SARS-CoV-2, along with SARS-CoV and MERS-CoV, forms part of the three highly pathogenic coronaviruses identified since the start of the millennium.1,2 While SARS-CoV was identified on 2003 and MERS-CoV on 2012, the initial reports of SARS-CoV-2 (the etiological agent of COVID-19) were first released at the end of December 2019.3,4 Now, after less than four months, the virus has distributed globally and has become the focus of extensive medical research, as the number of cases keeps rising Figure 1.
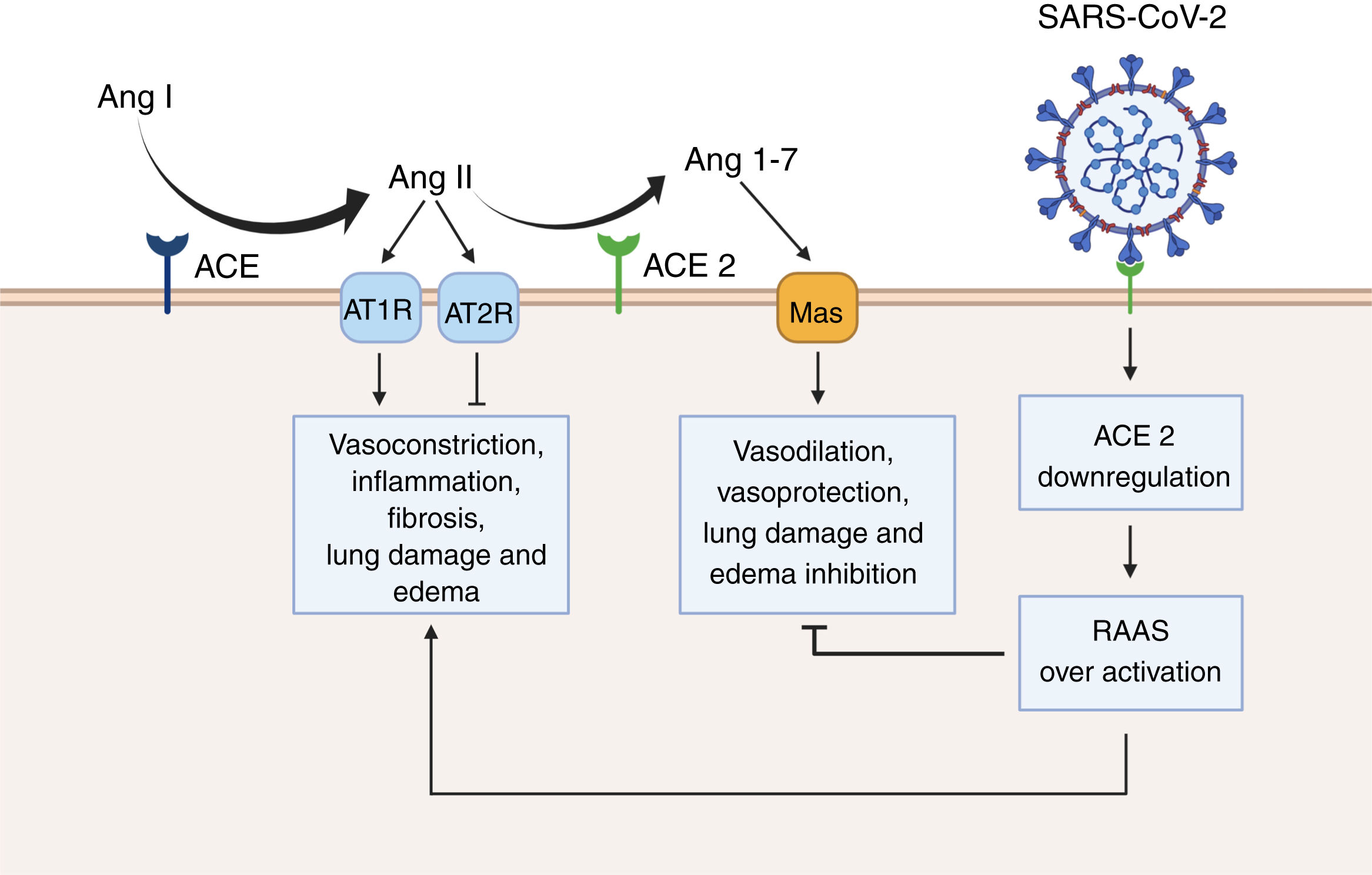
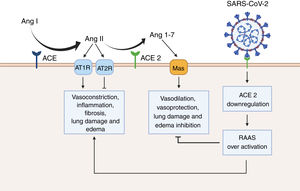
Renin angiotensin system (RAAS) overactivation as a result of SARS-CoV-2 infection. In physiological conditions, the angiotensin converting enzyme (ACE) metabolizes angiotensin I (Ang I) to angiotensin II (Ang II), thus leading to increased vasoconstriction, inflammation, fibrosis, lung damage and edema. Conversely, angiotensin converting enzyme 2 (ACE 2) inactivates Ang I by generating angiotensin 1-7 (Ang 1-7), which then interacts with the G-protein-coupled receptor Mas. This interaction is known to be vasoprotective, since it antagonizes the actions of Ang I. However, SARS-CoV-2 downregulates the expression of ACE2, thus leading to RAAS overactivation and to increased lung damage and edema. Created with BioRender.com.
A significant part of the investigative effort has been directed to the search for an effective therapy or intervention that could stop the spread of the disease or be used to effectively treat infected patients. Likewise, potential predisposing factors to develop a more severe clinical presentation are progressively being identified. Some of the more relevant are older age and the presence of certain comorbidities, such as cerebrovascular and coronary heart disease, hypertension and diabetes.5–8 It is important to highlight that the last two are chronic conditions commonly treated with ACE-inhibitors and angiotensin II type-I receptor blockers.9–11 However, the evidence suggests that these medications can upregulate the expression of angiotensin converting enzyme 2 (ACE2), the cellular receptor for both SARS-CoV and SARS-CoV-2.11–16 Thus, a group of researchers hypothesized that ACE2-increasing drugs could raise the risk of infection and prompt a more severe clinical course or a fatal outcome in diabetic and hypertensive patients.11
As a result, the safety of ACE-inhibitors and angiotensin receptor blockers in COVID-19 patients was put into question and an intense debate arose. Nonetheless, although the idea of the intensification of ACE2 receptor expression, leading to an increased risk of COVID-19 infection is reasonable, hypertension and diabetes are also frequent comorbidities of MERS-CoV.17 The latter is relevant since MERS-CoV uses a distinct host-cell receptor, namely the dipeptidyl peptidase 4 (DPP4), also known as CD26. Hence, it would seem that the relation between chronic conditions and SARS-CoV-2 could be explained by the general pathology of coronavirus infection itself rather than by the type of receptor the virus uses.
Furthermore, we suggest that SARS-CoV-2 infection should not just be considered as a single but as a dual phase phenomenon. Therefore, in the first phase of SARS-CoV-2 infection, expressing a higher number of ACE2 receptors could indeed promote viral entry.11 However, in a second or a later phase of the infection, ACE2 receptors may be protective of SARS-CoV-2 mediated acute lung injury.18,19 In fact, experimental evidence shows that SARS-CoV infection is paired with a decrease in the expression of ACE2.18–20 As a consequence, the physiological antagonism of ACE2 over the classical renin-angiotensin-aldosterone system (RAAS) is blunted. In normal circumstances, the vasoconstrictor peptide angiotensin II is metabolized by ACE2 to produce angiotensin 1-7 (Ang 1-7).18 Ang 1-7 is then able to interact with Mas, a G-protein-coupled receptor with known vasoprotective effects.18,19,21,22 Moreover, Ang 1-7 has been shown to reduce inflammation, tissue damage and lung edema in the set of acute respiratory distress syndrome.23 This suggests that along with the viral load and the host immune response to SARS-CoV-2, an over-active RAAS could contribute to the exacerbated lung injury generated by COVID-19. Thus, recombinant ACE2 may be an effective therapeutic approach to treat COVID-19 cases, as some studies already suggest.24 Additionally, cyclic Ang 1-7, which shows increased resistance to human peptidases, such as angiotensin-converting enzyme (ACE) and dipeptidyl peptidase 3 (DPP3), could counteract the downregulation of ACE2 caused by SARS-CoV-2 infection.25,26
Finally, it is important to mention that the protease (Ang 1-7 generation) and the SARS-CoV receptor functions of ACE2 are independent.18 In consequence, catalytically inactive ACE2 receptors can still interact with viral particles. Moreover, ACE2 is still active while the SARS spike proteins is bound.18,27 This is important, since it opens the possibility for the development of selective drugs that specifically target the ACE2 domain used by the SARS-CoV-2 to enter the host-cell without affecting the production of Ang 1-7. Nonetheless, intensive experimental and clinical research should validate the feasibility of this approach.
Conflicts of interestThe authors report no conflicts of interest.
FundingNone.
None.