Although the benefits of exercise and changes in lifestyle on components of the metabolic syndrome (MS) have been described, little is known about the effect of dancing and nutritional changes on the cardiovascular system.
ObjectiveEvaluate the effect of an intervention based on dancing and nutrition education on hemodynamic and autonomic status in adults with MS.
MethodsA randomized controlled clinical trial was conducted involving 59 adults with MS. The intervention lasted 12 weeks and consisted of an aerobic exercise program (dancing) at an intensity of 60-75% of heart rate reserve, 60minutes 3 times a week, and muscle strength training at an intensity of 50% of a maximum repetition, 30minutes twice a week. The nutrition education program consisted of 2-hour workshops each week. Assessment of impedance cardiography and function of nervous system with analysis of heart rate variability (HRV) were made before and after the intervention.
ResultsIn the intervention group, a decrease in mean arterial pressure of -7.8mmHg (95% CI, -12.84 to -2.75; P=0.004) was found as well as in the systemic vascular resistance (SVR) index of -864.29 dyn·s·m2/cm5 (95% CI, -1506.31 to -222.26; P=0.010). Increase was observed in the cardiac output index of 0.48 L/min/m2 (95% CI, 0.14 to 0.83; P=0.007). In the spectral analysis of HRV a reduction in LF/HF ratio of -0.52 (95% CI, -1.02 to -0.02; P=0.040) was also found.
ConclusionsAn intervention with dancing and nutrition education lowers arterial blood pressure and SVR and has favorable effects on the sympathovagal balance in patients with MS.
Aunque se describieron los beneficios del ejercicio y cambios en el estilo de vida sobre los componentes del síndrome metabólico (SM), poco se sabe del efecto del baile y la nutrición en el sistema cardiovascular.
ObjetivoEvaluar el efecto de una intervención de baile y educación nutricional sobre el estado hemodinámico y autonómico en adultos con SM.
Materiales y métodosEnsayo clínico controlado que incluyó 59 adultos con SM. La intervención duró 12 semanas y consistió en un programa de baile a una intensidad del 60 a 75% de la frecuencia cardiaca de reserva, 60 minutos, 3 veces a la semana, y entrenamiento de la fuerza a una intensidad del 50%, 30 minutos dos veces a la semana. El programa nutricional consistió en talleres de 2 horas cada semana. La cardiografía de impedancia y el análisis de la variabilidad de la frecuencia cardiaca (VFC) fueron realizados antes y después de la intervención.
ResultadosEl grupo de intervención disminuyó la presión arterial media en -7,8mmHg (IC 95%, -12,84 a -2,75; p=0,004) y el índice de resistencia vascular sistémica (RVS) en -864,29 dyn·s·m2/cm5 (IC 95%, -1506,31 a -222,26; p=0,010); y aumentó, el índice de gasto cardiaco en 0,48 L/min/m2 (IC 95%, 0,14 a 0,83; p=0,007). En la VFC se reportó una reducción en la relación LF/HF de -0,52 (IC 95%, -1,02 a -0,02; p=0,040).
ConclusionesUna intervención de baile y educación nutricional disminuye la presión arterial y la RVS y tiene efectos favorables en el balance simpático-vagal en pacientes con SM.
Metabolic syndrome (MS) is a clinical condition with a high prevalence,1 mainly due to changes in the Western lifestyle, which predispose to an increased risk of coronary events.2 Early recognition and proper diagnosis of people with MS are clinically relevant because patients require aggressive strategies of pharmacological and non-pharmacological treatment.2 MS is associated with insulin resistance and abdominal obesity and includes a series of alterations in vascular and metabolic function.3
Insulin resistance observed in patients with MS leads to an increase in insulin secretion (hyperinsulinemia), which has deleterious effects on the cardiovascular system.4 Although insulin has a vasodilatory effect,5 when endothelial dysfunction is present, activation of the sympathetic autonomic nervous system (ANS) is predominant, as are its corresponding hemodynamic consequences, such as increased heart rate, myocardial contractility, stroke volume, cardiac output, systemic vascular resistance (SVR), and arterial blood pressure.6,7 An increase in sympathetic tone increases the risk of cardiovascular death, and at the same time is related to metabolic disorders such as obesity, insulin resistance, type 2 DM, and MS.6
Exercise training and a healthy diet have favorable effects on different cardiovascular risk factors such as arterial hypertension, dyslipidemia, DM, and obesity.8 Exercise and good eating habits change body composition,8 increase insulin sensitivity8 and baroreflex sensitivity,9 improve endothelial function,10 hemorheology, hemostasis and sympathovagal balance,10,11 reduce inflammation,10 and slow the progression of atherosclerotic lesions.8 However, despite the current evidence on the beneficial effects of exercise and diet, adherence to intervention programs is low.12
Dancing is a type of exercise that can be adapted culturally in each region in order to increase adherence which has been associated with a lower risk of MS in middle-aged and older people.13 Dancing has also been used as treatment and rehabilitation of patients with different risk factors and chronic diseases,14 because it improves aerobic power, lower body muscle endurance, strength and flexibility, balance, agility, and gait in the elderly.15
Although there have been reports of the combined and individual benefits of exercise and nutrition education programs on cardiovascular risk factors,8,16,17 to our knowledge no studies have been conducted to measure the effect of dancing and diet on hemodynamic and autonomic variables simultaneously in high-risk patients.
The aim of this study was to evaluate the effect of an intervention based on dancing and nutrition education on hemodynamic and autonomic status in adults with MS. It was hypothesized that this intervention decreases arterial blood pressure, SVR and sympathetic tone.
MethodsA randomized controlled clinical trial was conducted on adults with MS in the municipality of Valparaíso, Antioquia (Colombia). The assignment of subjects to the intervention or control group was randomized through a sequence of random numbers generated by computer at a 1:1 ratio. Those who measured the outcomes were blinded to the group assignments.
SubjectsMen and women diagnosed with MS who met three or more criteria were included, according to the latest Intersociety Consensus.2 They were aged 30-60 years and lived in the urban area of the municipality of Valparaíso or in a rural area near the county seat. People excluded from the study were those with a body mass index>40kg/m2, those who had a history of a previous cardiovascular event, chronic diseases such as stage 4 or 5 kidney failure, chronic obstructive pulmonary disease, cancer, acquired immunodeficiency syndrome, type 1 DM, or uncontrolled blood pressure>160/100mm Hg, physical or cognitive limitations, pregnant women, and those subjects who had planned undergoing bariatric surgery.
InterventionBefore starting the intervention, the control group and the intervention group received general recommendations about exercise and healthy eating, and continued with the conventional treatment of the disease in the local hospital.
The intervention group received an exercise and nutrition education program for 12 weeks. An introduction was performed in the week prior to the start of the intervention; motivation and familiarization sessions were conducted with the exercise routine and the education program. Exercise program planning was carried out by a medical specialist in medicine applied to physical activity and sport, a dance teacher with extensive experience in the area, and a physical educator. A female teacher previously trained in the protocol conducted the sessions.
The aerobic training component consisted of dancing classes of continuous type, at intensity between 60-75% of heart rate reserve, 3 days a week with a duration of 60minutes per session, including 5minutes of warm-up and 5minutes of cool-down. In addition, muscle strengthening was performed, including stimulation of five large muscle groups twice a week on alternate days for 30minutes. The exercises were performed as circuits, which permitted strength resistance exercises. The initial load was approximately 50% of one maximum repetition (3 sets of 20 repetitions), which increased progressively every 3 weeks, depending on the evolution of each individual. The total exercise volume was 240minutes per week. Aerobic exercise intensity was controlled by a Polar Team2® (Polar, Finland) system and the Borg scale of perceived exertion.
The nutritional education component was based on the transtheoretical model18 and included a 2-hour educational session each week. The strategy of Information, Education, and Communication was applied19 including lectures, workshops, one goal educational primer, one erasable poster of nutritional labels, and one banner to highlight the goal of each week. In the first two sessions, a psychologist addressed issues related to habits, satiety, and appetite. Subsequently, a previously trained dietitian-nutritionist developed 10 workshops on nutrition education, with a focus on knowledge, attitudes, and practices, stressing the protective and risk factors for cardiovascular disease derived from food. The methods used in this study have been previously reported.20
Evaluation of oxygen consumption and muscle strengthMaximal oxygen consumption was assessed in the physiology laboratory of Indeportes-Antioquia, through the Bruce protocol21 on a Quinton® 1845 (Quinton Cardiology Inc., EEUU) treadmill. Prior to the test, the procedure to be performed was explained to each subject; heart rate and blood pressure were determined according to the recommendations of the European Society of Hypertension,22 after a rest period of 5minutes in the sitting position. The test ended when the patient requested termination due to fatigue, heart rate stabilization despite the increase in stress intensity, or in the presence of symptoms such as dizziness, chest pain, or dyspnea. Based on the time spent, maximal oxygen consumption (VO2 max) was estimated with a regression formula.21
Strength was evaluated from a test of 10 to 12 maximal repetitions of the flexor and extensor muscle groups of the elbows, as well as flexors and extensors of the legs. From a regression formula, the maximum weight that the individual could lift in each exercise was calculated.23 Additionally, the number of repetitions of abdominal crunches and sit-ups that each subject performed in 1minute was recorded using a standardized test.23
Assessment of body compositionHeight was measured with a Seca® (Seca, Germany) measuring rod; body weight with Health Meter® (Sunbeam Products Inc., EEUU) scales that had capacity of 181.4kg and accuracy of 0.1kg, and waist circumference with a glass-fiber anthropometric tape. In addition, four skinfolds (biceps, triceps, subscapular, and suprailiac) were measured with a Slim Guide® (Creative Engineering Inc., EEUU) caliper according to the technique described by Lohman.24 Body fat percentage was obtained from the formula of Durnin and Womersaley.25
Hemodynamic and autonomic evaluationEach individual who participated in the study was instructed to avoid the consumption of tobacco, liquor, or hallucinogenic substances and to avoid physical activity for 24hours before evaluation. Hemodynamic and autonomic function were recorded between 8 and 10 a.m. in an environment at 20°C without visual or auditory distractors after a rest period of 15minutes, in the sitting position for 5minutes, and then in the standing position for another 5minutes. The assessment was performed with MP150 (BIOPAC Systems, Inc., Goleta, CA), according to current recommendations for performing impedance cardiography.26 For measurement, analysis, and processing of data, AcqKnowledge software was used. Arterial blood pressure was measured at the end of each stage with a calibrated sphygmomanometer as recommended by the European Society of Hypertension.22 Taking into account arterial blood pressure, electrocardiographic recording, and impedance cardiography data, estimates were obtained for the RR interval, heart rate, stroke volume, cardiac output, mean arterial pressure, SVR, pre-ejection period, ejection time, speed index, acceleration index, and left ventricular work as described.26
Autonomic function was assessed from the RR interval record obtained from the electrocardiograph included in MP150 (BIOPAC Systems, Inc., Goleta, CA) under control of breathing to 15 cycles per minute. The electrocardiograph data were visually reviewed for anomalies and movement artifacts. These were analyzed off-line using Kubios HRV 2.2 software (University of Eastern Finland, Kuopio, Finland), which calculates measurements in the time-domain, frequency-domain and nonlinear dynamics of heart rate variability (HRV).27
We used three time-domain measures of HRV, which are derived from direct measurements of RR intervals: i) the SD of all normal-to-normal RR intervals in the entire recording (SDNN); ii) the square root of the mean of the sum of squares of difference between adjacent RR intervals (RMSSD); and iii) the percentage of differences between adjacent RR intervals that are greater than 50ms (pNN50).
We used an autoregressive model to estimate the power spectrum densities for the frequency-domain indexes of HRV from a data set length of 256 beats. The power spectra were quantified by measuring the area in three frequency bands: i) very low frequency (VLF, 0 to 0.04Hz); ii) low frequency power (LF, 0.04 to 0.15Hz); and iii) high frequency power (HF, 0.15 to 0.40Hz). Additionally, the LF and HF oscillatory components are presented in normalized units (%) and LF/HF ratio was calculated. The normalized unit expresses the power centered in the frequency of interest divided by total power minus very low frequency power.27
In addition to time-domain and frequency-domain measures of HRV, four nonlinear dynamics methods were used: i) Poincaré plot (SD1 and SD2); ii) approximate entropy; iii) sample entropy; and iv) correlation dimension (D2).27
Laboratory testsAll subjects received the same food at dinner the night before the assessment, and measurements were taken after rest and controlled fasting for 12hours. Cobas® (Roche Diagnostics, EEUU), which uses the absorbance method, was used to quantify fasting glucose, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglycerides. Chemiluminescence was used for blood insulin. The HOMA insulin resistance index was calculated from fasting glucose and insulin levels.28 Total cardiovascular disease risk was calculated with the Framingham equation.29 To quantify physical activity, the Global Physical Activity Questionnaire was used.30
Statistical analysisA necessary sample size of 60 subjects (30 in each group) was calculated, taking into account a mean difference in SVR between the two groups of 650 dyn·s·/cm5, a standard deviation of 840 dyn·s/cm5, a 95% confidence, a power of 80%, and a potential loss of 10%.
The Shapiro-Wilk test was used to evaluate whether the variables came from a normally distributed population. For the description of quantitative variables, the average, standard deviation, median, and interquartile range were used. For a description of the nominal variables, proportions were used. Quantitative variables were compared between the groups before the intervention with Student's t-test or the Mann-Whitney U-test, depending on whether they came from a normally distributed population. Qualitative variables were compared with the χ2 test. Intra-group differences before and after the intervention were obtained by the paired Student's t-test or Wilcoxon test. To establish the differences between groups after the intervention, the linear model with baseline correction was used (analysis of covariance; ANCOVA).31 For all analyses, a statistical significance level of α=0.05 was used, and the IBM SPSS Statistics software, version 21.0, was employed.
Informed consent was obtained from all individual participants included in the study; standards for health research from the Ministry of Social Protection of Colombia in Resolution 008430 of 1993 and the principles of the Declaration of Helsinki were taken into account.32 The Ethics Committee of Indeportes-Antioquia approved the research protocol.
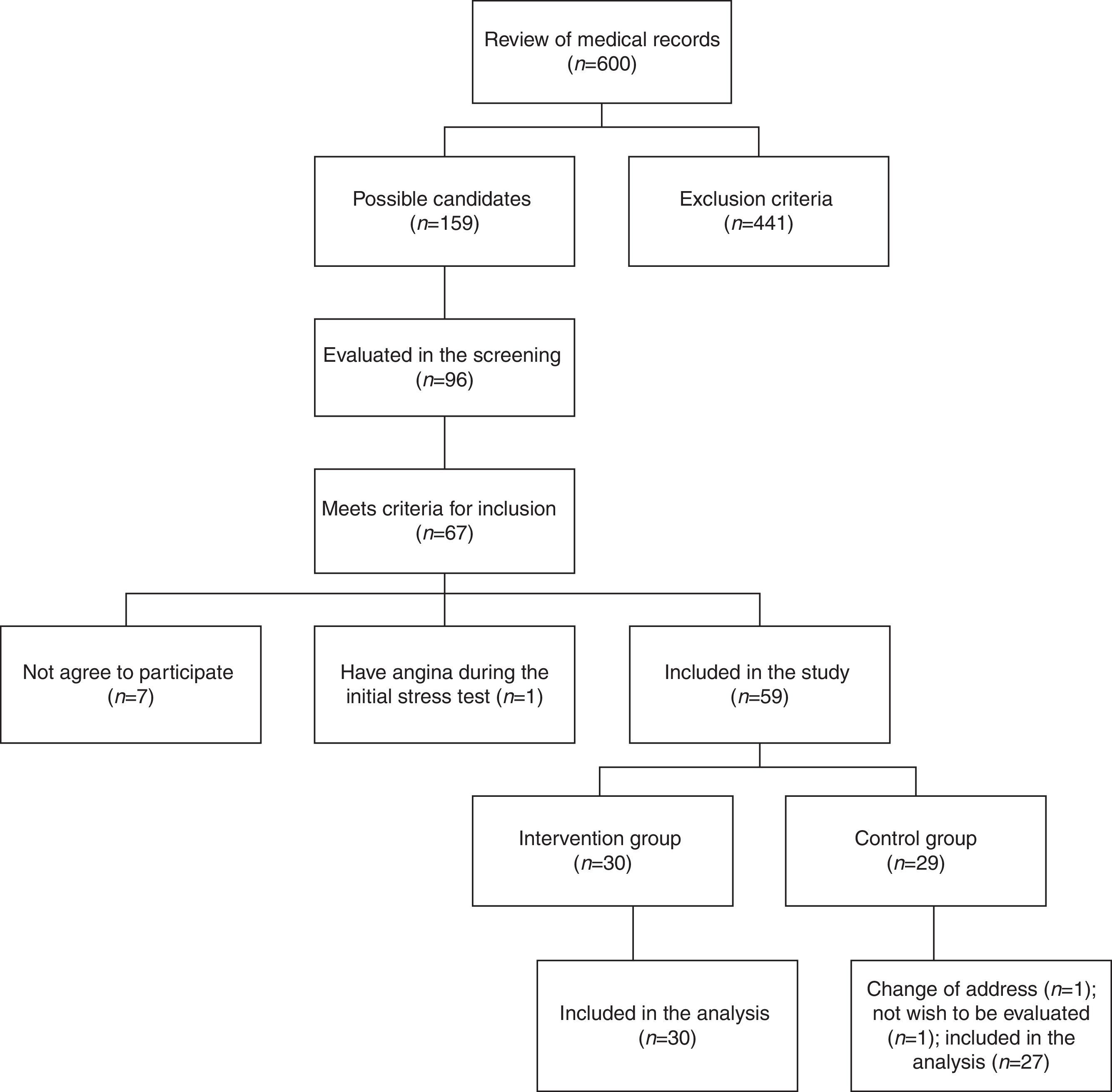
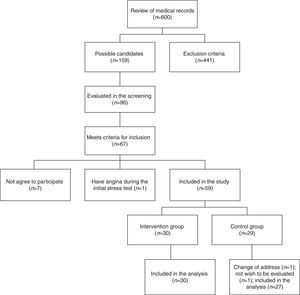
Results159 medical records that belonged to potential candidate subjects were selected initially to participate in the study for presenting multiple cardiovascular risk factors. In an initial screening, 96 people were evaluated; of these, 67 met the inclusion criteria, but only 60 agreed to participate. During the stress test at baseline, one female patient presented angina and required referral to rule out coronary disease. From a total of 59 individuals (10 men and 49 women), 30 were randomly assigned to the intervention group and 29 to the control group. At follow-up, 2 subjects in the control group were excluded. Ultimately, 30 subjects in the intervention group and 27 subjects of the control group were included in the analysis (Fig. 1).
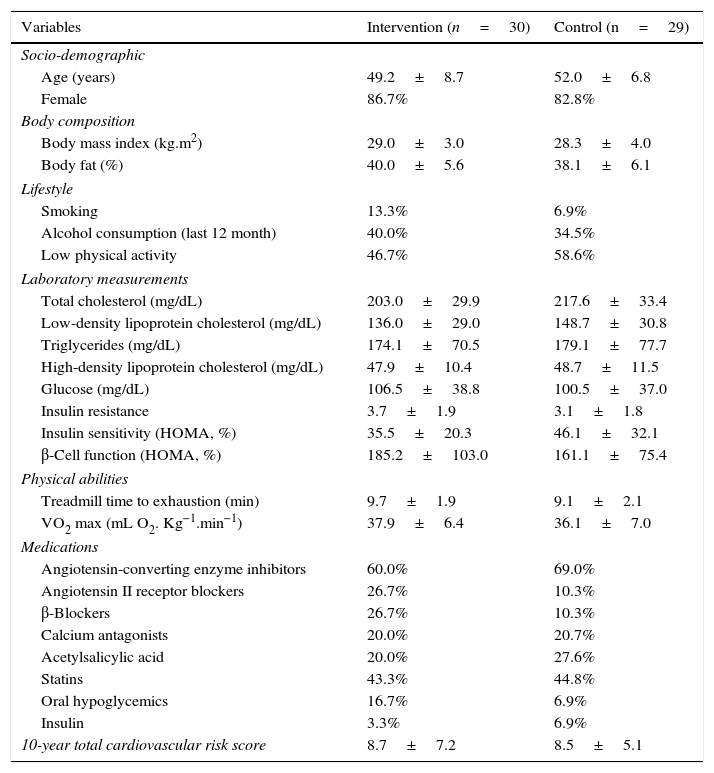
When baseline socio-demographic, clinical, and anthropometric characteristics, as well as those related to lifestyle, laboratory measurements, and physical abilities, were compared between the individuals assigned to the intervention group and those included in the control group, no differences were found (p>0.05) (Table 1).
Patient characteristics.
| Variables | Intervention (n=30) | Control (n=29) |
|---|---|---|
| Socio-demographic | ||
| Age (years) | 49.2±8.7 | 52.0±6.8 |
| Female | 86.7% | 82.8% |
| Body composition | ||
| Body mass index (kg.m2) | 29.0±3.0 | 28.3±4.0 |
| Body fat (%) | 40.0±5.6 | 38.1±6.1 |
| Lifestyle | ||
| Smoking | 13.3% | 6.9% |
| Alcohol consumption (last 12 month) | 40.0% | 34.5% |
| Low physical activity | 46.7% | 58.6% |
| Laboratory measurements | ||
| Total cholesterol (mg/dL) | 203.0±29.9 | 217.6±33.4 |
| Low-density lipoprotein cholesterol (mg/dL) | 136.0±29.0 | 148.7±30.8 |
| Triglycerides (mg/dL) | 174.1±70.5 | 179.1±77.7 |
| High-density lipoprotein cholesterol (mg/dL) | 47.9±10.4 | 48.7±11.5 |
| Glucose (mg/dL) | 106.5±38.8 | 100.5±37.0 |
| Insulin resistance | 3.7±1.9 | 3.1±1.8 |
| Insulin sensitivity (HOMA, %) | 35.5±20.3 | 46.1±32.1 |
| β-Cell function (HOMA, %) | 185.2±103.0 | 161.1±75.4 |
| Physical abilities | ||
| Treadmill time to exhaustion (min) | 9.7±1.9 | 9.1±2.1 |
| VO2 max (mL O2. Kg−1.min−1) | 37.9±6.4 | 36.1±7.0 |
| Medications | ||
| Angiotensin-converting enzyme inhibitors | 60.0% | 69.0% |
| Angiotensin II receptor blockers | 26.7% | 10.3% |
| β-Blockers | 26.7% | 10.3% |
| Calcium antagonists | 20.0% | 20.7% |
| Acetylsalicylic acid | 20.0% | 27.6% |
| Statins | 43.3% | 44.8% |
| Oral hypoglycemics | 16.7% | 6.9% |
| Insulin | 3.3% | 6.9% |
| 10-year total cardiovascular risk score | 8.7±7.2 | 8.5±5.1 |
Data are presented as mean±SD when appropriate
HOMA: The homeostasis model assessment. VO2 max: Maximal oxygen consumption
In the intervention group, there was an average compliance percentage of attendance to exercise and nutrition education sessions of 89.2% and 88.4%, respectively. After the intervention, the group performing the exercise program and attending nutrition education activities obtained an increase in treadmill time to exhaustion during exercise test (0.53min, 95% CI, 0.05 to 1.01; p<0.05) and VO2 max (1.7mL O2·kg−1 · min−1; 95% CI, 0.1 to 3.3; p<0.05) and strength in elbow flexors (11.8 lbs; 95% CI, 9.4 to 14.2; p<0.001) and knee extensors (16.5 lbs; 95% CI, 12.9 to 20.0; p<0.001), and they performed a greater number of repetitions in the abdominal test (7.8 repetitions, 95% CI, 4.8 to 10.8; p<0.001) and sit-ups (5.6 repetitions, 95% CI, 3.1 to 8.1; p<0.001). Additionally, at the end of the study, the intervention group decreased body weight (-2.8kg; 95% CI, -3.8 to -1.8; p<0.001), body mass index (-1.2kg/m2; 95% CI, -1.6 to -0.7; p<0.001), waist circumference (-5.2cm; 95% CI, -6.9 to -3.5; p<0.001), body fat percentage (-2.1%; 95% CI, -2.8 to -1.4; p<0.001), and the 10-year total cardiovascular risk score (-1.5%; 95% CI, -2.7 to -0.3; p<0.05). The control group showed no changes in the variables described above (data not shown).
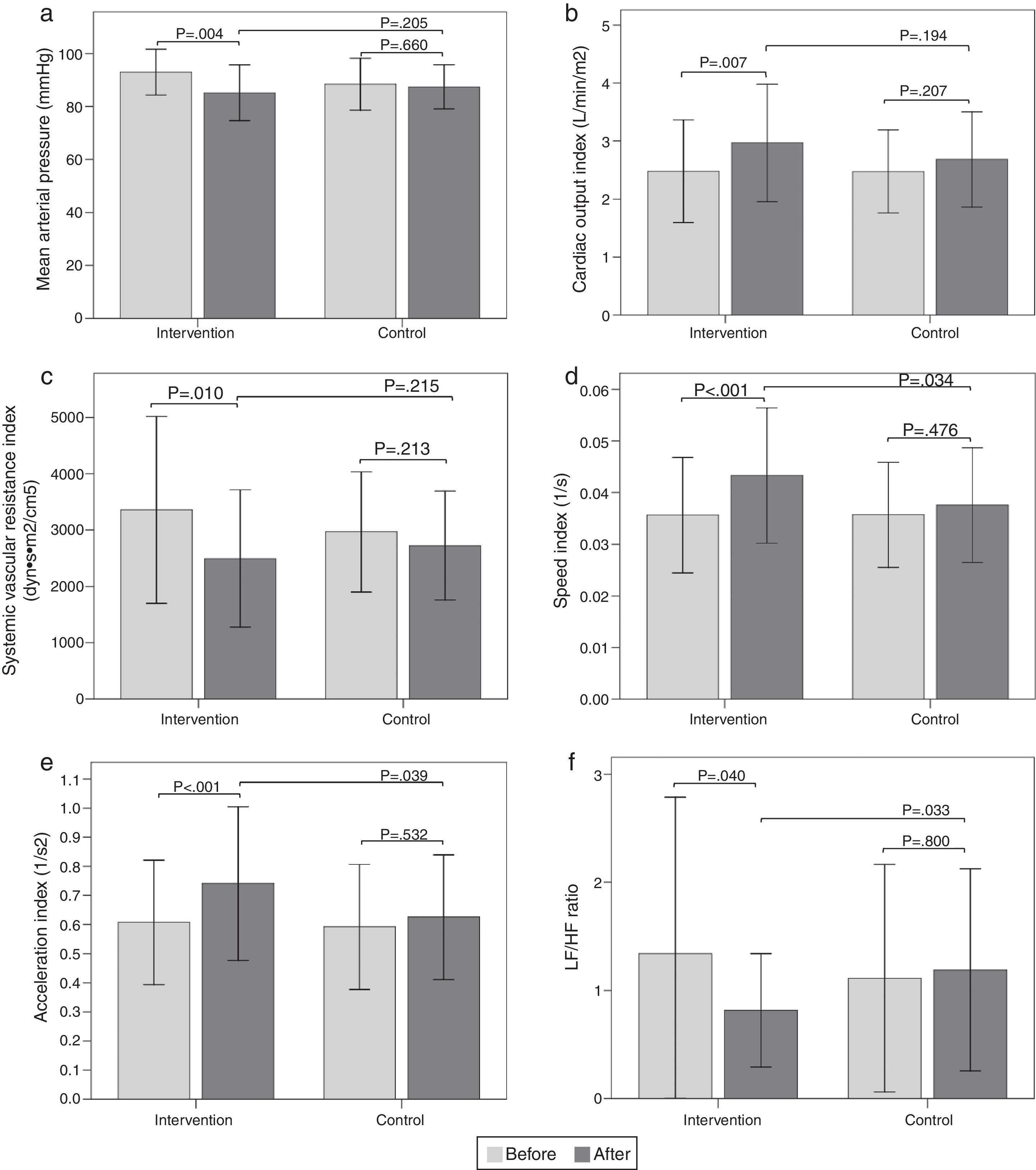
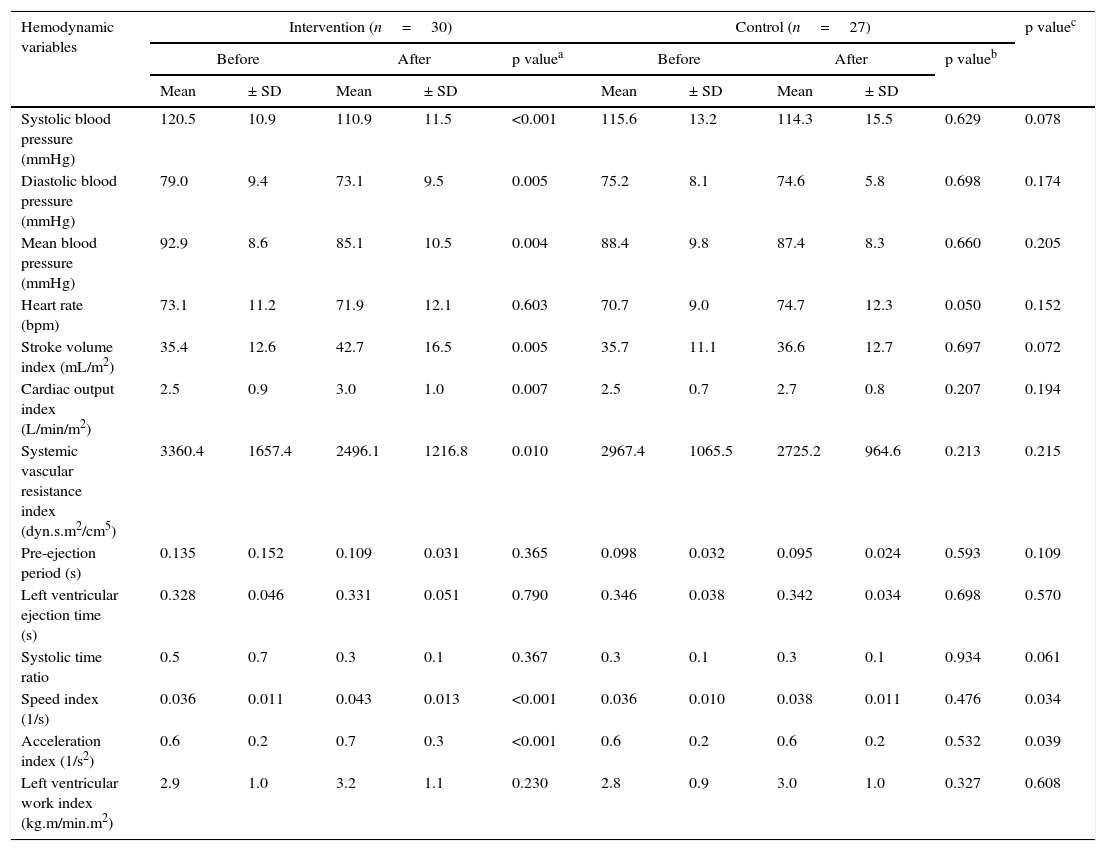
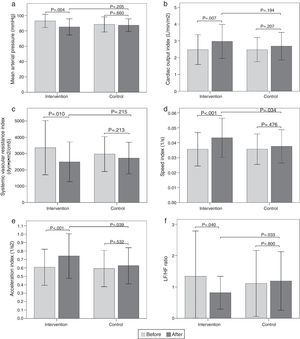
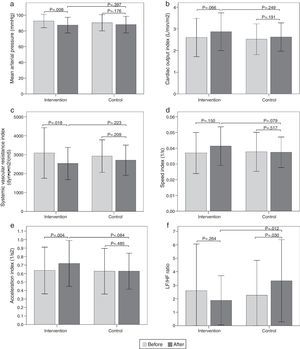
Hemodynamic assessmentWhen comparing the before and the after in the sitting position in the intervention group, a decrease in systolic blood pressure of -9.6mmHg (95% CI, -14.32 to -4.87; p<0.001) was found, as well as a diastolic blood pressure of -5.9mmHg (95% CI, -9.79 to -1.93; p=0.005), a mean arterial pressure of -7.8mmHg (95% CI, -12.84 to -2.75; p=0.004) (Fig. 2a), and a SVR index of -864.29 dyn·s·m2/cm5 (95% CI, -1506.31 to -222.26; p=0.010) (Fig. 2c). Increases were also observed in the stroke volume index of 7.26mL/m2 (95% CI, 2.32 to 12.20; p=0.005), cardiac output index of 0.48 L/min/m2 (95% CI, 0.14 to 0.83; p=0.007) (Fig. 2b), speed index of 0.008 1/s (95% CI, 0.003 to 0.011; p<0.001) (Fig. 2d), and acceleration index of 0.13 1/s2 (95% CI, 0.07 to 0.18; p<0.001) (Fig. 2e). When comparing the before and the after, the control group showed no changes in the sitting position. Comparisons of hemodynamic variables between the two groups at 12 weeks showed an increase in the speed index and acceleration index in the intervention group (p<0.05) (Table 2 and Fig. 2).
Figura 2 Bar graph showing comparison of hemodynamic and autonomic variables in the sitting position between the two groups before and after intervention. a) Mean arterial pressure; b) Cardiac output index; c) Systemic vascular resistance index; d) Speed index; e) Acceleration index; f) LF/HF ratio. Values are means ± SD.
Comparison of hemodynamic variables in the sitting position between the two groups before and after intervention.
| Hemodynamic variables | Intervention (n=30) | Control (n=27) | p valuec | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | p valuea | Before | After | p valueb | ||||||
| Mean | ± SD | Mean | ± SD | Mean | ± SD | Mean | ± SD | ||||
| Systolic blood pressure (mmHg) | 120.5 | 10.9 | 110.9 | 11.5 | <0.001 | 115.6 | 13.2 | 114.3 | 15.5 | 0.629 | 0.078 |
| Diastolic blood pressure (mmHg) | 79.0 | 9.4 | 73.1 | 9.5 | 0.005 | 75.2 | 8.1 | 74.6 | 5.8 | 0.698 | 0.174 |
| Mean blood pressure (mmHg) | 92.9 | 8.6 | 85.1 | 10.5 | 0.004 | 88.4 | 9.8 | 87.4 | 8.3 | 0.660 | 0.205 |
| Heart rate (bpm) | 73.1 | 11.2 | 71.9 | 12.1 | 0.603 | 70.7 | 9.0 | 74.7 | 12.3 | 0.050 | 0.152 |
| Stroke volume index (mL/m2) | 35.4 | 12.6 | 42.7 | 16.5 | 0.005 | 35.7 | 11.1 | 36.6 | 12.7 | 0.697 | 0.072 |
| Cardiac output index (L/min/m2) | 2.5 | 0.9 | 3.0 | 1.0 | 0.007 | 2.5 | 0.7 | 2.7 | 0.8 | 0.207 | 0.194 |
| Systemic vascular resistance index (dyn.s.m2/cm5) | 3360.4 | 1657.4 | 2496.1 | 1216.8 | 0.010 | 2967.4 | 1065.5 | 2725.2 | 964.6 | 0.213 | 0.215 |
| Pre-ejection period (s) | 0.135 | 0.152 | 0.109 | 0.031 | 0.365 | 0.098 | 0.032 | 0.095 | 0.024 | 0.593 | 0.109 |
| Left ventricular ejection time (s) | 0.328 | 0.046 | 0.331 | 0.051 | 0.790 | 0.346 | 0.038 | 0.342 | 0.034 | 0.698 | 0.570 |
| Systolic time ratio | 0.5 | 0.7 | 0.3 | 0.1 | 0.367 | 0.3 | 0.1 | 0.3 | 0.1 | 0.934 | 0.061 |
| Speed index (1/s) | 0.036 | 0.011 | 0.043 | 0.013 | <0.001 | 0.036 | 0.010 | 0.038 | 0.011 | 0.476 | 0.034 |
| Acceleration index (1/s2) | 0.6 | 0.2 | 0.7 | 0.3 | <0.001 | 0.6 | 0.2 | 0.6 | 0.2 | 0.532 | 0.039 |
| Left ventricular work index (kg.m/min.m2) | 2.9 | 1.0 | 3.2 | 1.1 | 0.230 | 2.8 | 0.9 | 3.0 | 1.0 | 0.327 | 0.608 |
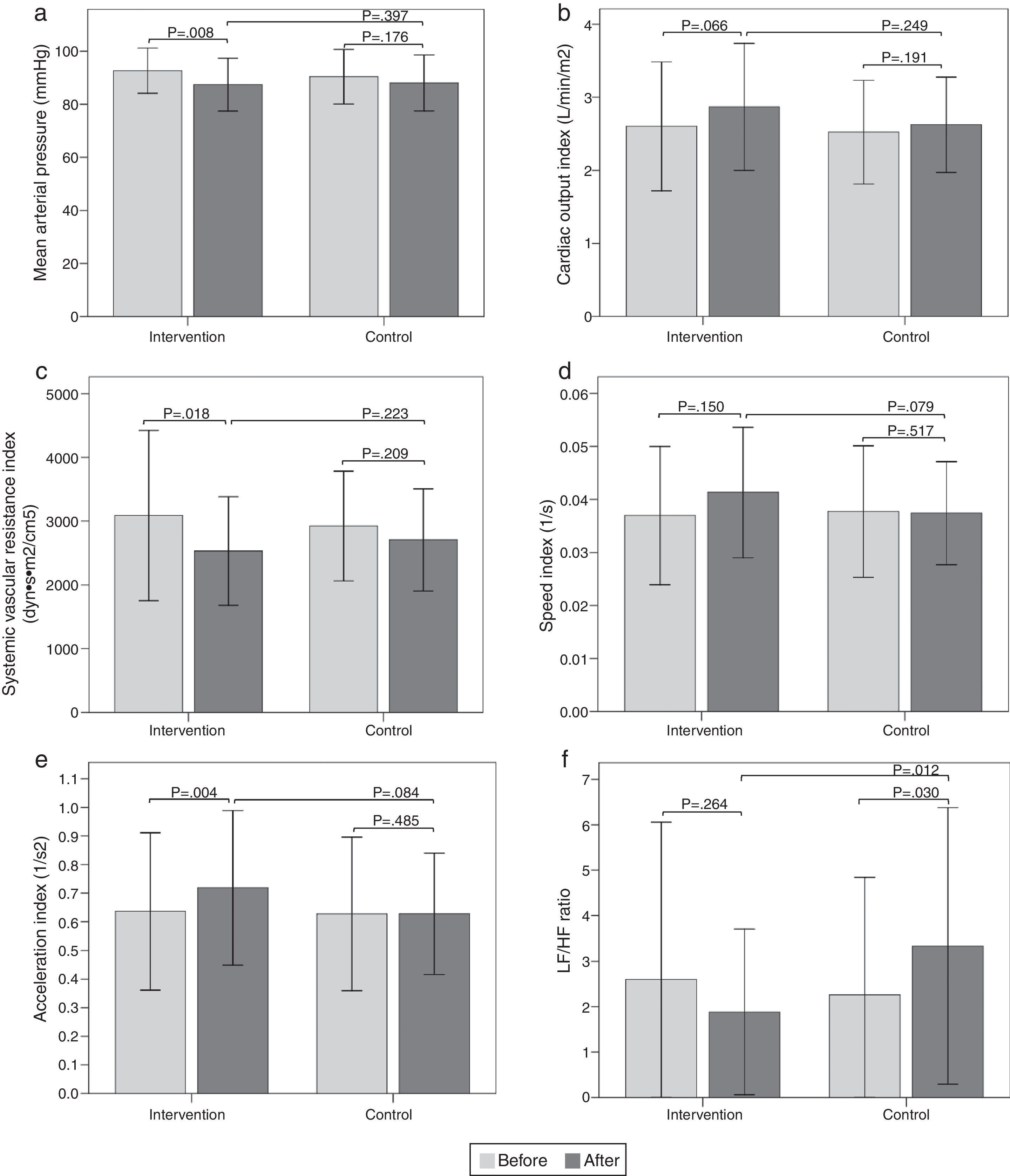
The results of the intragroup and intergroup comparisons in the standing position showed similar findings in hemodynamic variables to those reported in the sitting position (Fig. 3).
Bar graph showing comparison of hemodynamic and autonomic variables in the standing position between the two groups before and after intervention. a) Mean arterial pressure; b) Cardiac output index; c) Systemic vascular resistance index; d) Speed index; e) Acceleration index; f) LF/HF ratio. Values are means ± SD.
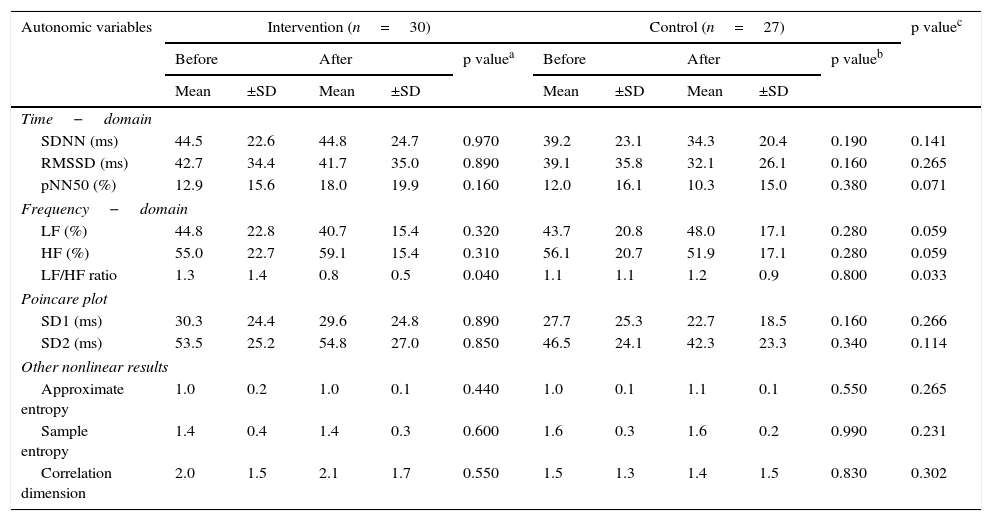
When comparing the before and the after, the intervention group showed a decrease in the sitting-position LF/HF ratio of -0.52 (95% CI, -1.02 to -0.02; p=0.040) (Fig. 2f). There were no differences in the other variables analyzed. When comparing the before and the after, the control group showed no changes in sitting-position variables. Comparisons of the autonomic variables between the two groups at 12 weeks showed a decrease of the LF/HF ratio in the intervention group (p<0.05) (Table 3 and Fig. 2).
Comparison of autonomic variables in the sitting position between the two groups before and after intervention.
| Autonomic variables | Intervention (n=30) | Control (n=27) | p valuec | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | p valuea | Before | After | p valueb | ||||||
| Mean | ±SD | Mean | ±SD | Mean | ±SD | Mean | ±SD | ||||
| Time−domain | |||||||||||
| SDNN (ms) | 44.5 | 22.6 | 44.8 | 24.7 | 0.970 | 39.2 | 23.1 | 34.3 | 20.4 | 0.190 | 0.141 |
| RMSSD (ms) | 42.7 | 34.4 | 41.7 | 35.0 | 0.890 | 39.1 | 35.8 | 32.1 | 26.1 | 0.160 | 0.265 |
| pNN50 (%) | 12.9 | 15.6 | 18.0 | 19.9 | 0.160 | 12.0 | 16.1 | 10.3 | 15.0 | 0.380 | 0.071 |
| Frequency−domain | |||||||||||
| LF (%) | 44.8 | 22.8 | 40.7 | 15.4 | 0.320 | 43.7 | 20.8 | 48.0 | 17.1 | 0.280 | 0.059 |
| HF (%) | 55.0 | 22.7 | 59.1 | 15.4 | 0.310 | 56.1 | 20.7 | 51.9 | 17.1 | 0.280 | 0.059 |
| LF/HF ratio | 1.3 | 1.4 | 0.8 | 0.5 | 0.040 | 1.1 | 1.1 | 1.2 | 0.9 | 0.800 | 0.033 |
| Poincare plot | |||||||||||
| SD1 (ms) | 30.3 | 24.4 | 29.6 | 24.8 | 0.890 | 27.7 | 25.3 | 22.7 | 18.5 | 0.160 | 0.266 |
| SD2 (ms) | 53.5 | 25.2 | 54.8 | 27.0 | 0.850 | 46.5 | 24.1 | 42.3 | 23.3 | 0.340 | 0.114 |
| Other nonlinear results | |||||||||||
| Approximate entropy | 1.0 | 0.2 | 1.0 | 0.1 | 0.440 | 1.0 | 0.1 | 1.1 | 0.1 | 0.550 | 0.265 |
| Sample entropy | 1.4 | 0.4 | 1.4 | 0.3 | 0.600 | 1.6 | 0.3 | 1.6 | 0.2 | 0.990 | 0.231 |
| Correlation dimension | 2.0 | 1.5 | 2.1 | 1.7 | 0.550 | 1.5 | 1.3 | 1.4 | 1.5 | 0.830 | 0.302 |
The results of the intragroup and intergroup comparisons in the standing position showed similar findings in the autonomic variables to those reported in the sitting position (Fig. 3).
DiscussionThe main finding of our study was that an intervention of dancing and nutrition education conducted for 12 weeks reduced blood pressure (systolic and diastolic) and SVR; it also increased the cardiac output and indicators of left ventricular systolic function (speed index and acceleration index), and improved sympathovagal balance in patients with MS.
Different studies conducted in patients with MS undergoing exercise and nutrition interventions have also shown decreases in systolic and diastolic arterial blood pressure similar to our results.33,34 However, the effect is greater when both strategies are simultaneously combined.34 These results as well as ours suggest the need to include changes in diet and exercise as part of treatment for patients with MS, to produce a greater effect on arterial blood pressure.
Regular aerobic exercise at moderate intensity reduces arterial systolic blood pressure between 6-10mmHg and arterial diastolic blood pressure between 4-8mmHg in patients with essential hypertension.35–37 Additionally, a restriction of sodium intake to 2,300mg per day, increasing the intake of vegetables, fruit, and fiber, and lowering saturated fats in the diet can lower systolic blood pressure between 2-14 mmHg.38 It has been estimated that a reduction of 5mmHg in systolic blood pressure in the population produces a decrease of 14% in stroke mortality, 9% in coronary heart disease mortality, and 7% in all-cause mortality.38
Since blood pressure is determined by cardiac output and SVR, a reduction in blood pressure after an exercise and nutrition education intervention could be mediated by a decrease in one or both variables. Our findings show that the main mechanism explaining the reduction in resting arterial blood pressure after aerobic exercise training was a decrease in SVR. A Finnish cohort study that followed 1,741 young adults for 6 years and assessed the relationship between MS and different hemodynamic variables (measured with impedance cardiography) also reported a greater decreased SVR in those subjects who recovered from MS with changes in lifestyle.4
After an intervention with exercise and nutrition education, SVR reduction could be mediated by neuro-hormonal33,35 adaptations in the vascular structure35,39 or function.10,35 Regarding the potential neuro-hormonal adaptations that may explain the reduction of SVR and blood pressure as a result of exercise training, a decrease in central sympathetic activity, norepinephrine release, and plasma renin activity has been described, as well as an increase in baroreflex sensitivity and an inhibition of renal sympathetic outflow.33,35,40 Regarding changes in vascular structure, there is evidence that exercise can produce remodeling (increase in length, cross-sectional area, and diameter) and angiogenesis in muscle vessels.35 However, the major finding explaining the decrease in blood pressure and SVR is the reduction in precapillary vascular resistance, possibly due to the increased number of muscle precapillary vessels, which increases the cross-sectional area of resistance vessels.39 The changes in vascular function that result from exercise training include a lower response of α-adrenergic receptors to norepinephrine stimulation,41 a decrease in endothelin-1 levels,42 and an increase in the production of nitric oxide,10 which contributes to a reduction in vascular tone and SVR.
After the intervention of dancing and nutrition education, we found increased indicators of systolic function such as the speed index, acceleration index, stroke volume, and cardiac output. We cannot exclude that these findings may be explained by an adaptation of the myocardium to the intervention applied, but these most likely may be secondary to decreased SVR leading to a reduction in afterload.
Patients with MS have a decreased HRV,43 which is explained by the state of hyperinsulinemia, increased noradrenaline secretion in sympathetic nerves, and impairment of arterial baroreflex function.6 A reduction in HRV increases the risk of mortality from cardiovascular disease.44 However, regular exercise induces various changes in the ANS that can counteract the neuro-endocrine disorders presented by patients with MS.11 Regular aerobic training decreases resting heart rate and increases HRV due to increased baroreflex sensitivity, greater efferent parasympathetic activity, and reduced efferent sympathetic activity.11
A controlled clinical trial evaluating the effect of an aerobic exercise program of moderate intensity at 70-80% of maximum heart rate, at high or moderate volume, for 8 weeks in sedentary people reported favorable changes in cardiac autonomic regulation in both groups, characterized by an increase in the HF component and a decrease in the LF component and the LF/HF ratio of the HRV spectral analysis, after the exercise training program.45 The results of that study are consistent with our findings, which indicated a decrease in the LF/HF ratio of HRV in the intervention group, and may explain the decrease in SVR and arterial blood pressure due to an improvement in sympathovagal balance.
Alterations of heart rate regularity in patients with MS measured by nonlinear dynamic methods such as approximate entropy,46 have been described. Entropy is a tool for the analysis of nonlinear signals, which provides an independent model to measure the irregularity and complexity of different over-time series. In individuals with MS, greater heart rate irregularity has been reported, and entropy increases with the number of MS components.46 In this study, a relationship between entropy and the impedance of the aorta as determined by volumetric expansion was found, demonstrating that MS causes an imbalance in sympathetic tone and alters the intra-thoracic mechanical capacity of the cardiovascular system.46 Although some studies have shown the effect of aerobic exercise on measurements of nonlinear dynamics, such as an increase in the standard deviation in the Poincaré plot,45,47 we observed no changes as a result of the evaluated intervention, either for the entropy or for other measurements of nonlinear dynamics.
To our knowledge, few reports have evaluated the combined effect of an intervention with exercise and nutrition education on hemodynamic and autonomic variables in patients with MS in a rural area.34 However, the results obtained in this study are consistent with the cardiovascular changes resulting from exercise training that have been observed in healthy individuals with risk factors and in those with cardiovascular disease.10,35 One of the main strengths of this study was the high adherence to the intervention unlike those reported in other studies,48 which can be explained by the implementation of a strategy in group and the inclusion of dancing as a form of aerobic exercise training.
The limitations of the study include the inability to blind the intervention to participants. Also, due to the site where they lived (rural area) and the close relationship between the subjects involved in this research, it is possible that some contamination might have existed. Even though this latter limitation could be contrary to the study hypothesis, despite this, some important hemodynamic and autonomic changes were found in the intervention group.
In our study, the group of patients with MS who received the intervention of dancing and nutrition education showed decreases in body weight, body mass index, waist circumference, and body fat percentage. However, our design did not allow us to estimate the extent to which the decrease in arterial blood pressure and SVR was explained by changes in body composition or by the intervention applied. Finally, we made no adjustments for multiple comparisons which introduce an increased chance of a type I error. However, the consistency of our findings with our prespecified hypotheses makes unlikely that our conclusions were reached by chance alone.
ConclusionsAn intervention with dancing and nutrition education lowers arterial blood pressure and SVR, increases indicators of systolic function and has favorable effects on the sympathovagal balance in patients with MS.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingThis research was supported with funds from the program “For Your Health, Move” of Indeportes-Antioquia and the SICOR Clinical and Research Center.
Conflict of interestThe authors declare no conflict of interest.
The authors thank all those who agreed to participate, the San Juan de Dios Local Hospital of Valparaiso Antioquia, the staff from Indeportes-Antioquia, and the SICOR Clinical and Research Center. DPC was a research trainee in the hypertension and cardiovascular risk line, partially supported by Colciencias under the contact 436-2009.