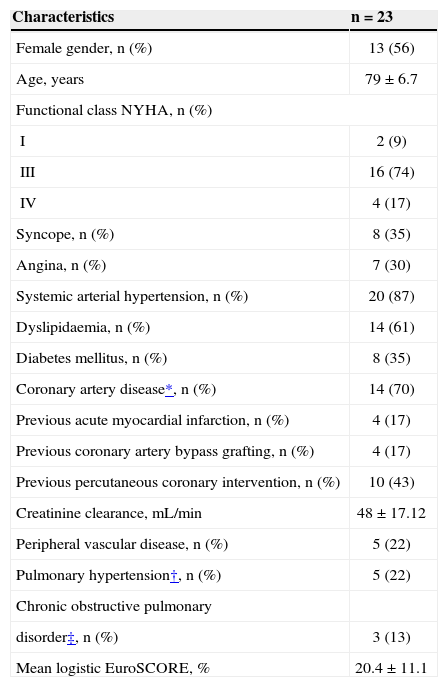
The patients had an average aortic valve area of 0.53 ± 0.14cm2, 35% had reported episodes of syncope, 30% had angina, and 96% had dyspnoea on exertion, with 9% in functional class II, 74% in functional class III, and 17% in functional class IV, as categorised according to the criteria set forth by the New York Heart Association (NYHA). The mean logistic EuroSCORE was 20.4 ± 11.1%. The main baseline characteristics are presented in Table 1.
Baseline clinical characteristics of patients undergoing transcatheter aortic valve implantation (TAVI)
| Characteristics | n = 23 |
|---|---|
| Female gender, n (%) | 13 (56) |
| Age, years | 79 ± 6.7 |
| Functional class NYHA, n (%) | |
| I | 2 (9) |
| III | 16 (74) |
| IV | 4 (17) |
| Syncope, n (%) | 8 (35) |
| Angina, n (%) | 7 (30) |
| Systemic arterial hypertension, n (%) | 20 (87) |
| Dyslipidaemia, n (%) | 14 (61) |
| Diabetes mellitus, n (%) | 8 (35) |
| Coronary artery disease*, n (%) | 14 (70) |
| Previous acute myocardial infarction, n (%) | 4 (17) |
| Previous coronary artery bypass grafting, n (%) | 4 (17) |
| Previous percutaneous coronary intervention, n (%) | 10 (43) |
| Creatinine clearance, mL/min | 48 ± 17.12 |
| Peripheral vascular disease, n (%) | 5 (22) |
| Pulmonary hypertension†, n (%) | 5 (22) |
| Chronic obstructive pulmonary | |
| disorder‡, n (%) | 3 (13) |
| Mean logistic EuroSCORE, % | 20.4 ± 11.1 |
n = number of patients; NYHA = New York Heart Association.
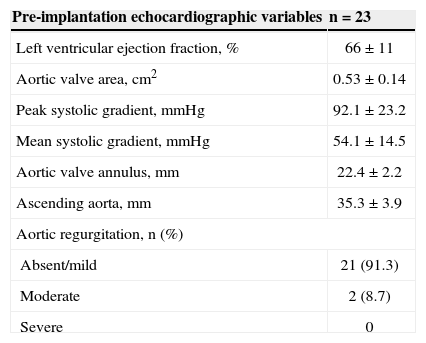
The annulus diameter of the aorta was 22.4 ± 2.2mm and the ascending aorta was 35.3 ± 3.9mm. The left ventricle ejection fraction was 66 ± 11%, with three patients showing left ventricular systolic dysfunction. The maximum peak gradient observed between the left ventricle and the aorta was 92.1 ± 23.2mmHg and the mean was 54.1 ± 14.5mmHg. Echocardiographic data are shown in Table 2.
Pre-transcatheter aortic valve implantation echocardiographic characteristics
| Pre-implantation echocardiographic variables | n = 23 |
|---|---|
| Left ventricular ejection fraction, % | 66 ± 11 |
| Aortic valve area, cm2 | 0.53 ± 0.14 |
| Peak systolic gradient,mmHg | 92.1 ± 23.2 |
| Mean systolic gradient,mmHg | 54.1 ± 14.5 |
| Aortic valve annulus,mm | 22.4 ± 2.2 |
| Ascending aorta,mm | 35.3 ± 3.9 |
| Aortic regurgitation, n (%) | |
| Absent/mild | 21 (91.3) |
| Moderate | 2 (8.7) |
| Severe | 0 |
n = number of patients.
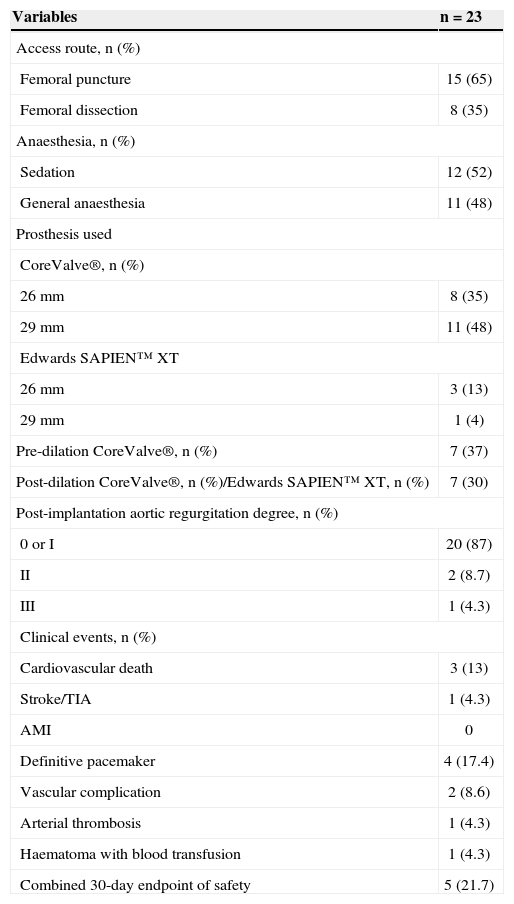
Sedation was used in 12 procedures (52%), while the remainder of the procedures were performed under general anesthesia. A right femoral artery puncture was used in 15 (65%), while the other patients underwent surgical dissection. The CoreValve® prosthesis was implanted in 19 cases and the Edwards SAPIEN™ XT, in four. A pre-dilatation balloon catheter was used in seven (37%) patients receiving the CoreValve® prosthesis. Postdilatation was performed in seven (30%) procedures. The main procedural data are shown in Table 3.
Characteristics related to the procedure and in-hospital clinical events
| Variables | n = 23 |
|---|---|
| Access route, n (%) | |
| Femoral puncture | 15 (65) |
| Femoral dissection | 8 (35) |
| Anaesthesia, n (%) | |
| Sedation | 12 (52) |
| General anaesthesia | 11 (48) |
| Prosthesis used | |
| CoreValve®, n (%) | |
| 26mm | 8 (35) |
| 29mm | 11 (48) |
| Edwards SAPIEN™ XT | |
| 26mm | 3 (13) |
| 29mm | 1 (4) |
| Pre-dilation CoreValve®, n (%) | 7 (37) |
| Post-dilation CoreValve®, n (%)/Edwards SAPIEN™ XT, n (%) | 7 (30) |
| Post-implantation aortic regurgitation degree, n (%) | |
| 0 or I | 20 (87) |
| II | 2 (8.7) |
| III | 1 (4.3) |
| Clinical events, n (%) | |
| Cardiovascular death | 3 (13) |
| Stroke/TIA | 1 (4.3) |
| AMI | 0 |
| Definitive pacemaker | 4 (17.4) |
| Vascular complication | 2 (8.6) |
| Arterial thrombosis | 1 (4.3) |
| Haematoma with blood transfusion | 1 (4.3) |
| Combined 30-day endpoint of safety | 5 (21.7) |
TIA= transient ischaemic attack; AMI = acute myocardial infarction.
Technical success of the implant was achieved in 95.7% of cases. In one patient, the prosthesis failed to implant in the correct position, and progressed to moderate heart failure. This called for a repositioning of the prosthesis, which was successfully performed using a loop snare. The maximum gradient between the left ventricle and the aorta after valve implantation was 17.9 ± 7.3mmHg. The left ventricular ejection fraction achieved was 71% ± 6.6% after the procedure, with a percentage increase of 7.3% from before the procedure. Mild or absent aortic regurgitation was observed in 17 (74%) patients.
Clinical outcomesThe mean follow-up time for patients was 22 ± 12.8 months. Six deaths, regardless of cause, were observed (26.1%) during this period, three of which occurred in the first 30 days (13%) and two of which (21.7%) occurred by the end of the first year. There were three cardiovascular deaths, all in the first month after the procedure, three non-cardiovascular deaths at four months due to pneumonia, and another two at 12 months and 33 months after the procedure due to gastrointestinal complications.
The combined safety endpoint at 30 days was 21.7% (5/23). There were three cardiovascular deaths, one arterial thrombosis and one bleeding event requiring transfusion. One patient (4.3%) had a transient ischaemic attack in the hospital, but there were no episodes of stroke or myocardial infarction in both the periprocedural period or during the clinical follow-up period.
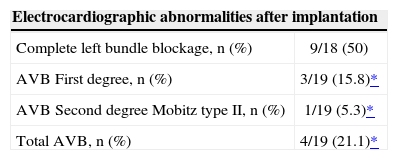
Among the study patients, four had pacemakers implanted before the procedure, four required definitive pacemaker implants in the hospital, and one patient had the device implanted three months after TAVI. Left bundle branch blockage before the procedure was observed in five patients, while nine patients (50%) presented new left bundle branch blockages. Electrocardiographic alterations after TAVI are described in Table 4.
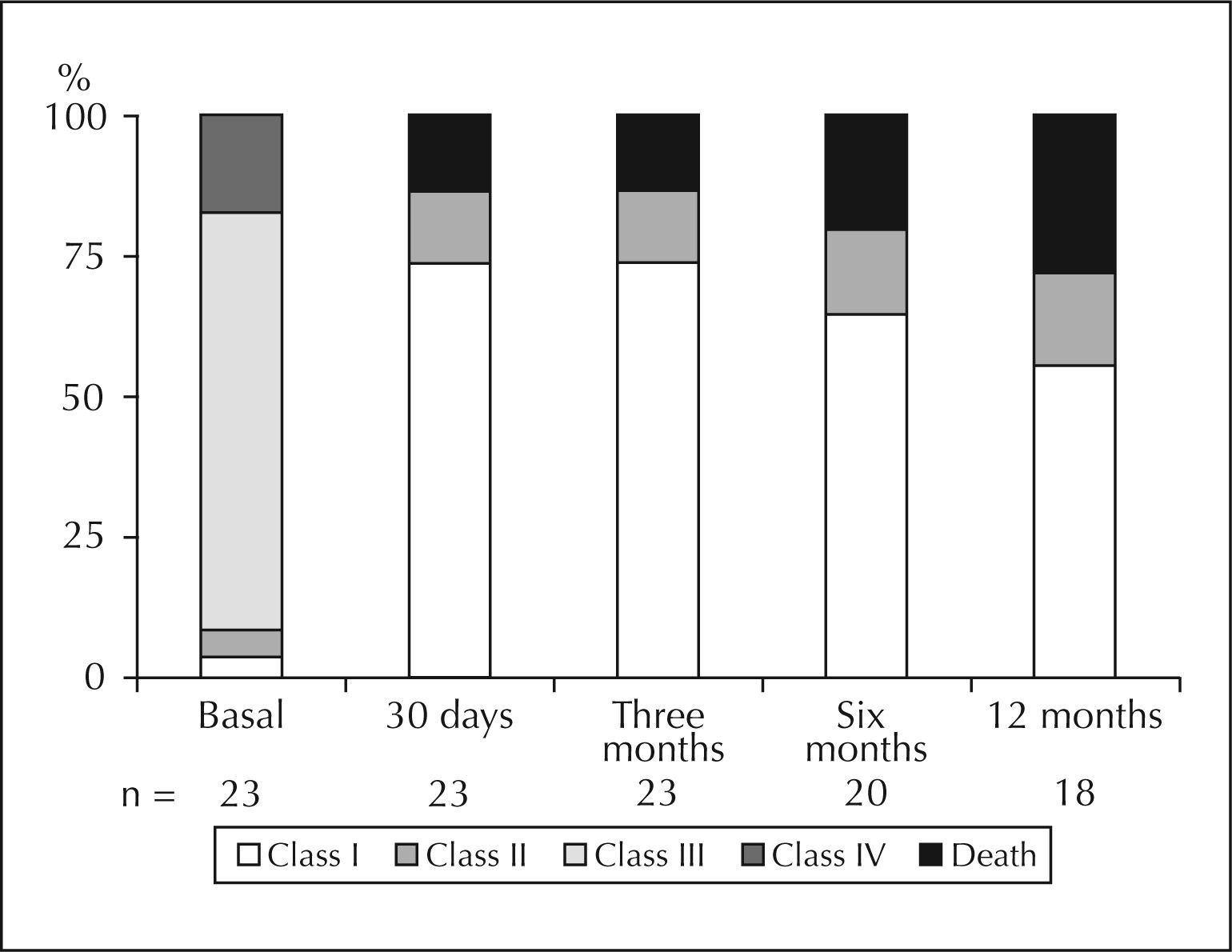
The functional class of patients during evolution is shown in Figure 3.
The combined efficacy endpoint at 12 months was 78.3% (18/23), with the occurrence of two additional non-cardiovascular deaths between 30 days and one year. There were no admissions due to aortic stenosis treatment failure during this period and no malfunctions were detected in the implanted prosthesis.
DISCUSSIONTAVI enables the approach of patients with surgical contraindications, such as high-risk patients or those with exclusionary features, such as a porcelain aorta. In the present sample, patients with high-risk profiles, those who were elderly (mean age of 79 years), and those with multiple risk factors for atherosclerosis and a EuroSCORE of 20.4 were selected.
TAVI was successful in 22 of the 23 patients. The individual defined as a failure presented moderate aortic regurgitation at the end of the procedure, but was resolved with prosthesis repositioning two days after the implantation.
A 13% overall mortality at 30 days is similar to previously published reports: Gilard et al.,20 9.7%; Lemos et al.,21 8.4%; Gurvitch et al.,22 9.4%; and Stähli et al.,23 11.5%. The 21.7% safety outcome obtained in this series is comparable to that of Gurvitch et al.22 (18.4%) and Stähli et al.23 (20.8%). This outcome evaluates events related to the technical complications of the procedure within 30 days. The 78.3% combined efficacy endpoint at one year was similar to the endpoint reported by Stähli et al.23 (70.2%). This outcome of effectiveness evaluates therapeutic failure-free survival, with the proper performance of the prosthesis.
TAVI provided marked clinical improvement, as shown in Figure 3. At baseline, 74% of patients were in functional class III and 17% were in functional class IV. Immediate after the operation and at their 30-day assessment, 94.7% and 91.3% of patients, respectively, were in functional class I or II, and remained at satisfactory levels in late follow-up.
Procedural completion through the femoral puncture technique and hemostasis using the Perclose ProGlide system was feasible, with only one vascular complication in the present study. One patient required surgical treatment for femoral artery thrombosis.
The main adverse effect observed with this technique was the need to implant a pacemaker, which occurred in four patients (21.1%), all of whom had received the CoreValve™ prosthesis. In comparison, this complication can occur in up to 8% of those undergoing the conventional surgical procedure.24,25 Some series have shown rates of up to 30.4% for patients needing pacemakers after TAVI.25
Study limitationsThe study was limited by its observational nature, its single-centre design, and its small sample size.
CONCLUSIONSThese results demonstrate that TAVI is an attractive alternative for the treatment of severe calcified aortic stenosis in high-risk surgical patients. A thorough evaluation is crucial for procedural success and to reduce complications. However, the long-term effectiveness needs to be demonstrated, and technological developments may further improve outcomes and expand the indications for patients at lower risk.
CONFLICT OF INTERESTThe authors declare no conflicts of interest.