Preliminary data have shown the Firebird™ and the Cypher® stents have similar safety and efficacy profiles. However, to date, no study has evaluated the percutaneous coronary intervention (PCI) with the Firebird™ stent in diabetic patients.
METHODSThe performance of the Firebird™ stent in diabetic patients with multivessel coronary artery disease (CAD) (n=100) was compared to that of the Cypher® stent, using historical data from the ARTS-II study (n=159). We compared the major adverse cardiovascular events (MACE) at one year.
RESULTSMost of the patients in the Firebird™ group were male (65%), with mean age of 63.3±10.4years and 5% were receiving insulin. Stable coronary syndromes were prevalent (60%), 45% had three-vessel CAD and ventricular function was preserved (56.6±13.7%). In patients with three-vessel CAD, 135 lesions were treated with≥3 stents in 78% of the cases and 2 stents in the remaining ones. In patients with two-vessel CAD, 110 lesions were treated with≥2 stents in 80% of the cases and 1 stent in the remaining ones. The incidence of MACE at one year of the Firebird™ stent was 21%, death was observed in 3% of the patients, myocardial infarction in 2% and a new revascularization procedure in 18%, predominantly at the expense of a new PCI in 14% of the cases. Comparison with the Cypher® group did not show differences for any of the evaluated endpoints.
CONCLUSIONSIn our study, the use of the Firebird™ stent showed similar results to those of patients in the ARTS-II study, which makes it attractive for use in the complex scenario of diabetic patients with multivessel CAD.
Desempenho do Stent Farmacológico Firebird™ em DiabéticosPortadores de Doença Coronária Multiarterial
IntroduçãoDados preliminares têm demonstrado que o perfil de segurança e eficácia do stent Firebird™ é semelhante ao do stent Cypher®. No entanto, até o presente momento, nenhum estudo avaliou a intervenção coronária percutânea (ICP) com o Firebird™ em diabéticos.
MétodosComparamos, em diabéticos portadores de doença arterial coronária (DAC) multiarterial, o desempenho do Firebird™ (n = 100) ao do Cypher®, utilizando os dados históricos do estudo ARTS-II (n = 159). Foram comparados os eventos cardiovasculares adversos maiores (ECAM) em um ano.
ResultadosA maioria dos pacientes do grupo Firebird™ era do sexo masculino (65%), com média de idade de 63,3 ± 10,4 anos e 5% estavam em uso de insulina. Predominaram os quadros clínicos estáveis (60%), 45% eram portadores de DAC triarterial e a função ventricular era preservada (56,6 ± 13,7%). Nos pacientes com DAC triarterial, foram tratadas 135 lesões, com 3 ou mais stents em 78% dos casos e 2 stents nos demais. Nos pacientes com DAC biarterial, foram tratadas 110 lesões com 2 ou mais stents em 80% dos casos e 1 stent nos demais. A incidência de ECAM em um ano do Firebird™ foi de 21%, óbito ocorreu em 3% dos pacientes, infarto do miocárdio em 2%, e novo procedimento de revascularização miocárdica em 18%, predominantemente à custa de nova ICP em 14% dos casos. A comparação com o grupo Cypher® não mostrou diferenças para nenhum dos desfechos avaliados.
ConclusõesEm nosso estudo, o uso do stent Firebird™ apresentou resultados similares aos dos pacientes do estudo ARTS-II, o que o torna atrativo para ser utilizado no complexo cenário de pacientes diabéticos portadores de DAC multiarterial.
The efficacy of drug-eluting stents in reducing the need for target-lesion revascularisation and adverse cardiovascular events compared with baremetal stents, especially in diabetic patients, has been established.1–3
The Firebird™ drug-eluting stent (Microport Co., Ltd. – Qinghai, China) has been approved for clinical use in China since 2005, and 350,000 units have been used worldwide.4 This stent has a thin stainless steel strut platform with an ethylene vinyl acetate copolymer coating that releases sirolimus. Preliminary data have demonstrated safety and efficacy profiles similar to the first-generation sirolimus-eluting stent Cypher® (Cordis Cardiology – Miami Lakes, USA).5 However, to date, no study has evaluated the performance of the Firebird™ stent in diabetic patients.
The objective of the present study was to evaluate the performance of the Firebird™ stent in patients with diabetes mellitus and multi-vessel coronary artery disease (CAD), and to compare it with the Cypher® stent using the historic diabetic data of the Arterial Revascularisation Therapies Study (ARTS-II).6
METHODSPatientsFrom January of 2006 to December of 2010, 354 patients were treated in this service with the Firebird™ stent, 100 (28.2%) of whom had diabetes mellitus and multi-vessel coronary artery disease and were selected for this analysis. Patients whose initial presentation included ST-elevation myocardial infarction or percutaneous coronary intervention (PCI) in saphenous grafts were excluded. This group was compared with the historic data of 159 diabetic patients with multivessel disease from the ARTS-II study who were treated with the Cypher® stent.
The current study followed the Helsinki Declaration regarding human studies, and all patients signed an informed consent.
The stentThe drug-eluting Firebird™ stent combines a fine (0.0040 inches) stainless steel (316L) strut platform with open cells and an N connection in a design that incorporates larger and smaller waves and an expansible balloon. It is coated with an ethylene vinyl acetate copolymer matrix that releases a dose of 9μg/mm2 of sirolimus.
Percutaneous coronary interventionPercutaneous coronary interventions were performed according to current guidelines,7 and the final procedure strategy was at the discretion of the surgeon.
During the procedure, 70IU/kg to 100IU/kg of non-fractionated heparin were administered, and the use of glycoprotein IIb/IIIa inhibitors was left to the discretion of the surgeon. Pre-dilation was not mandatory, and post-dilation was recommended in the case of residual stenosis>20% by a visual estimate.
The dual anti-platelet therapy consisted of acetylsalicylic acid (100g/day) and clopidogrel (75mg/day). Clopidogrel should have been started at least 24 hours before the procedure, at a loading dose of 300mg (or 600mg if PCI<24 hours). After the PCI, therapy with acetylsalicylic acid was continued indefinitely, with clopidogrel therapy for at least 12 months.
Outcomes and clinical follow-upThe primary outcome of the study was the development of major adverse cardiac events (MACE) during the 12-month follow-up. MACE was defined as death, non-fatal acute myocardial infarction, or the need for new revascularisation. All deaths were considered to be cardiac in origin unless a non-cardiac cause could be clearly established by clinical and/or pathological studies. The diagnosis of acute myocardial infarction was based on the development of new pathologic Q waves in more than two contiguous electrocardiographic leads and/or elevation of the isoenzyme CK-MB over three times the upper normal limit after the procedure during the index hospitalisation, or over two times the upper normal limit after hospital discharge. All revascularisations, surgical or percutaneous, were considered.
A clinical follow-up was performed 12 months after the procedure, consisting of a phone contact according to a predefined institutional protocol.
Statistical analysisCategorical variables were described as percentages and were compared using the chi-squared test or Fisher exact test. Continuous variables were described as means and standard deviations and were compared using the Student’s test. One-year clinical events were compared with the diabetic subgroup of the ARTS-II study. A value of P<0.05 was considered statistically significant.
RESULTSIn this study, 100 patients treated with the Firebird™ stent were compared with 159 diabetic patients with multivessel disease from the ARTS-II study treated with the Cypher® stent.
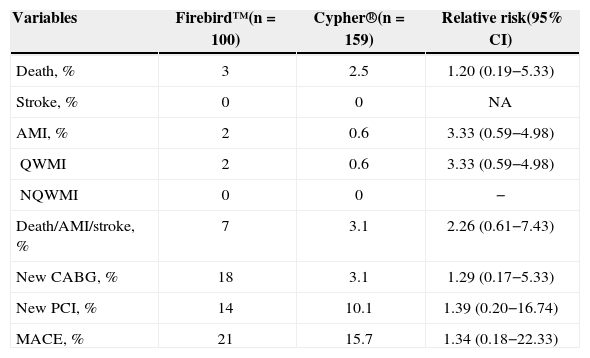
Clinical and anatomical characteristics of the patients are summarised in Table 1, and no significant differences were observed. Most patients in the Firebird™ group were male (65%), with a mean age of 63.3±10.4years, and 5% were on insulin. On clinical presentation, stable patients predominated (60%); 45% had three-vessel disease and 55% presented two-vessel disease, and ventricular function was preserved (56.6±13.7%). In patients with three-vessel disease, 135 lesions were treated with three or more stents in 78% of the cases and with two stents in the remainder (22%). In patients with two-vessel disease, 110 lesions were treated with two or more stents in 80% of cases and with one stent in the remaining 20%. The one-year incidence of MACE in the Firebird™ group was 21%: 3% mortality 2% acute myocardial infarction, and new myocardial revascularisation was performed in 18% predominantly through a new PCI in 14% of cases. In this group, stroke was not observed. Compared with the Cypher® group, significant differences in the outcomes were not observed (Table 2).
Clinical and Angiographic Characteristics
| Firebird™(n=100) | Cypher®(n=159) | P | |
|---|---|---|---|
| Age, mean±standard deviation | 63.3±10.4 | 64.5±8.7 | 0.61 |
| Male gender, % | 65 | 66.7 | 0.56 |
| Body mass index, kg/m2 | 27±4.3 | 28.8±4.5 | 0.11 |
| Current smoking, % | 15 | 11.9 | 0.93 |
| Hypertension, % | 72 | 79.9 | 0.77 |
| Dyslipidaemia, % | 75 | 74.1 | 0.88 |
| Diabetes on insulin, % | 5 | 4.6 | 0.76 |
| Prior AMI, % | 31 | 29.6 | 0.88 |
| Prior angioplasty, % | 2 | 0 | 0.86 |
| Prior surgery, % | 2 | 1.9 | 0.81 |
| Clinical presentation, % | 0.17 | ||
| Stable angina | 60 | 53.5 | |
| Unstable angina | 30 | 32.1 | |
| Silent ischaemia | 14 | 14.5 | |
| Ejection fraction, % | 56.6±13.7 | 59.5±11.8 | 0.66 |
| Lesion type (AHA/ACC), % | 0.22 | ||
| A/B1 | 30 | 27 | |
| B2/C | 70 | 73 | |
| Number of vessels, % | 0.88 | ||
| One-vessel | 0 | 0.6 | |
| Two-vessel | 55 | 49.1 | |
| Three-vessel | 45 | 50.3 |
AHA/ACC=American Heart Association/American College of Cardiology; AMI=acute myocardial infarction; n=number of patients.
One-year Major Adverse Cardiovascular Events
| Variables | Firebird™(n=100) | Cypher®(n=159) | Relative risk(95% CI) |
|---|---|---|---|
| Death, % | 3 | 2.5 | 1.20 (0.19−5.33) |
| Stroke, % | 0 | 0 | NA |
| AMI, % | 2 | 0.6 | 3.33 (0.59−4.98) |
| QWMI | 2 | 0.6 | 3.33 (0.59−4.98) |
| NQWMI | 0 | 0 | − |
| Death/AMI/stroke, % | 7 | 3.1 | 2.26 (0.61−7.43) |
| New CABG, % | 18 | 3.1 | 1.29 (0.17−5.33) |
| New PCI, % | 14 | 10.1 | 1.39 (0.20−16.74) |
| MACE, % | 21 | 15.7 | 1.34 (0.18−22.33) |
AMI=acute myocardial infarction; 95% CI=95% confidence interval; MACE=major adverse cardiovascular events; CABG=coronary artery bypass graft; NQWMI=non-Q wave myocardial infarction; PCI=percutaneous coronary intervention; QWMI=Q-wave myocardial infarction; NA=non-applicable.
In the present study, it was demonstrated that the one-year clinical results of the Firebird™ stent in a high complexity clinical and anatomical scenario, i.e., in patients with diabetes mellitus and multivessel coronary artery disease, were similar to the results of the historic data of the subgroup of diabetic patients with multivessel coronary artery disease from the ARTS-II study treated with the Cypher® stent.
Drug-eluting stents significantly reduce late luminal loss and angiographic restenosis as well as the need for new revascularisation, when compared with bare-metal stents. Specifically, the Cypher® stent has demonstrated its efficacy in diabetic patients in randomised clinical studies and large registries.1,8–11
Its safety was also demonstrated in several studies, including the e-Cypher registry in which 15,157 patients (18,295 lesions) treated with the sirolimus-eluting stent had acute, subacute, and late thrombosis rates of 0.13%, 0.56%, and 0.19%, respectively, for a total of 0.88% over 12 months. Despite the low thrombosis rates of the Cypher® stent, diabetes treated with insulin and the treatment of multiple lesions were independent predictors of one-year stent thrombosis, along with acute coronary syndrome, advanced age, post-procedure TIMI flow<3, and very calcified or occluded lesions.12
The very few studies that have evaluated the Firebird™ stent presented favourable clinical results in patients representing the daily clinical practice. The prospective and multicentre FIREMAN Registry included 1,029 consecutive patients with complex lesions, and evaluated their clinical evolution over one year. The most commonly observed angiographic characteristics included long lesions (59.2%), multivessel disease (50.4%), and small-calibre vessels (31.6%). Major adverse cardiovascular events were observed in 5.1% of patients, including cardiac death in 0.6%, non-fatal acute myocardial infarction in 1.1%, and target-lesion revascularisation in 3.5%. Definitive or probable stent thrombosis affected 1.36% of patients. Diabetes, small-calibre coronary arteries, and chronic occlusions were predictors of stent thrombosis.13
Zhang et al.14 investigated the clinical efficacy and safety of the Firebird™ stent over a longer follow-up period. This study included 509 consecutive patients, 65.4% of whom were treated for off-label indications. The three-year MACE-free survival was 92.1%. In diabetic patients, the MACE rates (13.7% vs. 6.4%; P<0.05) and need for new revascularisation (9.8% vs. 4%; P<0.05) were higher, and the MACE-free survival (86.4% vs. 93.6%; P<0.05) was lower at the end of this period.
Recently, Xu et al. 5 performed the only comparison available between the Firebird™ and Cypher® stents. In their study, 3,979 consecutive patients were evaluated; 2,274 treated with the Firebird™ stent and 1,705 treated with the Cypher® stent, followed-up for 2 years. The risk ratios for MACE (1.27; 95% confidence interval [95% CI] 0.90–1.79), mortality (1.22; 95% CI 0.57–2.60), myocardial infarction (1.38; 95% CI 0.61–2.40), target-lesion revascularisation (1.10; 95% CI 0.65–1.80), and definitive or probable stent thrombosis (1.22; 95% CI 0.57–2.60) did not show significant differences after adjustment for 30 variables between the groups.
A new version of this stent, the Firebird 2™, has had structural modifications and now has a new cobalt-chromium platform with a smaller profile, with cell connections in S, reducing the shortening of this stent; at the same time, its design, with sinuous and uniform waves, combines radial strength and flexibility. It also has an expandable balloon, and its new polymer is more biocompatible and releases sirolimus more rapidly (90% within one month). The Firebird 2™ cObalt-Chromium alloy sirolimus-elUting Stent (FOCUS) registry15 recruited 5,084 patients from 83 centres who used the Firebird 2™ stent and demonstrated, in a six-month follow-up of 99.5% of patients, that the development of MACE was 1.8%, with death in 0.6%, myocardial infarction in 0.95%, and the need for new revascularisation in 0.3%.
The incidence of stent thrombosis was 0.43% (22/5,058), including eight cases of acute, 11 cases of subacute, and three cases of late thrombosis, confirming the six-month efficacy and safety in patients in daily practice.
The present findings in diabetic patients with multivessel disease, along with those of other studies that demonstrated satisfactory results in the in-stent restenosis subgroup16 and in patients who represent the national clinical practice, expand the knowledge regarding the Firebird™ stent.17,18
Study limitationsThis study has several limitations: 1) it was a retrospective, single centre study; 2) it had a small study population; and 3) patients in the Firebird™ group were evaluated predominantly using the clinical information obtained by the team or by telephone contact with their physicians.
CONCLUSIONSThe present study suggests that the Firebird™ stent has a safety and efficacy profile similar to that of the Cypher® stent, and that the Firebird™ stent is an option for use in the treatment of the complex scenario of diabetic patients with multivessel disease.
CONFLICTS OF INTERESTThe authors declare no conflicts of interest.