Little is known about the late clinical outcomes of patients undergoing saphenous vein graft percutaneous coronary intervention (SVG-PCI), and there are controversies regarding the role of lesion location (aorto-ostial or graft body).
MethodsSingle-center registry including patients undergoing SVG-PCI between 2006 and 2011. Aorto-ostial lesion was defined as a lesion within the first 8mm of the graft; graft body lesion was defined as a lesion located in the remaining portions of the graft. Interventions approaching only the distal anastomosis or the native coronary bed were excluded. We evaluated the rates of major adverse cardiac events (MACE), death, myocardial infarction (MI), and target vessel revascularization (TVR) between the groups.
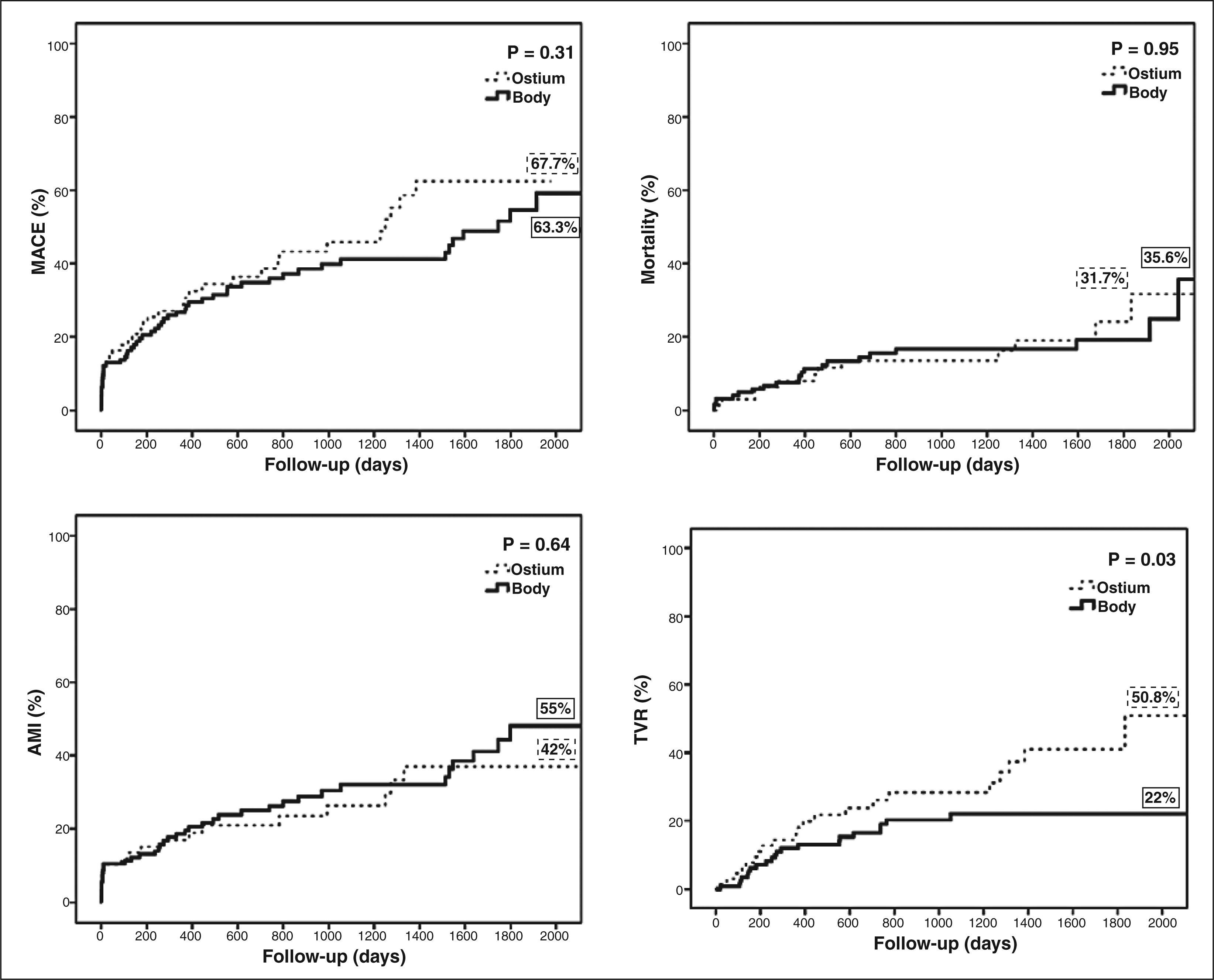
ResultsOne hundred and ninety-five patients were evaluated, 69 in the Aorto-Ostial Group and 126 in the Graft Body Group. Mean age was 69.6 ± 10.2 years, 41% were diabetic, 65.1% had acute coronary syndromes and most of them were treated with bare metal stents (82.5%). There was no statistical difference between groups for most of the characteristics evaluated. In the late follow-up, the TVR rate (50.8% vs. 22%; P = 0.03) was greater in the Aorto-Ostial Group. The MACE rate (67.7% vs. 63.3%; P = 0.33), death (31.7% vs. 35.6%; P = 0.95) and MI (55% vs. 42%; P = 0.64) were similar between the two groups.
ConclusionsThis population included a high-risk subgroup, with high late mortality rates, regardless of the location of the lesion in the graft. In patients treated predominantly by baremetal stents, aorto-ostial lesions had a higher reintervention rate when compared to graft body lesions.
Influência da Localização das Lesões nos Desfechos Clínicos Tardios apósIntervenção Coronária Percutânea em Enxertos de Veia Safena
IntroduçãoA evolução tardia de pacientes submetidos à intervenção coronária percutânea em enxertos de veia safena (ICP-Saf) é pouco discutida na literatura, havendo, inclusive, controvérsias sobre a influência da localização da estenose (em posição aorto-ostial ou no corpo do enxerto).
MétodosRegistro unicêntrico, que incluiu pacientes submetidos à ICP-Saf, entre os anos de 2006 e 2011. A lesão aorto-ostial foi definida como aquela localizada nos primeiros 8mm do enxerto; as lesões de corpo foram as localizadas nas porções remanescentes. Foram excluídas as intervenções que abordaram somente a anastomose distal ou o leito coronário nativo. Foram avaliadas as taxas de eventos cardíacos adversos maiores (ECAM), óbito, infarto agudo do miocárdio (IAM) ou revascularização do vaso-alvo (RVA) entre os grupos.
ResultadosForam avaliados 195 pacientes, sendo 69 no Grupo Óstio e 126 no Grupo Corpo. A média de idade da população total foi de 69,6 ± 10,2 anos, 41% dela era diabética, e 65,1% apresentaram síndromes coronárias agudas, sendo a maioria tratada com stents convencionais (82,5%). Não houve diferença entre os grupos na maioria das características estudadas. No seguimento tardio, a taxa de RVA (50,8% vs. 22%; P = 0,03) foi maior no Grupo Óstio. As taxas de ECAM (67,7% vs. 63,3%; P = 0,33), morte (31,7% vs. 35,6%; P = 0,95) e IAM (55% vs. 42%; P = 0,64) foram semelhantes entre os grupos.
ConclusõesEssa população compôs um subgrupo de risco elevado, com alta mortalidade tardia, independentemente da localização da lesão no enxerto. Em pacientes tratados predominantemente com stents não farmacológicos, lesões aorto-ostiais apresentaram maior taxa de reintervenção em relação às lesões de corpo.
Percutaneous coronary interventions in saphenous vein grafts (PCI-Saph) usually occur in a complex scenario. On one side, there’s the patient with chronic coronary artery disease, history of CABG, with or without previous percutaneous interventions, besides multiple comorbidities; on the other, there are the peculiarities inherent to the percutaneous procedure itself. This intervention can result in distal embolization of atherothrombotic debris, often resulting in elevated markers of myocardial necrosis and/or in reduced antegrade flow (no-reflow phenomenon).1,2
The lesions may be located on the aorto-ostial portion or in the body of the graft. The aorto-ostial lesions have a high rate of restenosis compared to non-aorto-ostial lesions, due to the greater likelihood of suboptimal angiographic results, resulting in greater resistance of the lesions to dilation.3−7 Conversely, some trials have shown that lesions of the graft body are associated with post-procedural complications and with early worse clinical outcome.8−11 Thus, there is controversy regarding the location of the lesions in the graft influencing late clinical outcomes.
This study aimed to characterize, from the evolutionary point of view, the PCI-Saph in a representative sample of Brazilian reality, and to assess whether the lesion location influences clinical outcomes in a very late follow-up.
METHODSStudy populationThe study population consisted of patients already treated with coronary artery bypass graft (CABG) surgery who required PCI-Saph. Patients consecutively treated between January of 2006 and March of 2011 in the Department of Interventional Cardiology, Instituto do Coração, Hospital das Clinicas, Faculdade de Medicina, Universidade of São Paulo (Incor-HCFMUSP), São Paulo, SP, Brazil, were included in the present study.
The patients were divided into two groups according to lesion location: aorto-ostial or body graft. The ostium group included patients with at least one lesion located in the first 8mm of the graft, and the body group included patients with localized lesions in the remaining portions of the graft. Interventions that addressed only the anastomosis or the native coronary bed after anastomosis were discarded. With this criterion in mind, there was absolutely no restriction for inclusion regarding clinical status at admission, age, comorbidities, angiographic characteristics, or ventricular function.
ProcedureThe platelet anti-aggregation consisted of the administration of a loading dose of clopidogrel 300-600mg (with recommendation for 600mg if the time between the administration of the drug and the intervention was six hours), followed by 75mg/day for one month in the case of conventional stents and for at least one year in the case of drug-eluting stents (DES). Furthermore, patients were instructed to use acetylsalicylic acid (100mg/day) indefinitely. In patients with acute coronary syndrome without ST-segment elevation, antiplatelet therapy was initiated with acetylsalicylic acid and glycoprotein IIb/IIIa inhibitor (tirofiban), with replacement of this agent by clopidogrel after PCI. After obtaining a vascular access (> 6F) and introducing the catheter, unfractionated heparin 70 to 100 IU/kg was administered. The stent was implanted according to the technique currently established, i.e., direct stent introduction, whenever possible. When necessary, pre-dilation was performed with short low-pressure balloons. The stent was implanted in order to ensure full coverage of the lesion and, when necessary, more than one stent was used with overlapping of its edges. Post-dilation, when indicated, was performed with less lengthy balloons compared to the stent, taking care not to exceed its borders, thus avoiding lesions to adjacent segments not covered by the stent. The use of a distal protection filter and of glycoprotein IIb/IIIa inhibitors and the selection of the type of stent were left to the surgeon’s discretion.
Data collection and analysisIn-hospital progression data were collected by trained physicians during the index hospitalization, according to the completion of previously standardized forms. The collection included clinical and procedural characteristics, laboratory test results, and clinical evolution until discharge. The collection of information about the late evolution was performed by outpatient follow-up, review of hospital records, or telephone contact.
Objectives and definitionsThe primary outcome evaluated was the occurrence of major adverse cardiac events (MACE), comprising death, acute myocardial infarction (AMI), and target vessel revascularization (TVR). As secondary outcomes, the rates of death, AMI, and TVR were analyzed separately. The primary objective of the study was to compare the occurrence of the primary outcome and of secondary outcomes between the Ostium and Body groups.
Periprocedural AMI was defined as the emergence of new Q waves in two contiguous leads of the electrocardiogram and/or elevation of creatine kinase MB fraction (CK-MB) or a level of troponin I greater than three times the upper limit of normal. Markers of myocardial necrosis were requested at least twice in the first 24 hours post-PCI.
The diagnosis of stable angina was established using the Canadian Cardiovascular Society criteria.12 It was strongly recommended, but not mandatory, that non-invasive tests for ischemia in patients with atypical symptoms were performed. The diagnosis of AMI with ST-segment elevation was performed in the occurrence of persistent ST elevation > 1mm in two contiguous leads, or of a new left branch block in the electrocardiogram. Acute coronary syndrome with non-ST-segment elevation was diagnosed by the presence of clinical symptoms suggestive of ischemia associated with electrocardiographic alterations compatible with ischemia (inverted T wave or ST-segment depression) and/or troponin elevation above the normal limit. TVR was defined as a new surgical or percutaneous intervention in lesions > 50% in the treated graft, or new interventions in the correspondent native coronary territory. TVR was conducted only in the presence of symptoms and/or signs of ischemia. Angiographic success was defined as a reduction of target lesion 30%, with maintenance or restoration of a normal antegrade flow (Thrombolysis in Myocardial Infarction [TIMI] 3).
Statistical analysisQualitative variables were expressed as numbers and percentages, and quantitative variables as means and standard deviations. The comparison of numeric variables was performed through the Mann Whitney test, or Pearson’s test, when appropriate. The chi-squared test or Fisher’s exact test was used to compare qualitative variables. Survival free of MACE, death, AMI, and TVR were estimated using the Kaplan-Meier method.
The groups were compared using the log-rank test. The differences were considered statistically significant when P 0.05, and all P-values reported were two-tailed. Data were analysed using SPSS, version 12.0 (SPSS Inc. – Chicago, USA) software.
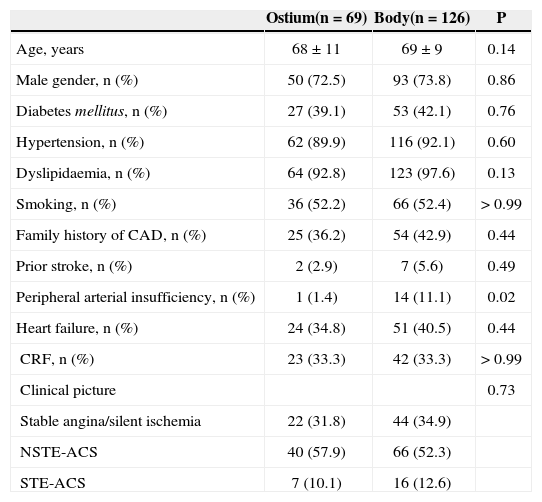
RESULTSBaseline procedural characteristics195 patients were included, with mean age of 69.6 ± 10.2 years; 73% were male, 41% were diabetics, and 65% had acute coronary syndrome on admission. Most were treated with bare-metal stents (82.5%). The Ostium and Body groups had similar clinical characteristics, except for a higher prevalence of peripheral arterial disease in the second group (1.4% vs. 11.1%. P = 0.02) (Figure 1). The baseline clinical characteristics are shown in Table 1.
Baseline clinical characteristics
| Ostium(n = 69) | Body(n = 126) | P | |
|---|---|---|---|
| Age, years | 68 ± 11 | 69 ± 9 | 0.14 |
| Male gender, n (%) | 50 (72.5) | 93 (73.8) | 0.86 |
| Diabetes mellitus, n (%) | 27 (39.1) | 53 (42.1) | 0.76 |
| Hypertension, n (%) | 62 (89.9) | 116 (92.1) | 0.60 |
| Dyslipidaemia, n (%) | 64 (92.8) | 123 (97.6) | 0.13 |
| Smoking, n (%) | 36 (52.2) | 66 (52.4) | > 0.99 |
| Family history of CAD, n (%) | 25 (36.2) | 54 (42.9) | 0.44 |
| Prior stroke, n (%) | 2 (2.9) | 7 (5.6) | 0.49 |
| Peripheral arterial insufficiency, n (%) | 1 (1.4) | 14 (11.1) | 0.02 |
| Heart failure, n (%) | 24 (34.8) | 51 (40.5) | 0.44 |
| CRF, n (%) | 23 (33.3) | 42 (33.3) | > 0.99 |
| Clinical picture | 0.73 | ||
| Stable angina/silent ischemia | 22 (31.8) | 44 (34.9) | |
| NSTE-ACS | 40 (57.9) | 66 (52.3) | |
| STE-ACS | 7 (10.1) | 16 (12.6) |
CAD = coronary artery disease; CRF = chronic renal failure (creatinine clearance 60 mL/min); NSTE-ACS = non-ST-segment elevation acute coronary syndrome; STE-ACS = ST-segmenelevation acute coronary syndrome.
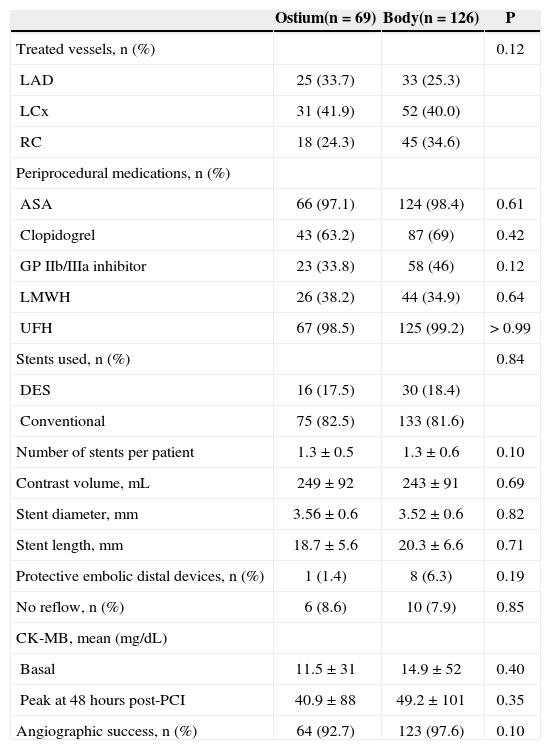
The angiographic and procedural characteristics are presented in Table 2. The most frequently treated territory was supplied by the left circumflex artery and its marginal branches (approximately 40%). In the index procedure, 1.19 ± 0.52 stents were implanted, on average, per vessel; in most of these patients, baremetal stents were used (82.5% in the Ostium group vs. 81.6% in the Body group; P = 0.84).
Angiographic and procedural characteristics
| Ostium(n = 69) | Body(n = 126) | P | |
|---|---|---|---|
| Treated vessels, n (%) | 0.12 | ||
| LAD | 25 (33.7) | 33 (25.3) | |
| LCx | 31 (41.9) | 52 (40.0) | |
| RC | 18 (24.3) | 45 (34.6) | |
| Periprocedural medications, n (%) | |||
| ASA | 66 (97.1) | 124 (98.4) | 0.61 |
| Clopidogrel | 43 (63.2) | 87 (69) | 0.42 |
| GP IIb/IIIa inhibitor | 23 (33.8) | 58 (46) | 0.12 |
| LMWH | 26 (38.2) | 44 (34.9) | 0.64 |
| UFH | 67 (98.5) | 125 (99.2) | > 0.99 |
| Stents used, n (%) | 0.84 | ||
| DES | 16 (17.5) | 30 (18.4) | |
| Conventional | 75 (82.5) | 133 (81.6) | |
| Number of stents per patient | 1.3 ± 0.5 | 1.3 ± 0.6 | 0.10 |
| Contrast volume, mL | 249 ± 92 | 243 ± 91 | 0.69 |
| Stent diameter, mm | 3.56 ± 0.6 | 3.52 ± 0.6 | 0.82 |
| Stent length, mm | 18.7± 5.6 | 20.3 ± 6.6 | 0.71 |
| Protective embolic distal devices, n (%) | 1 (1.4) | 8 (6.3) | 0.19 |
| No reflow, n (%) | 6 (8.6) | 10 (7.9) | 0.85 |
| CK-MB, mean (mg/dL) | |||
| Basal | 11.5 ± 31 | 14.9 ± 52 | 0.40 |
| Peak at 48 hours post-PCI | 40.9 ± 88 | 49.2 ± 101 | 0.35 |
| Angiographic success, n (%) | 64 (92.7) | 123 (97.6) | 0.10 |
LAD = left anterior descending; LCx = left circumflex artery; RC = right coronary artery; ASA = acetylsalicylic acid; GP = glycoprotein; LMWH = low molecular weight heparin; UFH = unfractionated heparin; DES = drug eluting stent; CK-MB = creatine kinase MB fraction; PCI = percutaneous coronary intervention.
In relation to periprocedural medications, there was also no significant difference between groups. A trend towards a lower rate of angiographic success in Ostium group (92.7% vs. 97.6%; P = 0.10) was noted. The mean diameter and length of the stents were, respectively, 3.56 ± 0.6mm vs. 3.52 ± 0.6mm (P = 0.82) and 18.7 ± 5.6mm vs. 20.3 ± 6.6mm (P = 0.71). The mean peak CK-MB in the first 48 hours after the procedure was 40.9 ± 88 mg/dL vs. 49.2 ± 101 mg/dL (P = 0.35). Distal embolic protection (Filter Wire®) devices were rarely used: one in the Ostium group and eight in the Body group (P = 0.19). The occurrence of no-reflow phenomenon was similar between groups (8.6% vs. 7.9%; P = 0.85).
The median follow-up time was 31.7 ± 22.9 months. There was no significant difference between groups regarding mortality (31.7% vs. 35.6%; P = 0.95), occurrence of MACE (67.7% vs. 63.3%; P = 0.31), or occurrence of AMI (42% vs. 55%; P = 0.64). However, in the Ostium group, a higher incidence of TVR (50.8% vs. 22%; P = 0.03) was observed.
DISCUSSIONThe main finding of the present study was the high occurrence of MACE at late follow-up, regardless of the location of the lesion, with greater occurrence of TVR in ostial lesions. These findings should be interpreted in the context of this population, which is representative of the Brazilian reality and of the scenario often encountered in PCI-Saph.13−15 This is a high risk population for new cardiovascular events; over 60% of patients had acute coronary syndrome, approximately 40% were diabetics and over one-third of patients had heart failure and chronic renal failure. Furthermore, the rate of use of distal embolic protection devices was low, and conventional stents were used in over 80% of patients, because most of these procedures are linked to the Brazilian Unified Health System (Sistema Único de Saúde - SUS).
Some studies have demonstrated that lesions of the saphenous graft body are more strongly associated with post-procedural complications and with a worse early clinical outcome, due to distal embolization of atherothrombotic debris into the native coronary circulation, often resulting in an increase of markers of myocardial necrosis or in a reduction of the antegrade flow (no-reflow phenomenon).1 Analyzing only the subgroup of patients with stable coronary artery disease (n = 66), a higher, although not statistically significant incidence of periprocedural myocardial infarction was noted in Body group vs. the Ostium group (18.1% vs. 27.2% P = 0.42), possibly due to the small number of patients with stable coronary disease.
Hong et al.8 published a study that evaluated, through intracoronary ultrasound, the characteristics of aorto-ostial and body of saphenous graft lesions, in addition to clinical evolution, showing a higher incidence of death and myocardial infarction for the body lesions and similar rates of TVR between groups. Unlike the present study, in the trial by Hong et al., approximately 80% of patients were approached with DES, and only 10% to 15% of these patients had a diagnosis of AMI, indicating a low-risk population.
Toutouzas et al.5 demonstrated that aorto-ostial lesions of native coronary artery or of saphenous grafts are related to the high incidence of restenosis, showing no benefit of the conventional stent coated with politetrafluoroethylene (PTFE). Traditionally, the presence of an aorto-ostial stenosis puts the interventionist into a difficult situation, since these lesions are most often associated with suboptimal angiographic results due to the greater resistance of the lesion to dilation, the presence of calcification, and the increased elastic recoil of the vessel.9 Iakovou et al.4 published clinical and angiographic results demonstrating that, when compared with conventional stents in aorto-ostial lesions, the implantation of DES is safe and effective, with a reduction in restenosis rates.
Caños et al.16 published data suggesting that grafts with early failure (one year after surgery) had more aggressive and diffuse degenerative/atherosclerotic disease, and the culprit lesions were located predominantly in the aorto-ostial or proximal portion (62% vs. 42%, respectively). Ellis et al.17 demonstrated that the disease in saphenous vein grafts has a rapidly progressive character, resulting, in large part, from the evolution of the disease in untreated segments, and that the occurrence of TVR following the intervention in saphenous grafts after the first year may occur more commonly in untreated locations, rather than in the target lesion. Restenosis and progression of atherosclerotic disease are the causes of long-term failure of PCI-Saph,17,18 which could explain the unfavorable evolution in both groups and the higher incidence of TVR in the Ostium group.
Limitations of the studyThe main limitations of this study were its retrospective and observational character, with limitations inherent to this type of research; the small number of patients treated with DES; and the low rate of use of distal embolic protection devices.
Nevertheless, it was possible demonstrate the difficulties inherent to PCI-Saph, especially regarding the high incidence of adverse events at the late follow-up of this Brazilian population in clinical practice. TVR rates in aorto-ostial lesions showed significant differences when compared to lesions in the body of the saphenous graft. Specific studies are needed to clarify the best conduct and follow-up strategy for patients undergoing PCI-Saph, particularly in aorto-ostial lesions.
CONCLUSIONSPatients with previous CABG requiring percutaneous coronary interventions in saphenous vein grafts comprise a subgroup of high risk, with high late mortality, regardless of lesion location in the graft. In patients predominantly treated with conventional stents (i.e., non-DES), aorto-ostial lesions had higher re-intervention rates versus lesions in the body of saphenous grafts.
CONFLICTS OF INTERESTThe authors declare no conflicts of interest.