Nutritional status and unhealthy dietary habits may have an influence on Systemic Lupus Erythematosus (SLE) course and activity.
ObjectivesThis work aimed to determine the nutritional status and dietary intake of SLE patients and its relation to disease activity.
Material and methodsSixty-five SLE patients were recruited from Kasr Alainy outpatient clinic from October 2017 to December 2017 in a cross-sectional study. Data were collected using a structured interview questionnaire including inquiry about the socioeconomic status, nutritional status using anthropometric measurements, semi-quantitative food frequency questionnaires (FFQ), and hemoglobin level measurement. Disease activity level was assessed using SLE disease activity index (SLE-DAI).
ResultsThe median age of the enrolled female patients was 30.0 (24.0–37.0), with median disease duration of 3.0 years (1.0–9.0). BMI assessment revealed that more than three quarters of SLE patients were overweight and obese. The semi-quantitative FFQ revealed a decreased consumption of fresh fruit, vegetables, milk and other dairy products and an increased intake of fats and oils. Disease activity (SLE-DAI) correlated with increased BMI (r=0.299, p=0.016), body weight (r=0.276, p=0.026), and disease duration (r=0.246, p=0.049).
ConclusionsInadequate nutrient intake and the high percentage of overweight and obesity among SLE patients with excessive consumption of lipids and low intake of fibers were revealed. Also, disease activity (SLE-DAI) correlated with increased BMI.
El estado nutricional y los hábitos alimenticios poco saludables pueden influir en el curso y la actividad del lupus eritematoso sistémico (LES).
ObjetivosEste trabajo tuvo como objetivo determinar el estado nutricional y la ingesta dietética de los pacientes con LES y su relación con la actividad de la enfermedad.
Material y métodosSe reclutaron 65 pacientes con LES de la clínica ambulatoria de Kasr Alainy desde octubre de 2017 hasta diciembre de 2017 en un estudio transversal. Los datos se recopilaron mediante un cuestionario de entrevista estructurada que incluía una investigación sobre el estado socioeconómico, el estado nutricional mediante mediciones antropométricas, los cuestionarios de frecuencia de alimentos semicuantitativos (FFQ) y la medición del nivel de hemoglobina. El nivel de actividad de la enfermedad se evaluó utilizando el índice de actividad de la enfermedad del LES (LES-DAI).
ResultadosLa mediana de edad de las pacientes reclutadas fue de 30,0 (24,0-37,0) años, con una duración media de la enfermedad de 3,0 años (1,0-9,0). La evaluación del índice de masa corporal (IMC) reveló que más de las tres cuartas partes de las pacientes con LES tenían sobrepeso y eran obesas. La FFQ semicuantitativa reveló una disminución del consumo de frutas frescas, verduras, leche y otros productos lácteos, y una mayor ingesta de grasas y aceites. El índice del LES-DAI se correlacionó con un aumento del IMC (r=0,299; p=0,016), peso corporal (r=0,276; p=0,026) y duración de la enfermedad (r=0,246; p=0,049).
ConclusionesSe reveló una ingesta inadecuada de nutrientes y el alto porcentaje de sobrepeso y obesidad entre los pacientes con LES con un consumo excesivo de lípidos y una baja ingesta de fibras. Además, la actividad de la enfermedad (LES-DAI) se correlacionó con un aumento del IMC.
Systemic Lupus Erythematous (SLE) is a chronic connective tissue disease characterized by a multi-system inflammatory disorder with immune system imbalance.1 There is an increasing trend in SLE prevalence with time all over the world. Women were more frequently affected than men at every age2 The peak incidence of SLE falls at the relatively young age of 20–40 years.3 Gastrointestinal symptoms can be present in approximately 50% of lupus patients.4 Both inflammatory processes and gastrointestinal involvement may lead to malnutrition among lupus subjects.5 Thus, it has been suggested that the autoimmunity and the inflammation of SLE are directly related to the nutritional profile of patients and may have an impact on their overall outcome.6,7
Different factors are involved in nutritional status impairment among systemic lupus patients: anorexia, dietary restrictions, depression, and malabsorption of some nutrients, such as vitamin D, continuous use of immunosuppressive drugs, physical inactivity, and systemic inflammation therapy with corticosteroids.8 Some clinical characteristics, such as disease duration and activity and systemic and polyarticular forms, are associated with nutritional worsening.1 Moreover, these patients are at high risk of developing low bone mineral density and anemia, On the other hand, reducing levels of body fat may lead to the reduction of inflammation and associated comorbidities.9 These aspects suggest that the nutritional status and food intake of patients with SLE may interfere in the disease course.1
The inflammatory and autoimmunity processes of lupus are related to the metabolism of lipoproteins and release of different nutritional regulatory adipokines that enhance the inflammation through the tumor necrosis factor-α (TNF-α) and the anti-lipoprotein antibodies.10,11
Previous studies assessing the nutritional status among SLE patients revealed that SLE patients have inadequate nutritional status with excessive intake of lipids and proteins and low intakes of micronutrients and that overweight was the primary nutritional disorder seen among them.12
In Egypt, there is a gap in the literature about the nutritional status of the Egyptian SLE patients and the relation between nutritional status and disease activity. Although a few studies have been conducted, they have tended to focus on the estimate of the prevalence of specific micronutrient deficiency such as vitamin D deficiency in SLE patients and its relation to the disease.13 Accordingly, this descriptive cross-sectional study was conducted to determine the nutritional status and disease activity of a group of systemic lupus patients and their relation with different demographic, and laboratory factors.
Materials and methodsStudy setting and designThe current study is a descriptive cross-sectional study in which systemic lupus patients aged>18 years attending the Rheumatology outpatient clinic, Internal Medicine department at Cairo University Hospital during the study period (October 2017–December 2017) were recruited to assess the nutritional status and disease activity among them.
Sample size and sampling techniqueA consecutive sample of patients attending the rheumatology outpatient clinic who met the inclusion criteria and agreed to participate were included in the study. The inclusion criteria for enrollment of patients were fulfilling the American College of Rheumatology revised classification (ACR),13,14 and aged>18 years. While patients who were pregnant, with severe hepatic and renal dysfunction and whose disease duration was less than 3 months were excluded. The total number was Sixty-Seven SLE patients.
Data collection toolsA pre-tested anonymous structured interview questionnaire was used to collect the data. Most of the questions were close-ended and were pre-coded before data collection to facilitate data entry and analysis
Pilot testing: The preliminary data collection form was tested on 10 women (beyond the sample) to assess the clarity and comprehension of questions, and the time needed to answer the questionnaire.
The questionnaire included questions about the following data- 1.
Sociodemographic characteristics: Age, education, occupation, and residence.
- 2.
Assessment of nutritional status of the studied patients using:
- A.
Anthropometric measures:
Anthropometric measurements (namely; weight, height and accordingly the body mass index (BMI) were conducted for the enrolled patients. Participants were classified as normal weight if their BMI was between 18.5 and 24.9kg/m2, overweight if their BMI was between 25.0kg/m2 and 29.9kg/m2, obese if their BMI was 30kg/m2 or higher, and underweight if their BMI was <18.5kg/m2.15
- B.
Dietary history:
Food intake was assessed using a semi-quantitative food frequency questionnaire:
The semi-quantitative food frequency questionnaire included 32 food items which then were grouped into food groups: grains, vegetables, fruits, meat, milk and dairy products, beans, oils, and sugars.
The patients were asked about the frequency of consumption of each food item (how many times per day or week or month and the amount consumed in household measures), this was subsequently converted to daily servings. The portions of food eaten from each group were calculated.16,17
- C.
Laboratory data:
Collected from the patient's file (within the previous three months): Hemoglobin levels to diagnose anemia at sea level (gm/dl),18 Total leucocyte count (TLC),19 and Albumin.20
- 3.
Systemic Lupus Erythematosus Disease Activity Index (SLE-DAI)
The SLEDAI is a physician-administered instrument accounting for the preceding ten days. It assesses 16 clinical features and eight laboratory indices. The weight which has been applied to each index gives this tool a range of 0–105 points. The activity is classified into mild if scores 0–≤2, moderate if score 3–≤12, and severe if score >12.21
- 4.
Patients files:
A separate sheet was generated to collect the required data about the medical history from the patient's medical record: Disease duration, age at onset of diseases, steroid duration
- 5.
One of the researchers conducted 1–2 visits per week to the clinic to interview 2.–3 cases with inclusion criteria per day. An estimated period of 30min was on average required to complete the questionnaire.
The data were coded, entered and analyzed using the statistical package SPSS version 21.22 Continuous variables were examined for normality. They were expressed using median and interquartile range (non-normal variables). Mann–Whitney test was used to compare differences between two independent groups. Chi-square or Fisher's exact test for categorical variables. Spearman's rho nonparametric correlation was used to test the association between quantitative variables. P value≤0.05 was considered as statistically significant. Tables and graphs were used to illustrate information.
Nutrient intake analysis was carried out for the different macro and micronutrients using the National Nutrition Institute food composition tables for Egyptian foods.23 As regards adequacy, nutrient intake was classified according to the level of consumption compared to the FAO, WHO and UNU24 human energy requirements and the human vitamin and mineral requirements,16 where<50% was considered an unsafe level of consumption, >50–75% was considered that needs improvement, >75–120% regarded as an acceptable level of consumption and >120% was considered over consumption.18,24 Calcium and Iron were nutritionally assessed because SLE patients present a high risk for the development of anemia and low bone mineral density.
Ethical considerationsThe Medical Research Committee in the Internal Medicine Department at Faculty of Medicine Cairo University revised and approved the study protocol. Informed consent from each participant was obtained after proper orientation of them regarding the study objectives. Only those who agreed were included, and those who refused were excluded from the study. All procedures for data collection were treated with confidentiality according to Helsinki declarations of biomedical ethics.25
Regarding the possible biases, and what was thought to avoid them: It is the Recall bias, however, we had applied here four methods of nutritional assessment both subjective and objective measurements (data collection methods) and the interviews lasted sufficient time for adequate recall. In addition, the current study was conducted to explore the situation in this area of inquiry regarding the nutritional status of SLE patients. It was not used to infer causal relationships.
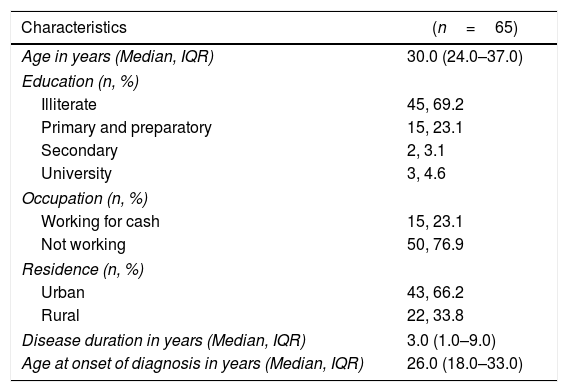
ResultsA total of sixty-five systemic lupus patients were enrolled in the current study during the study period. As revealed in Table 1 more than three quarters (76.9%) were not working. About two-thirds of the enrolled patients (66.2%) reside in urban areas. All of the enrolled patients were females with median disease duration of 3.0 years (1.0–9.0). The range of steroid dose is 5mg to 60mg/d.
Demographic and clinical characteristics of the enrolled systemic lupus patients at Kasr Al Aini rheumatology outpatient clinic.
| Characteristics | (n=65) |
|---|---|
| Age in years (Median, IQR) | 30.0 (24.0–37.0) |
| Education (n, %) | |
| Illiterate | 45, 69.2 |
| Primary and preparatory | 15, 23.1 |
| Secondary | 2, 3.1 |
| University | 3, 4.6 |
| Occupation (n, %) | |
| Working for cash | 15, 23.1 |
| Not working | 50, 76.9 |
| Residence (n, %) | |
| Urban | 43, 66.2 |
| Rural | 22, 33.8 |
| Disease duration in years (Median, IQR) | 3.0 (1.0–9.0) |
| Age at onset of diagnosis in years (Median, IQR) | 26.0 (18.0–33.0) |
IQR=interquartile range.
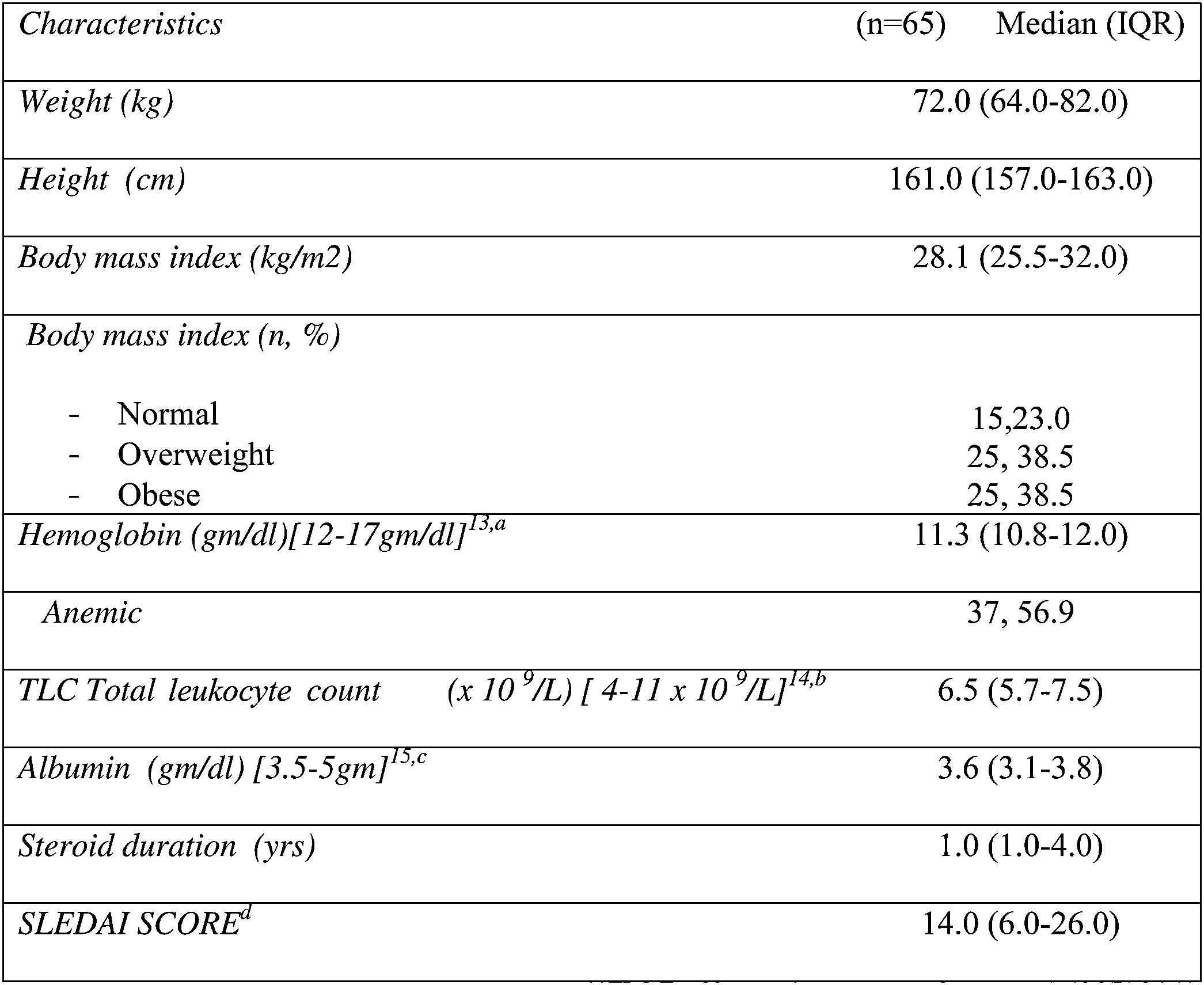
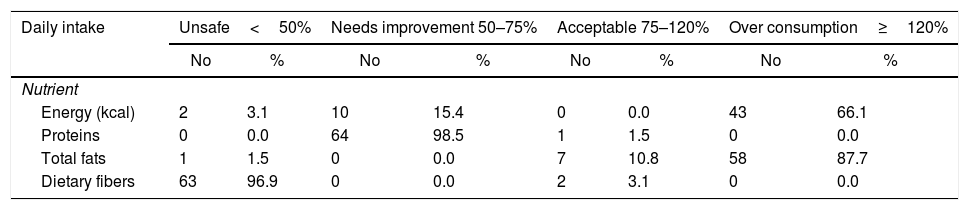
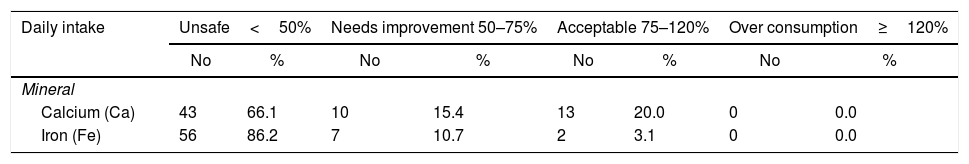
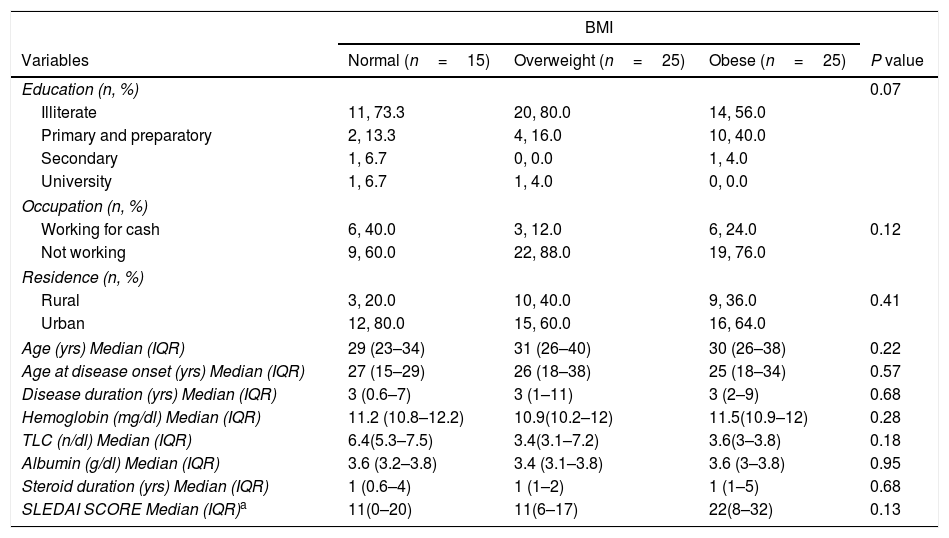
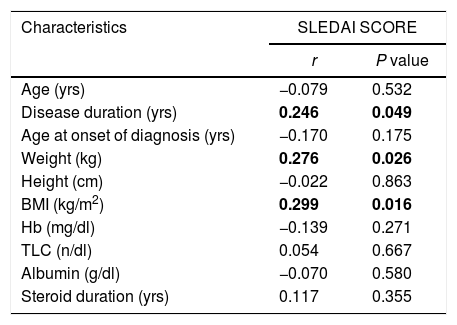
As demonstrated in Table 2, more than three quarters of them were overweight and obese (77%). The median BMI was 28.1 (25.5–32.0), while the median SLEDAI SCORE was 14.0 (6.0–26.0). Table 3 shows that the majority (87.7%) of the studied patients overconsumed fats and about two thirds (66.1%) overconsumed calories. However, dietary fibers were unsafely consumed by the majority of the patients (96.9%). Calcium and Iron were unsafely consumed by the majority of the studied patients, needing improvement in 15.4% and 10.7 of the studied patients respectively as shown in Table 4. There is no significant difference between overweight, normal and obese regarding different background and clinical characteristics as displayed in Table 5. There was a weak positive significant correlation between SLEDAI score and disease duration (r=0.246, p=0.049), BMI (r=0.299, p=0.016), and body weight (r=0.276, p=0.026) as illustrated in Table 6. There was no significant correlation between SLEDAI score and the semi quantitative food frequency intake of the enrolled systemic lupus patients (untabulated results).
The daily caloric and macronutrients’ intake from the diet of the enrolled systemic lupus patients at Kasr Al Aini rheumatology outpatient clinic (n=65).
| Daily intake | Unsafe<50% | Needs improvement 50–75% | Acceptable 75–120% | Over consumption≥120% | ||||
|---|---|---|---|---|---|---|---|---|
| No | % | No | % | No | % | No | % | |
| Nutrient | ||||||||
| Energy (kcal) | 2 | 3.1 | 10 | 15.4 | 0 | 0.0 | 43 | 66.1 |
| Proteins | 0 | 0.0 | 64 | 98.5 | 1 | 1.5 | 0 | 0.0 |
| Total fats | 1 | 1.5 | 0 | 0.0 | 7 | 10.8 | 58 | 87.7 |
| Dietary fibers | 63 | 96.9 | 0 | 0.0 | 2 | 3.1 | 0 | 0.0 |
The daily intake of calcium and iron from the diet among the studied systemic lupus patients at Kasr Al Aini rheumatology outpatient clinic (n=65).
| Daily intake | Unsafe<50% | Needs improvement 50–75% | Acceptable 75–120% | Over consumption≥120% | ||||
|---|---|---|---|---|---|---|---|---|
| No | % | No | % | No | % | No | % | |
| Mineral | ||||||||
| Calcium (Ca) | 43 | 66.1 | 10 | 15.4 | 13 | 20.0 | 0 | 0.0 |
| Iron (Fe) | 56 | 86.2 | 7 | 10.7 | 2 | 3.1 | 0 | 0.0 |
Percent distribution of the enrolled systemic lupus patients by BMI and different demographic, medical and laboratory variables.
| BMI | ||||
|---|---|---|---|---|
| Variables | Normal (n=15) | Overweight (n=25) | Obese (n=25) | P value |
| Education (n, %) | 0.07 | |||
| Illiterate | 11, 73.3 | 20, 80.0 | 14, 56.0 | |
| Primary and preparatory | 2, 13.3 | 4, 16.0 | 10, 40.0 | |
| Secondary | 1, 6.7 | 0, 0.0 | 1, 4.0 | |
| University | 1, 6.7 | 1, 4.0 | 0, 0.0 | |
| Occupation (n, %) | ||||
| Working for cash | 6, 40.0 | 3, 12.0 | 6, 24.0 | 0.12 |
| Not working | 9, 60.0 | 22, 88.0 | 19, 76.0 | |
| Residence (n, %) | ||||
| Rural | 3, 20.0 | 10, 40.0 | 9, 36.0 | 0.41 |
| Urban | 12, 80.0 | 15, 60.0 | 16, 64.0 | |
| Age (yrs) Median (IQR) | 29 (23–34) | 31 (26–40) | 30 (26–38) | 0.22 |
| Age at disease onset (yrs) Median (IQR) | 27 (15–29) | 26 (18–38) | 25 (18–34) | 0.57 |
| Disease duration (yrs) Median (IQR) | 3 (0.6–7) | 3 (1–11) | 3 (2–9) | 0.68 |
| Hemoglobin (mg/dl) Median (IQR) | 11.2 (10.8–12.2) | 10.9(10.2–12) | 11.5(10.9–12) | 0.28 |
| TLC (n/dl) Median (IQR) | 6.4(5.3–7.5) | 3.4(3.1–7.2) | 3.6(3–3.8) | 0.18 |
| Albumin (g/dl) Median (IQR) | 3.6 (3.2–3.8) | 3.4 (3.1–3.8) | 3.6 (3–3.8) | 0.95 |
| Steroid duration (yrs) Median (IQR) | 1 (0.6–4) | 1 (1–2) | 1 (1–5) | 0.68 |
| SLEDAI SCORE Median (IQR)a | 11(0–20) | 11(6–17) | 22(8–32) | 0.13 |
SD=standard deviation, IQR=interquartile range (range between 25th and 75th percentiles).
Correlation between SLEDAI score and different demographic, anthropometric and Laboratory Investigations of the Enrolled Systemic Lupus Patients (n=65).
| Characteristics | SLEDAI SCORE | |
|---|---|---|
| r | P value | |
| Age (yrs) | −0.079 | 0.532 |
| Disease duration (yrs) | 0.246 | 0.049 |
| Age at onset of diagnosis (yrs) | −0.170 | 0.175 |
| Weight (kg) | 0.276 | 0.026 |
| Height (cm) | −0.022 | 0.863 |
| BMI (kg/m2) | 0.299 | 0.016 |
| Hb (mg/dl) | −0.139 | 0.271 |
| TLC (n/dl) | 0.054 | 0.667 |
| Albumin (g/dl) | −0.070 | 0.580 |
| Steroid duration (yrs) | 0.117 | 0.355 |
r=Spearman correlation coefficient.
p- value < 0.05 is significant.
The present study aims to elucidate the nutritional status and disease activity among a group of SLE patients and their relation with different demographic, and laboratory factors. In the current study, more than three quarters of the participants were classified as overweight or obese in addition to the reported unhealthy dietary intake. More than half of the enrolled patients had active diseases, with median SLEDAI score of 14.0 (6.0–26.0). There was a weak significant positive correlation between the disease activity score measured by SLEDAI and the BMI, and body weight.
The high percentage of overweight and obesity are consistent with a study conducted by Borges et al. who observed excess weight among 63.0% of the Brazilian SLE patients.26 Also, Abou-Raya et al.27 had noticed that more than 40% of SLE patients were overweight. These results are along with what have been reported in previous studies.28,29 The current finding could be explained in the present research by the unhealthy dietary intake where the majority (87.7%) of the studied patients overconsumed fats and calories. On the other hand, dietary fibers were unsafely consumed by the majority of the patients (96.9%). The revealed finding is vital as overweight, and malnutrition can portray the immune response of the human and affect the disease pathway and comorbidities.30
The quality of the diet is critical in SLE patients as they are at high risk of developing dyslipidemia, low bone mineral density, and anemia.26 As revealed from the current study, high consumption of fats and oils was reported. The present study finding was in accordance with a previous study conducted by Abou-Raya et al.27 who noted high intake of lipids. This can be very detrimental in patients with SLE because they are more predisposed to the development of dyslipidemia and cardiovascular diseases30,31 Brown reported that excess calories, proteins, fats, zinc, and iron could aggravate the symptoms. On the other hand, some nutrients, for a diet with moderate quantities of protein, abundant in mono- and polyunsaturated fatty acids are highly recommended.32 Unfortunately, the majority of patients presented with inadequate intakes of calcium. This finding was in accordance with those revealed from the previous studies conducted among SLE patients by Shah et al.9 In the current study, the median steroid duration was 1.0 (1.0–4.0) this could make them more predisposed to develop osteoporosis.33
Fiber intake has been inversely related to SLE disease severity, and studies advocate that this outcome is somewhat due to the positive interaction between fiber, Vitamin B6, and B12, and folate.1However the current study revealed low fiber consumption among the enrolled patients and this is consistent with previously conducted studies.27,29
In the current study, more than half of the enrolled patients 56.9% (37 patients) were anemic. This was also in concordance with previous research.34 The results revealed from a study conducted by Fawzy et al. in which the average hemoglobin concentration was 9.4±2.2mg/dl.35 So anemia could be interrelated to the disease activity. In contrast to our results, de Miranda et al. showed that there were no abnormalities in the hemoglobin level and there was no correlation between hemoglobin levels and the BMI of their patients although the fact that those patients had high Systemic Lupus International Collaborating Clinics/ACR Damage Index.36 Regarding the inadequate intakes of iron as revealed from the current study this was an alarming finding because of the high percentage of anemia among the SLE patients. Therefore, the consumption of food sources rich in iron, such as meat, fish, and poultry, should be encouraged in SLE individuals.
As for the levels of the serum albumin, around 40% (24) of SLE patients have hypoalbuminemia. Mild to moderate suppression of serum albumin levels usually occur in patients with (SLE) and is attributed to many causes as renal loss or disease activity or protein-losing enteropathy.37 However, it is difficult to determine whether this hypoalbuminemia is due to the disease itself or malnutrition especially because we have found no statistically significant relation between albumin levels and body mass index (p-value 0.95) in this study.
More than half of the enrolled patients had active diseases, with median SLEDAI score 14.0 (6.0–26.0). There was a weak significant positive correlation between the disease activity score measured by SLEDAI and the BMI, and body weight. The possible explanation is that excess weight is considered a surrogate factor in flaring up the inflammatory response and consequently exacerbating the disease activity and oppressing life quality of lupus patients.26 In another study done by de Miranda et al., they found higher values of SLICC/ACR damage index in overweight and obese patients than in eutrophic patients.36 However, Oeser et al. did not observe the same association.28
The current study finding should be viewed with the following limitation, the descriptive nature of the study. However, it was conducted to explore the situation in this area of inquiry as dietary factors can contribute to the geoepidemiology of autoimmune diseases such as SLE, and to generate hypotheses as no enough information is available regarding the nutritional status of SLE patients. It was not used to infer causal relationships.
ConclusionInadequate nutrient intake and the high percentage of overweight and obesity among SLE patients with excessive consumption of lipids and low intake of fibers were revealed. Also, disease activity (SLE-DAI) correlated with increased BMI. Therefore, proper attention should be paid to nutritional assessment and dietary management should be an integral part of their management plan.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
All authors have approved the final article.
Conflict of interestNone.
The authors are thankful to Medical Students Hala Saad, Shymaa Eisa and Hebatullah Ibrahim for their help in this work.