Coronary computed tomography angiography (CCTA) has evolved to be a flexible and cost-effective technique to classify the potential risk of cardiopulmonary pathologies presenting as acute chest pain (ACP) in the emergency department (ER). Mainly, CCTA has become a safe strategy for the diagnosis and stratification in ACP patients with low to intermediate likelihood of having acute coronary syndrome (ACS) and coronary artery disease (CAD). In fact, CCTA shows several advantages tested in prospective clinical trials including reduction in the time to diagnose CAD, increase in patient discharge from the ER, lower cost and reduction in hospital stay compared with standard of care due to rapid acquisition and very high accuracy (negative predictive value: 85-99% and sensitivity: 85-99%). In this review, we will focus on the early use of CCTA in the ER as a fundamental tool for risk stratification, prognosis and safety for discharge in patients with symptoms suggestive of ACS. This article includes CCTA indications, protocols and CAD characterization of this imaging technique over the last twenty years and future directions.
La angiotomografía computarizada coronaria ha evolucionado convirtiéndose en una técnica costo-efectiva y flexible que clasifica el riesgo potencial de enfermedades cardiopulmonares que acaecen con dolor torácico agudo en urgencias. Específicamente, es una estrategia segura y efectiva para el diagnóstico y la estratificación de pacientes con dolor torácico agudo, con probabilidad baja o intermedia de sufrir síndrome coronario agudo o enfermedad arterial coronaria. Igualmente, tiene diferentes ventajas publicadas en estudios clínicos prospectivos como por ejemplo, el diagnóstico oportuno de enfermedad arterial coronaria, el aumento de egresos en el departamento de urgencias y la disminución de costos en salud y en la estancia hospitalaria debido a su alto valor predictivo negativo (VPN=85%-99%) y sensibilidad (S=85%-99%). En esta revisión se hará énfasis en la importancia de su uso temprano para la estratificación del riesgo, el pronóstico y la seguridad en el egreso de los pacientes con síntomas sugestivos de síndrome coronario agudo. Este artículo incluye los protocolos, las indicaciones y la caracterización de esta técnica imagenológica en los últimos veinte años y en expectativas para el futuro.
Cardiovascular diseases (CVDs) are the first cause of mortality worldwide and were responsible for 17.7 million deaths in 2015.1–6 Within the CVD spectrum, CAD contributed to 41% (approximately 7.4 million deaths) of the overall mortality.6 Unfortunately, 3/4 of the cardiovascular deaths belong to low and middle-income countries.7 In fact, Colombia had a myocardial infarction incidence of 280 per 100,000 inhabitants in 2014, while United States (US) had 1122 CAD hospital admissions per 100,000 inhabitants in 2011.7,8
Also, Colombia had 32,338 CVD's deaths in 2014 compared to US mortality, where these conditions were responsible during 2014 for 360,000 deaths (1 in 7 deaths in US), with an overall prevalence of 6.3% and one new ACS every 40seconds.7,8 Furthermore, in the United States, the overall CAD-related cost was10.4 billion USD between 2012-2013.8 Due to its extremely high prevalence and incidence in addition to very high social financial burden, the medical field needs better and faster mechanisms to further stratify and diagnose CAD in ACP patients.
CCTA technical descriptionSince a decade ago, a 64-slice CCTA system is used for the visualization of coronary arteries to detect obstructive CAD.9 The first generation of 64-slice systems had temporal resolutions of 210 milliseconds (ms) and relative high radiation doses ranging from 5-15 mSv (milli-sieverts).9 Over time, second and third generation CCTA were developed with temporal resolutions of 76 and 66ms respectively, with coverage up to 16cm and high-pitch spiral acquisition.9,10 Currently, some CCTA protocols are based on dual-source systems with automatic correction algorithms increasing CCTA image quality.11 The ultimate CCTA has 256/320 multi-slice systems allow significant reductions in radiation exposure (down to 1 mSv or less) with the additional benefit of lower presence of artifacts.9,10,12 The closer future will be the multi-energy CCTA, which will bring multiple improvements in atherosclerosis CCTA morphology and myocardial perfusion.12
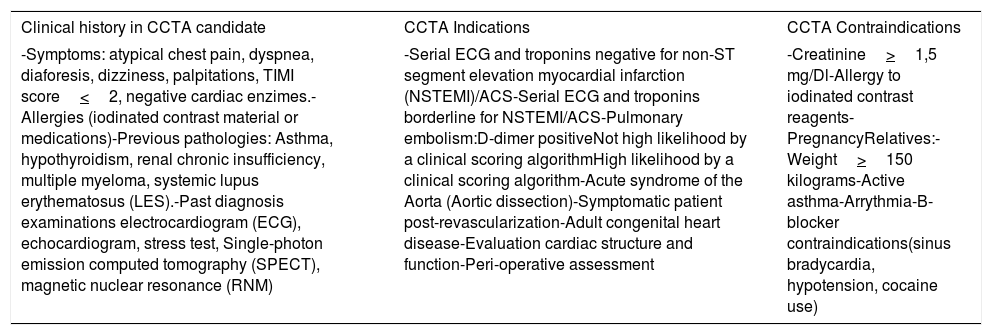
A key factor for optimal imaging quality is the adequate patient preparation in terms of apnea training and low heart rates to optimize image quality. Also, like any other non-invasive technique, the pre-scanning preparation must include sufficient clinical information, indications and contraindications for CCTA (Table 1).9,13 Optimizing these variables result in better image quality and higher accuracy.
Pre-CCTA aspects for consideration*
| Clinical history in CCTA candidate | CCTA Indications | CCTA Contraindications |
|---|---|---|
| -Symptoms: atypical chest pain, dyspnea, diaforesis, dizziness, palpitations, TIMI score<2, negative cardiac enzimes.-Allergies (iodinated contrast material or medications)-Previous pathologies: Asthma, hypothyroidism, renal chronic insufficiency, multiple myeloma, systemic lupus erythematosus (LES).-Past diagnosis examinations electrocardiogram (ECG), echocardiogram, stress test, Single-photon emission computed tomography (SPECT), magnetic nuclear resonance (RNM) | -Serial ECG and troponins negative for non-ST segment elevation myocardial infarction (NSTEMI)/ACS-Serial ECG and troponins borderline for NSTEMI/ACS-Pulmonary embolism:D-dimer positiveNot high likelihood by a clinical scoring algorithmHigh likelihood by a clinical scoring algorithm-Acute syndrome of the Aorta (Aortic dissection)-Symptomatic patient post-revascularization-Adult congenital heart disease-Evaluation cardiac structure and function-Peri-operative assessment | -Creatinine>1,5 mg/Dl-Allergy to iodinated contrast reagents-PregnancyRelatives:-Weight>150 kilograms-Active asthma-Arrythmia-B-blocker contraindications(sinus bradycardia, hypotension, cocaine use) |
In 2014, 4,703,012 ER visits were attributed to ACP in the United States.15 These patients are routinely evaluated using clinical history, physical examination, ECG, chest X-ray and traditional cardiac biomarkers,14 which allow physicians to have an initial approach of admission vs. outpatient work-up depending of their probability for having CAD. From those patients who are discharged, 2-3% have eventually ACS, which potentially represents fatal outcomes and is well known for justifying high number of malpractice inquiries.16 Conversely, 2-8% of the initial ACP diagnoses are eventually identified to be secondary to extra-cardiac pathologies.16 CCTA has emerged as an innovative cost-effective method to avoid missing CAD and ACS in the ER.
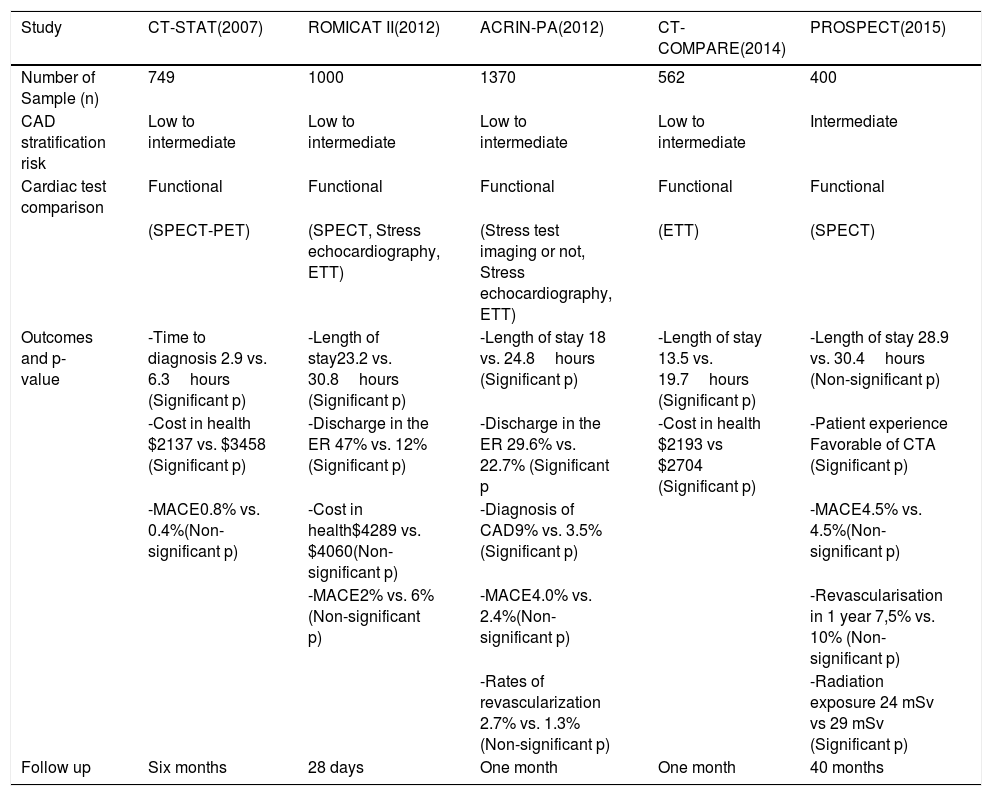
CCTA in the ER: Clinical evidenceSeveral prospective randomized trials with almost 4000 patients have provided relevant information about the utility of CCTA in the ER. These clinical trials demonstrated that patients with ACP who were stratified with CCTA show less length of stay, more ER discharges, increase CAD diagnosis in less time and more cardiac interventions and revascularization procedures in those with abnormal studies.2–5,17,18 However, some variables such as major adverse cardiovascular events (MACE) and the cost of care showed variable results between studies.12 Studies such as ROMICAT II, C-STAT, ACRIN-PA and PROSPECT demonstrated the mentioned benefits in terms of rapid diagnosis and early discharge although there is paucity of data regarding prognosis in the long term for patients with normal CCTA's.3–5,17 However, Schlett et al showed that patients with no evidence of CAD and absence of regional wall motion abnormalities, have excellent prognosis in the first 24 months after a normal CCTA.19Table 2 summarizes the key features and differences of CCTA trials.
Comparison between CCTA clinical trials in the ER*
| Study | CT-STAT(2007) | ROMICAT II(2012) | ACRIN-PA(2012) | CT-COMPARE(2014) | PROSPECT(2015) |
|---|---|---|---|---|---|
| Number of Sample (n) | 749 | 1000 | 1370 | 562 | 400 |
| CAD stratification risk | Low to intermediate | Low to intermediate | Low to intermediate | Low to intermediate | Intermediate |
| Cardiac test comparison | Functional | Functional | Functional | Functional | Functional |
| (SPECT-PET) | (SPECT, Stress echocardiography, ETT) | (Stress test imaging or not, Stress echocardiography, ETT) | (ETT) | (SPECT) | |
| Outcomes and p-value | -Time to diagnosis 2.9 vs. 6.3hours (Significant p) | -Length of stay23.2 vs. 30.8hours (Significant p) | -Length of stay 18 vs. 24.8hours (Significant p) | -Length of stay 13.5 vs. 19.7hours (Significant p) | -Length of stay 28.9 vs. 30.4hours (Non-significant p) |
| -Cost in health $2137 vs. $3458 (Significant p) | -Discharge in the ER 47% vs. 12% (Significant p) | -Discharge in the ER 29.6% vs. 22.7% (Significant p | -Cost in health $2193 vs $2704 (Significant p) | -Patient experience Favorable of CTA (Significant p) | |
| -MACE0.8% vs. 0.4%(Non-significant p) | -Cost in health$4289 vs. $4060(Non-significant p) | -Diagnosis of CAD9% vs. 3.5%(Significant p) | -MACE4.5% vs. 4.5%(Non-significant p) | ||
| -MACE2% vs. 6%(Non-significant p) | -MACE4.0% vs. 2.4%(Non-significant p) | -Revascularisation in 1 year 7,5% vs. 10% (Non-significant p) | |||
| -Rates of revascularization 2.7% vs. 1.3% (Non-significant p) | -Radiation exposure 24 mSv vs 29 mSv (Significant p) | ||||
| Follow up | Six months | 28 days | One month | One month | 40 months |
Own table based on 3,17,18 CT-STAT: Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment, ROMICAT: Rule Out Myocardial, Infarction Using Computer Assisted Tomography. ACRIN-PA: American College of Radiology Imaging Network and Pennsylvania Department of Health, CT-COMPARE: CT Coronary Angiography Compared to Exercise ECG, PROSPECT: Predictors of Response to CRT. MACE: Major adverse cardiovascular events, Exercise treadmill testing (ETT), Single-photon-emission computed tomography (SPECT)
For instance, the CT-STAT study published in 2007, compared CCTA vs. rest-stress myocardial perfusion –SPECT- in patients with low-intermediate risk in the ER and concluded CCTA use reduced by 58% the time to diagnosis (p=0.0001) and health expenses by 38% (p=0.0001).4 Also, ROMICAT II study recruited 1000 ACS patients with similar risk to determine safe and cost-effectiveness of CCTA in the ER. This study showed 35% more ER discharge (p <0.001) and shorter time for hospitalization (p<0.001) compared to standard of care stratification strategies.3 ACRIN-PA and CT-COMPARE concluded similar findings including faster discharge in the ER, decrease hospital stay (p<0.001) and additional findings like more CAD diagnosis and revascularizations in those with abnormal CCTA's.5,18
Finally, the PROSPECT trial had some atypical demographic features including patients with low socio-economic status, high number of females, ethnic minorities and patients with high prevalence of obesity. This study demonstrated less radiation exposure and better patient satisfaction with CCTA compared to SPECT17 although, there were no differences regarding to outcomes or health care cost.17
This compelling amount of data based on multiple, well-designed and executed randomized controlled trials resulted in the AHA/ACR guidelines recommending CCTA as an appropriate diagnosis tool for ACP in patients with low-intermediate risk.14
Another research study that supports CCTA use in CAD diagnosis is the CONFIRM study (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry).20 It has a sample of 23,854 patients –52% were ACP patients –and it demonstrated that CCTA had a high diagnostic performance for detection and exclusion of obstructive CAD defined as none (0%stenosis), mild (1% to 49% stenosis), moderate (50% to 69% stenosis), or severe (70% stenosis).20 Absence of CAD by CCTA was associated with a low rate of incident death and there is a differentiation in prognosis according with age or gender.20 Although patients older than 65 years had increased all-cause mortality, patients younger than 65 years had a higher mortality risk when they were diagnosed with two, three-vessels CAD or left main (LMCA) disease in comparison with non-obstructive or one-vessel CAD.20 When stratifying for gender, women had worse prognosis with higher mortality risk than males in those with significant 3-vessel or LMCA disease. Similar rates of death for non-obstructive 1-vessel and 2-vessel obstructive CAD where noticed.20
A controversial large non-randomized, administrative, retrospective analysis of 2,047,799 ACP patients presenting to the ER between 2006 to 2013 across US who were stratified with CCTA suggested the former strategy increased care cost, number of hospitalizations, prompted higher rates of PCI, coronary artery bypass grafting (CABG), repeated noninvasive testing and return ER in comparison with cardiac stress test.21 This has not been validated in rigorous prospective randomized controlled trials. Of notice, the study is based on a retrospective non-medical, administrative registry, with all the known intrinsic limitations to this type of study.
More recently, the Better Evaluation of Acute Chest Pain with Computed Tomography Angiography (BEACON) study, a prospective-randomized don in the Netherlands,22 recruited 500 patients with ACP randomizing them to CCTA vs. standard care with high sensitive (hs) troponins in a 30 days follow-up period. There was no significant difference between ER discharge, coronary revascularization within 30 days, length of stay or incidence in undetected ACP between the two arms in the early publication of results.22
Finally, the Rapid Assessment of Potential Ischemic Heart Disease with CCTA (RAPID-CTCA), is an ongoing randomized multi-center trial comparing early CCTA vs. standard care in high-risk ACP patients. This trial will provide pertinent information regarding additional uses of CCTA in the ER in a population that has not been tested in the past.23
CCTA Clinical applications in the ERCAD plaque morphologyCCTA allows thorough evaluation of atherosclerotic lesions with different accuracy depending on the type of plaque: non-calcified (Sensitivity 53% and Specificity 57.9%), calcified (Sensitivity 94%) and mixed plaques (Sensitivity 78% and Specificity 72%).13,24 Also, CCTA can identify the plaque attenuation pattern and divide it as homogeneous, heterogeneous and non-napkin-ring sign plaques.24
Coronary stentsTo evaluate coronary in-stent restenosis or occlusion, CCTA use may be challenging due to artifacts within the stent depending on its size.25 Therefore, Yoshioka et al. suggested a subtraction-CCTA technique using 3D datasets acquired before and after the contrast medium reaches the target coronary artery.26 The subtraction-CCTA was used to assess calcified plaques.27 As a result, in-stent restenosis was observed by subtraction CCTA in 398 patients noticing different accuracy of restenosis with conventional CCTA (62.7%) compared to subtraction CCTA (89.5%).25 Its important to note that ideal stent diameter for proper CCTA evaluation is 3mm, although some studies show 2.5-2.7mm as stent diameter cut-off value for optimal accuracy rates.28–30
Coronary artery bypass grafts (CABG)The assessment of grafts patency in CABG patients by CCTA has an elevated rate of sensitivity 97.6%, specificity (96.7%), positive (92.7%) and negative (98.9%) predictive values.31 Some of the advantages of CCTA in coronary grafts are that it has similar accuracy measurements for venous and arterial grafts and their relatively large diameter avoids CCTA artifacts seen in patients with prior stent implantation.31
Coronary anomaliesPrevalence of coronary anomalies has been reported to be present in up to 2% of the population depending in the geographical area. For instance, Korea has a prevalence 1.1% prevalence of this entity versus 1.3%-1.7% in US.32–34 The main congenital abnormalities of the coronary arteries are classified as anomalies of origin (number of coronary ostia, high take-off, ectopic coronary origin), and coronary artery fistula.35 Most of the anomalies in the coronary arteries are benign. However, some of them are associated with myocardial ischemia and sudden cardiac death, particularly the LMCA arising from the right coronary sinus with slit-like morphology, acute take-off angle and inter-arterial course –between the ascending aorta and the pulmonary artery.35 CCTA has demonstrated to be an extremely effective imaging modality to identify these anomalies in comparison with invasive coronary angiography (ICA) due to its unique capability of tomographic reconstruction. A study with a sample of 8864 subjects found the most common anomaly is the right coronary artery (RCA) originating from the left sinus of Valsalva (LSV) (39.8%) followed by absence of the (LMCA) (9.7%)32 with independent left anterior descending artery (LAD) and left circumflex artery (LCx).32
Future considerations of the CCTAThe CCTA potential future has several promising goals and the need to overcome some technical limitations. For instance, the multi-energy CCTA is getting images by changing low or high energy to get better plaque characterization and attenuation.12 In fact, functional tests are currently merging with CCTA to improve anatomic details and functional findings. Hence, dynamic myocardial perfusion imaging consists in a full-volume coverage system (>256 detector rows) to estimate cardiac ischemia and flow-obstructing stenosis in symptomatic patients with suspected CAD36 in a simultaneous fashion. Although this approach, initially described by Blankstein et al.,37 has been described in patient with stable chest pain, no data has been validated prospectively in ACP.
Equally important, the fractional flow reserve by CCTA (FFR-CT) appears to be very promising for the computational fluid analysis in vessel segmentation.38 An observational study with 171 patients, excluding patients with ACP, used the FFRCT method to determine whether (FFRCT) predicts coronary revascularization and whether its addition improves efficiency of referral to ICA after CCTA. The results showed FFRCT ≤ 0.80 was a better predictor of revascularization or major adverse cardiac events than severe stenosis on CCTA.39
Finally, the last encouraging approach is dual energy CT (DE-CT) which potentially can distinguish elements with high atomic number (such as calcium responsible for significant beam artifact in patients with high calcium score during regular CCTA) and involves ECG-gating with low-energy imaging facilitating intraluminal enhancement and allowing reduction in the total amount of contrast media and identify ischemia or scars.12
ConclusionsIn summary, CCTA has become an essential and innovative tool for diagnosing CAD and determining prognosis in patients with low-intermediate pre-test probability presenting with ACP. During the last ten years multiple rigorously designed prospective randomized trials have shown the benefits of using CCTA in ACP including lower time to diagnosis, lower length of stay a dearly discharge from the ER. This has translated to incorporate CCTA into national and international guidelines to stratify individuals with low to intermediate risk of having ACS presenting with ACP and has resulted in significant reduction in hospital stay, discharge from the ER and time for CAD diagnosis compared to standard of care.
There has been significant development of last generation scanners allowing very high temporal resolution and coverage which provides full cardiac coverage during one beat has resulted in spectacular decrease in radiation doses achieving 1 mSv or less during non-invasive coronary angiography (similar to 2-3 chest-Xrays). This represents a major advance in a field, which was initially criticized due to its high exposure to radiation for patients.
Potential resources such as FFRCT, dual CT, multi-energy systems, myocardial perfusion protocols and 256/320-slice scans are currently improving further the image quality and versatility of this technique. Also, they can help overcome traditional CCTA challenges such as artifacts due to heavily calcified plaques, time of breath holding, anatomic details and visualization of the whole cardiac cycle during stress.12,40
Conflict of interestsNone.