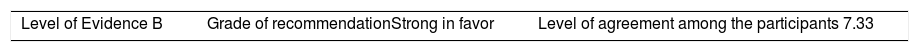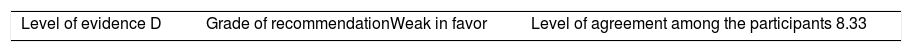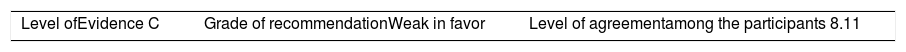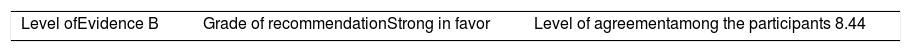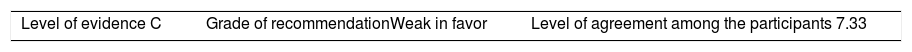The objective of this study was to establish recommendations for the reduction and discontinuation of biological disease-modifying antirheumatic drugs, with the aim of becoming a guide document for health professionals involved in the management of patients with rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis.
Materials and methodsThe recommendations were established through consensus by a panel of experts in rheumatology, and based on the analysis of available scientific evidence obtained from systematic reviews and the clinical experience of the panellists.
ResultsA total of 33 rheumatoid arthritis related studies were included, with 6 psoriatic arthritis related, and 9 ankylosing Spondylitis related. The recommendations for the reduction of biological therapies were made by establishing a plan to determine when and how to reduce the biological disease-modifying antirheumatic drugs in patients with these 3 diseases, and in some cases lead to the discontinuation of these treatments.
ConclusionThe recommendations established in this document will serve as a guide to improve the efficiency of biological therapy in these diseases, reduce the variability in clinical practice, and establish an adequate risk/benefit ratio.
El objetivo de este estudio fue establecer recomendaciones para la disminución y descontinuación de la terapia biológica con el fin de que se convierta en un documento guía para los profesionales de la salud involucrados en el manejo de pacientes con artritis reumatoide, espondilitis anquilosante y artritis psoriásica.
Materiales y métodosLas recomendaciones fueron establecidas mediante consenso desarrollado a través de un panel de expertos en reumatología, basado en el análisis de la evidencia científica disponible obtenida de revisiones sistemáticas y sobre la experiencia clínica de los panelistas.
ResultadosSe incluyeron 33 estudios relacionados con artritis reumatoide, 6 de artritis psoriásica y 9 de espondilitis anquilosante. Las recomendaciones para la disminución de las terapias biológicas se realizaron estableciendo un plan para determinar cuándo y cómo reducir los fármacos biológicos modificadores de enfermedades reumáticas en pacientes con estas 3 enfermedades y en algunos casos conducir a la descontinuación de estos tratamientos.
ConclusiónLas recomendaciones establecidas en este documento servirán de guía para mejorar la eficiencia de la terapia biológica en estas enfermedades, reducir la variabilidad en la práctica clínica y establecer de manera adecuada una relación riesgo/beneficio.
Rheumatoid arthritis (RA), ankylosing spondylitis (AS), and psoriatic arthritis (PsA) are chronic inflammatory diseases with a heterogeneous clinical spectrum and mainly affect the joints. These diseases generate a high social and public health impact because of their effect on quality of life and their association with increased morbidity and mortality. It is well recognized that around 1–3% of the population worldwide may present one of these conditions, and though there have been some approximate estimates in Colombia,1–5 the figures are not yet quite clear.6
For approximately twenty years, biologic therapy (BT) has been considered a follow-up therapy after conventional disease modifying antirheumatic drugs, since it has proven to be effective in controlling symptoms, and slowing radiological progression of the disease, when compared against standard therapies such as methotrexate (MTX) or other disease modifying agents.7,8
However, the broad variability in the clinical response to biologic medications from one person to another is due to: the heterogeneity of the clinical characteristics of the presentation of each one of these conditions; to the broad genotypic and phenotypic variability of the individuals presenting with these diseases; and, to behavioral differences of the serum concentrations that these medications may have in various populations and at different doses. Consequently, in clinical practice, the experts address the therapy in accordance with the different clinical characteristics of the disease, the type of patient, and their practical experience.8–11
Once the BT is started, therapeutic goals are set. These goals are measured using different tools that assess the activity, pain, functionality, and quality of life of the patient. The suggestion has been made to reduce the dose or to suspend the biologic therapy upon remission or until a low disease activity is achieved, since according to some publications, this may lower the risk associated with exposure to the drugs and reduce the costs to the healthcare systems.7,12,13
The process of dose reduction in BT is a question of balancing safety and efficacy. When efficacy has been achieved, tapering off the dose has the dual benefit of reducing the risk of severe infections – as established in recent publications14,15 – in addition to economic savings that may result in having more patients benefiting from the use of BT.
Over the last few years, rheumatic diseases are becoming increasingly relevant in the country because of the progressive increase in their diagnoses and due to the availability of biologic drugs as part of their treatment. These drugs have changed the history of medical practice and improved the quality of life of the patients.
The use of biologic agents accounts for 15.5% of the patients with these diseases; the “High CostAccount” in Colombia reports that there has been an increase in the costs of the healthcare system which results in barriers to treatment access.16
Consequently, the rational use and the definition of saving strategies when achieving the treatment goals in the management of the various diseases, encouraged the development of a consensus document.
This document is based on the evidence from the scientific literature, that enables an approximation to how to accomplish a dose reduction and discontinuation of the BT, in cases where the clinician considers relevant and timely to implement such dose reduction and discontinuation, with a view to optimizing the use of healthcare resources.
The purpose of this document is to issue the recommendations endorsed by the Colombian Association of Rheumatology (ASOREUMA), in order to implement the reduction or discontinuation of BT, based on the available scientific evidence. The intent is to provide a reference for rheumatologists and other practitioners involved in the management of patients with autoimmune inflammatory diseases.
Materials and methodsThe initiative of developing an evidence-based consensus document on the topic stemmed from a conference during the 16th Colombian Congress of Rheumatology, which gave rise to a proposal submitted to ASOREUMA.
This proposal was reviewed by the Education Committee and approved for implementation. Subsequently, the Board of Directors circulated an e-mail invitation to all members of the Association to participate in the development of the consensus document; then, under the guidance of a project coordinator, 8 additional rheumatologists (nominal group), participated in a first meeting at which the proposal was submitted.
During the first meeting, the diseases and biological medications to be considered for implementing the search strategy were established and, by consensus, the operating definitions of dose reduction, discontinuation, remission, low activity of the disease, and relapse were determined (Table 1). Additionally, the research questions according to the PICO (Population, Intervention, Comparator, and Outcome) structure were defined and used to conduct the systematic review.
Definitions: therapeutic goal, mild and severe relapse of rheumatic diseases, and tapering and discontinuation of biologic therapy.
| Definition of dose reduction and discontinuation of biologic therapy | |
|---|---|
| Dose reduction | Usually, tapering off the dose of the medication or extending the interval of administration (lengthening the dosing interval). It may include discontinuation (down to 0), but only after a slow reduction. |
| Discontinuation | Suspension or removal of a particular medication.7 |
| Definitions of therapeutic goal and relapse | |||
|---|---|---|---|
| Disease | Therapeutic goal | Mild relapse | Severe relapse |
| Rheumatoid arthritis and polyarticular psoriatic arthritis | DAS28 (ESR)≤3.2 | IncreaseDAS28≥0.6+DAS28Final≥3.2 | Increase DAS28≥1.2 |
| Axial spondylitis and axial psoriatic arthritis | BASDAI≤4ASDAS≤2.1 | Increase BASDAI≥1(Increase ASDAS≥1.1)+2≤BASDAI final≤4(ASDAS final≤2.1) | Increase BASDAI≥2Increase ASDAS≥2orBASDAI≥4(ASDAS final≥2,1)orMild relapse+CRP≥UNL |
| Psoriatic arthritis or oligoarticular enthesis | SJ and PJ≤1Pain according to VAS≤1.5Patient ODE≤2Painful enthesis≤1 | SJ 3 or painful enthesis≥3+CRP≥UNL | |
PJ: painful joints; SJ: swollen joints; ASDAS: ankylosing spondylitis disease activity scale; BASDAI: bath ankylosing spondylitis disease activity index; DAS: disease activity scale; AS: ankylosing spondylitis; ODE: overall disease evaluation; VAS: visual analogue scale; UNL: upper normal limit; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate.
To do the search, a research question was designed for each disease, in order to identify the specific evidence for each case, showing the effects derived from the discontinuation of biologic therapies.
The search was limited to studies published over the last 20 years in English, Portuguese, or Spanish. With regards to the type of study and the methodological quality, the evidence from meta-analyses or systematic reviews was included. When this type of evidence was not available, then clinical trials, cohort studies, or observational trials were included.
The information was screened by title and abstract, ruling out any studies that failed to consider the information related to the various research questions, as well as publications that were just research summaries, or Congress Minutes, that failed to report all of the results of the research projects. Subsequently, the search and the results were disclosed, with the support of an expert on the topic for validation purposes.17
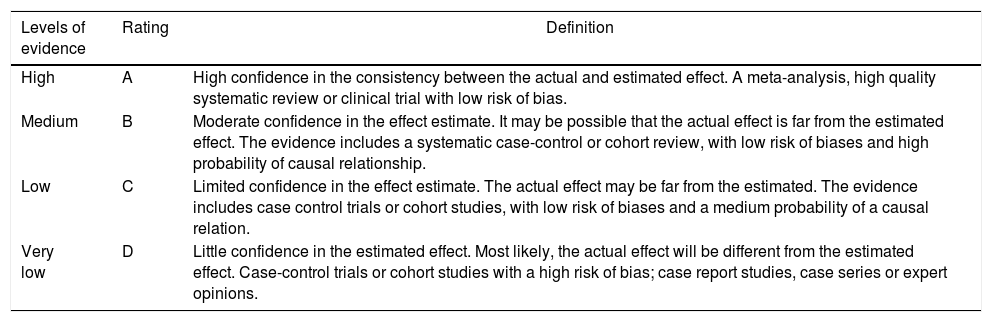
In accordance with the type of study identified, the evidence was rated in terms of methodological quality, using tools such as AMSTAR, PRISMA declaration, ISPOR, the Cochrane Collaboration risk of bias tool, the JADAD scale, and the declaration of the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) initiative. Based on the methodological quality, the Grading of Recommendations Assessment, Development and Evaluation (GRADE) classification tool was implemented (Table 2).18–20
GRADE system, levels of evidence.
| Levels of evidence | Rating | Definition |
|---|---|---|
| High | A | High confidence in the consistency between the actual and estimated effect. A meta-analysis, high quality systematic review or clinical trial with low risk of bias. |
| Medium | B | Moderate confidence in the effect estimate. It may be possible that the actual effect is far from the estimated effect. The evidence includes a systematic case-control or cohort review, with low risk of biases and high probability of causal relationship. |
| Low | C | Limited confidence in the effect estimate. The actual effect may be far from the estimated. The evidence includes case control trials or cohort studies, with low risk of biases and a medium probability of a causal relation. |
| Very low | D | Little confidence in the estimated effect. Most likely, the actual effect will be different from the estimated effect. Case-control trials or cohort studies with a high risk of bias; case report studies, case series or expert opinions. |
Once the level of evidence was established, the grade of recommendation for each question was determined pursuant to GRADE (Table 3). Subsequently, the project coordinator submitted a first report on the levels of evidence and the grades of recommendation to the 8 participating rheumatologists, who together with the coordinator, established the levels for each recommendation accordingly. These decisions were taken individually, anonymously, and independently, hence establishing the level of agreement among the 9 participants.
Levels of recommendation, GRADE system.
| Grade | Definition |
|---|---|
| Strong in favor | The desirable consequences clearly exceed the undesirable consequences. |
| Weak in favorWeak againstStrong againstExpert opinion | The desirable consequences probably exceed the undesirable consequences The undesirable consequences probably exceed the desirable consequencesThe undesirable consequences clearly exceed the desirable consequencesBased on the experience of the clinical practice of the developers |
The level of agreement of the recommendations was determined by scoring each element on a scale from 1 to 10 (1=total disagreement and 10=total agreement) and the average of the ratings given by the specialists was estimated. The percentage of rheumatologists that rated each recommendation was over 80%. The level of consistency was considered to be high when the recommendations reached an average score of more than 8 points and the percentage of rheumatologists that awarded a score of over 8 points for each recommendation exceeded 80%.
Five rheumatologists members of ASOREUMA, who were not part of the participants involved with the development of the consensus document, did an internal review and forwarded their comments, which were then taken into account for the drafting of this document.
Finally, the finished document was forwarded to a foreign external reviewer who has a vast experience in the area and enjoys international scientific and academic recognition (Dr. Isidoro González-Álvaro).
This research followed the ethical principles to carry out research in Colombia, pursuant to resolution 8430 of 1993. The document was developed with disclosure of conflict of interests, taking into account that there is a sponsor company that had no influence on the contents of the document, and respecting copyrights for ASOREUMA. It should be highlighted that neither the research company that supported the development of the document, nor the pharmaceutical company that sponsored the completion of the final document, have any rights over the document. Each author made a statement of conflict of interests.
ResultsThe literature search resulted in 11,819 articles related to RA, 4616 articles on PsA, 3055 articles on AS and spondyloarthritis. Of these, 70, 12 and 18 were evaluated as a full text, and 33, 6 and 9 (flowcharts 1, 2 and 3) were included, respectively.
The first recommendation was given for each of the diseases of interest, discriminating between tapering and discontinuation of the biologic therapy.
A recommendation was made about how to manage a relapse during the reduction of the BT. Similarly, we described the rituximab (RTX) dose reduction separately, due to the difference between this particular drug and the other biologics.
1. How long after achieving the therapeutic goal should the BT dose reduction be considered in patients with RA?
Recommendation:
The BT tapering process in early RA or in established RA may be initiated in patients that have maintained their therapeutic goal for a minimum of 12 months.
In the studies identified to make this recommendation, no comparison is made among the benefits of reducing the BT dose after a period of remission or low activity of the disease of 6 months versus 12 months. In the studies in general, tapering of the BT dose occurs after the patient achieves remission or low activity of the disease, in accordance with DAS28, ESR or CRP.9,21–31
However, in order to make such decision, the start of the BT dose tapering shall be assessed individually for each patient, bearing in mind that the literature reports that patients having the disease for over 10 years and with increased activity of the disease according to DAS28 prior to the start of treatment, are usually associated with higher relapse rates.12,23,24 Another recommendation to consider BT dose tapering in established RA is to reduce the dose in patients who remain in remission despite reducing the dose of steroids to the minimum required.
2. How long after reaching the therapeutic goal shall discontinuation of the BT dose be considered in patients with RA?
Recommendations:
a) The process of BT discontinuation in early RA may be started in patients that have maintained their therapeutic goal for at least 12 months.
Just as in the studies that evaluate a reduction of the BT dose in patients with RA, the studies evaluating BT discontinuation failed to compare the benefit of waiting 6 or 12 months to start such strategy. Most studies start discontinuation in early RA, 6–12 months after achieving the therapeutic goal, whether remission or low activity of the disease.24,32–36
In terms of he outcomes of radiological changes, according to the review by Galvao et al.,24 maintaining the BT with adalimumab or etanercept has a lower risk of radiological progression as compared to the discontinuation of therapy (NNT=12, 95% CI: 7–56).24 In the study by Smolen et al.,33 the radiographic progression was assessed with the modified Sharp scale, and it was slightly deteriorated in patients in whom adalimumab treatment was discontinued; nonetheless, 81% of the patients subject to discontinuation of the BT, did not present any radiological deterioration after 78 weeks of follow-up.
The PRIZE clinical trial, published by Emery et al.,31 showed that the discontinuation of etanercept plus MTX treatment, in patients with early RA, did not result in significant changes in terms of radiological progression, assessed with the total Sharp score modified by Genant, with an average joint damage of 7.9±12.7 in the x-rays.31b) The process of discontinuation of BT in established RA is NOT recommended, due to the risk of radiological progression and higher risk of relapse, following the discontinuation of the drug.22,27,30,37–40
The systematic review by Galvao et al.24 is the only one reporting factors associated with a higher risk of relapse, following BT discontinuation. This publication includes studies conducted in patients with early onset and established RA, in whom having an elevated baseline DAS28 score (HR of 1.39; 95% CI: 1.21–1.60) and more than 10 years with RA (HR of 1.29; 95%CI: 1.03–1.61) result in a higher risk of relapse.24
The systematic review by Van Herwaarden et al.29 estimated that the radiographic progression of patients who discontinued the BT with adalimumab gave rise to an increase in radiological changes, based on the Sharp modified scale, after 52 weeks of follow-up, with a mean difference of 0.66 (95% CI: 0.63–0.69).
The systematic revision by Kuijper et al.,41 though it does not specify the measurement scale used for radiological progression, when comparing between the discontinuation of etanercept use versus continuous therapy in patients with RA, the number of patients that discontinued therapy was higher than the number of patients that experienced just a dose reduction (0.60 units/year, versus −0.06 units/year, respectively, p=0.026). The review failed to identify tocilizumab trials in which radiological progression was reported.41
The STRASS trial published by Fautrel et al.,22 and the study by Brocq et al.,39 evaluated the radiological progression with the modified Sharp scale and reported that there were no significant changes in the patients in whom the frequency of the etanercept or adalimumab dose was reduced.
3. How long after achieving the therapeutic goal shall a reduction in the BT dose be considered in patients with PsA?
Recommendation:
The process of reducing the BT in patients with PsA may be initiated when the therapeutic goal has been maintained for a minimum of 12 months.
In patients with PsA, the tools used to establish the low activity of the disease or remission were PASI,42,43 the minimum disease activity criteria suggested by Coates et al.,44 or combinations of tools such as the Chimenti et al. trial,45 which used DAS28-CRP, absence of edema and morning joint stiffness<15min, absence of dactylitis, enthesitis or extra-articular involvement, PASI<75%, fatigue visual analogue scale, and pain visual analogue scale<10.
The observational study by Piaserico et al.,42 is the only one reporting factors related with a higher risk of relapse following BT reduction. The factors that increase the risk of relapse were: the use of etanercept (HR 3.6 [95% CI: 1.4–9.9], p=0.01); duration of the disease of more than 20 years (HR 2.8 [95% CI: 1.2–6.6], p=0.019); PASI>15 before starting the anti-tumor necrosis factor agents (TNF) (HR 2.9 [95% CI: 1.2–7.2], p=0.018).
In patients with PsA the evidence in terms of dose reduction is limited. There is only one study available by Cantini et al.,43 which reports that 88.6% (47 of 53) of the patients with PsA in remission, in whom the adalimumab dose was tapered down, maintained their remission.
It should be mentioned that there were no studies found that evaluated the consequences on radiological changes following the reduction of the BT dose in patients with PsA.
4. How long after reaching the therapeutic dose should discontinuation of the BT dose be considered in patients with PsA?
Recommendation:
The discontinuation of the BT in patients with PsA is NOT recommended in patients that have maintained their therapeutic goal.
The feasibility study conducted by Moverley et al.,46 evaluated the possibility to discontinue etanercept, adalimumab, infliximab, or golimumab therapy in patients with PsA who maintained the disease stable for 6 months. The observed relapse rate in this study was 54.6% (95% CI: 23.4-83.3%), which presented 8 weeks after the discontinuation of infliximab, etanercept, golimumab or adalimumab. This was the reason for discontinuation of the clinical trial.
Moreover, the observational study by Chimenti et al.45 assessed the discontinuation of etanercept in patients with PsA, comparing those that had received treatment for more than 72 weeks, versus those that received treatment for a shorter period of time. They found that there was not a statistically significant difference in the duration of the remission after discontinuation of therapy, which was 18.1 weeks (range: 46-12) in those that received less than 71 weeks of therapy versus 18.7 weeks (range: 52-11) in those treated for over 72 weeks with etanercept.
5. How long after reaching the therapeutic goal shall the BT dose reduction be considered in patients with AS?
Recommendation: The process of BT reduction in patients with AS may be initiated in patients that have maintained their therapeutic goal for a minimum of 12 months.
In order to assess the low activity of the disease or remission, the studies conducted in patients with SA report the use of the following tools: BASDAI,47–50 partial remission according to ASAS51,52 or a combination of scales including BASDAI and no peripheral joint involvement (arthritis or enthesitis).
The literature found on patients with SA, indicates that some trials start the process of BT tapering in patients that have maintained the standard dose of the biologic drug for up to 2 years. However, the panel considered that a BT dose tapering may be initiated 12 months after having achieved the therapeutic goal uninterruptedly.
In patients with spondyloarthritis, the clinical trial by Cantini et al.,47 reports that 86.3% (19 of 22) of the patients treated with etanercept weekly and 90.4% (19 of 21) of patients treated extending the interval of administration by one week, remained in remission, showing that there is no statistically significant difference between the two groups.
Moreover, in the observational study by Plasencia et al.,50 in patients with spondyloarthritis, the relapse occurred in 30% (22/74) of the patients that reduced the treatment with etanercept, adalimumab or infliximab, and in 19% (8/43) of the patients receiving standard therapy.
The observational study by Almirall et al.,51 showed that in patients with spondyloarthritis, as time progresses, the proportion of patients that relapse to a BASDAI≥4 increases, when the dose of the biologic therapy with etanercept, infliximab or adalimumab decreases, changing from 4.8% in the first 3 months, to 16.7% at 6 months, and to 23.8% at 12 months after starting to taper the dose.
In patients with AS, the study by Závada et al.,53 which compared the standard dose treatment versus a reduced those with anti-TNF, no statistically significant differences were identified. The BASDAI changed annually by 0.47 (95% CI: 0.18-0.76) in the patients receiving the standard therapy and by 0.36 (95% CI: 0.01-0.71) in patients in whom the dose was tapered, with a mean difference of −0.12 (95% CI: −0.57-0.34; p=0.615), and in the HAQ the change was of 0.07 (95% CI: 0.00-0.14) with the standard treatment and of 0.08 (95% CI: −0.01-0.17) with the reduced treatment and a means difference of 0.00 (95% CI: −0.11-0.12); p=0.942.
It should be mentioned that no studies were found that evaluated the consequences with regards to radiological changes subsequent to reducing the BT dose in patients with AS.
6. How long after achieving the therapeutic goal shall the discontinuation of the BT dose be considered in patients with AS?Recommendation: It is not recommended to discontinue the BT in patients with AS because the evidence has shown that by so doing, the activity of the disease gradually increases.49,52,54–56
The study by Sieper et al.52 estimated that in patients with axial spondyloarthritis, in whom the infliximab dose is discontinued, the average time until relapse was 23 weeks (95% CI: 14.43-25.14) in the naproxene group and 12.6 weeks (95% CI: 10.71-not estimated) in the group with no treatment. The mean duration of partial remission was 23 weeks (95% CI: 14.43-25.14) in the patients in whom infliximab was discontinued but not naproxene, and of 12.6 weeks in patients in whom all therapies were discontinued. The results of ASDAS, BASDAI, BASMI and BASFI increased gradually over the 2 evaluation periods (week 28 and week 52), with a mean difference in the naproxene group of 0.6±0.71; 0.6±1.06; 0.3±0.47; 0.5±0.79, respectively in each scale. However, in the group with no treatment, the mean difference was 0.6±0.63; 1.1±1.37; 0.3±0.63; 0.9±1.41, respectively, for each scale.
The rate of relapse in patients with spondyloarthritis in whom biologic therapy was discontinued as reported by Deng et al.49 was 60% (18 of 33 patients) with continued thalidomide treatment; 84.8% (28 of 33 patients) with continued sulfasalazine treatment; and 89.2% (33 of 37 patients) with continued treatment with a non-steroidal anti-inflammatory drug (NSAID).
7. Which is the BT dose tapering regimen we should follow when the decision is made to adopt this approach?
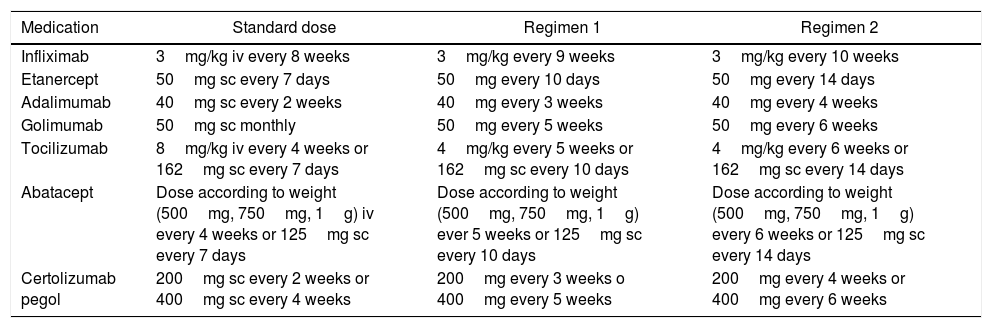
Recommendation: When the decision is made to taper the BT dose, the indicated and approved dose to start off with these medications, in these diseases, may be reduced by 25% of the initial dose, whether by decreasing the dose administered or extending the interval between administrations.
For each individual study and for each particular disease, a reduction regime for the biologic therapy is suggested; however, the Cárdenas et al.30 trial suggests 2 regimes to taper the dose, for 7 medications in patients with RA, in whom no statistically significant differences were identified, in terms of effectiveness to maintain the remission of the disease (Table 4).
Tapering regimens for biologic medications.
| Medication | Standard dose | Regimen 1 | Regimen 2 |
|---|---|---|---|
| Infliximab | 3mg/kg iv every 8 weeks | 3mg/kg every 9 weeks | 3mg/kg every 10 weeks |
| Etanercept | 50mg sc every 7 days | 50mg every 10 days | 50mg every 14 days |
| Adalimumab | 40mg sc every 2 weeks | 40mg every 3 weeks | 40mg every 4 weeks |
| Golimumab | 50mg sc monthly | 50mg every 5 weeks | 50mg every 6 weeks |
| Tocilizumab | 8mg/kg iv every 4 weeks or 162mg sc every 7 days | 4mg/kg every 5 weeks or 162mg sc every 10 days | 4mg/kg every 6 weeks or 162mg sc every 14 days |
| Abatacept | Dose according to weight (500mg, 750mg, 1g) iv every 4 weeks or 125mg sc every 7 days | Dose according to weight (500mg, 750mg, 1g) ever 5 weeks or 125mg sc every 10 days | Dose according to weight (500mg, 750mg, 1g) every 6 weeks or 125mg sc every 14 days |
| Certolizumab pegol | 200mg sc every 2 weeks or 400mg sc every 4 weeks | 200mg every 3 weeks o 400mg every 5 weeks | 200mg every 4 weeks or 400mg every 6 weeks |
iv: intravenous; sc: subcutaneous.
It is important to mention that in the case of IV medications, tapering may be done reducing the milligrams per Kg of weight in each infusion, or increasing the interval between administrations. The decision of which option to use will depend on the opinion of the rheumatologists together with the patient.
This dose reduction shall be implemented in accordance with the clinical characteristics of the particular patients, including low activity vs. remission, time of evolution of the disease, the presence of poor prognosis factors established for each disease, and the particular characteristics of the patient.
8. How to do the follow-up once the BT dose tapering starts?
Recommendation: As of the moment when the BT dose reduction starts, the recommendation is to do the first control at 2 months and then every 2 months for the first 6 months; if the patient continues within the therapeutic goal initially established, any subsequent visits may be scheduled according to the recommendations of the T2T strategy.
There are different studies both in RA as in PsA and AS, establishing the average time to relapse, following the reduction or the discontinuation of the BT; these studies have varying ranges in months and do not exceed our recommended intervals between visits.21,24,29,31,42,43,45,46,49,50 It is important to know that when the rheumatologist makes the decision to taper off or discontinue, patients’ access to control visits should be ensured.
9. What should we do in case of a relapse during the BT dose reduction or discontinuation process?
Recommendations:
a) In the case of patients with RA who experience a relapse during the process of dose reduction, the dose must be readjusted again in order to accomplish the therapeutic goal; in order to decide whether to prescribe the standard dose approved for the drug or go back to the dose used prior to the relapse, each particular case must be assessed, establishing the severity and the cause for the relapse. In case of a mild relapse, the recommendation is to go back to the dose prior to the relapse and in case of a severe relapse, we must go back to the initial dose.21,24,29,31
b) In the case of patients with Ps A, whether polyarticular or a PsA with axial involvement, who experience a relapse during the tapering process, the recommendation is to return to the approved standard dose of the medication, in order to regain the therapeutic goal.42,43,45,46
c) In case of the patients with SA that experience a mild relapse during the tapering process, the recommendation is to use NSAID at full dose for a minimum of 4 weeks, and in accordance with a new evaluation, decide whether to return to the dose prior to the relapse or return to the approved standard dose for each medication, if the therapeutic goal is not accomplished. In case of a severe relapse, the initial standard dose approved for the medication should be prescribed again.49–51
Under any of these circumstances, each case has to be assessed individually, establishing the severity and the cause of the relapse; support from paraclinical tests may be needed, including acute phase reactants, and in some cases, imaging studies for a more objective evaluation of the disease activity.
10. Is it possible to reduce the dose of MTX in patients with RA?
Recommendation: There is still little evidence to make a precise recommendation about a tapering regimen in patients with RA receiving RTX; however, according to some studies described herein, one might suggest that once the patient accomplishes the therapeutic goal, the dose may be reduced to one half and then assess. In case of relapse, resume the approved standard dose.
No studies are available on discontinuation or dose reduction of RTX treatment after achieving remission or low disease activity. The available RTX studies assess different treatment regimens in patients with established disease. No recommendations can be made with regards to time of initiation, indicating a regimen, or establishing the efficacy and safety of tapering or discontinuing therapy in patients who are in remission or have low disease activity, because of the lack of evidence.
Moreover, the Mariette et al.57 trial evaluates treatment of patients with RA in 2 groups: one receiving RTX 1000mg, as a single dose, and the other receiving RTX 1000mg, in 2 doses (day 1 and day 15). All patients received methylprednisolone 100mg, 30min prior to each infusion and after every infusion the patients continued treatment with MTX.57
The study included 143 patients with RA who were randomized, 70 patients in the RTX single dose group, and 73 in the RTX 2-doses group. The outcomes were evaluated according to DAS28-CRP and adverse events.57
The results of the DAS28-CRP at week 24 were 4.2±1.2 in all the patients assessed; the adjusted mean difference between the randomized groups was 51.4 (95% CI: 131.2–234).57
In terms of safety, a total of 1058 adverse events were reported in 204 patients, with no statistically significant differences between the group re-treated with a single dose of RTX, versus the group receiving 2 doses. The rate of infections was 70 and 59% respectively, with an estimated rate of serious infections of 12 and 3%,57 respectively.
The MIRROR58 trial assessed the efficacy and safety of 3 dosing regimens and the repetition of the RTX plus MTX therapy in patients with active RA. The patients with active RA, despite the stable MTX (10–25mg/week), were randomly assigned to one of the 3 treatment regimens that comprised 2 courses of RTX, 24 weeks apart: 2×500mg and 2×500mg; 2×500 and 2×1000mg (dose increase); and 2×1000 and 2×1000mg. The primary outcome was the proportion of patients that accomplished ACR20 at week 48. The RTX 2×500mg and 2×1000mg regimens could not be clearly differentiated, although the results indicate improved outcomes in the RTX 2×1000mg group. Retreating at week 24 lead to a sustained suppression of the disease activity until week 48.
Finally, the study by Henry et al.,15 which has a good sample size and good 5-year follow-up, shows that the use of reduced doses of RTX, for retreatment in patients with RA, does not alter the long term maintenance of treatment and was associated with a significantly lower rate of severe infections. The reduced dose of RTX allows for a 40% reduction as compared against the standard dose strategy, and for a 65% reduction as compared against re-treatment at full dose every 6 months.
Discussion13 recommendations were analyzed in this consensus, based on the evidence described in the procedure to reduce the BT dose in patients with RA, PsA, and AS. The evidence collected and evaluated shows that in patients with early RA, there is more evidence associated with discontinuation, whilst in established RA – though the evidence favors both BT tapering and discontinuation – our recommendation is that BT may be discontinued in patients with early RA and in case of a relapse, the biologic therapy should be reinitiated, as per the recommendation.21,27,30–32,34
In the case of patients with established RA, we do not recommend discontinuing the BT because of the risk of radiological progression and increased risk of relapse, following the discontinuation of the drug.22,27,30,37–40
The studies conducted in patients with PsA and AS, in whom BT is tapered or discontinued, are of lesser quality as compared against the studies available in patients with RA.
In PsA, the evidence indicates that the biological therapy dose may be decreased, whilst the evidence for discontinuation is contradictory, since there is one unfinished study because of the high relapse rates that led to the interruption of the discontinuation strategy46; in yet another publication, they were able to discontinue the BT, but the patients experienced a relapse short time later.45
The search considered the difference of the effects that may present in patients with RA treated with RTX, but the clinical trials conducted with the medication fail to assess the long term impact of discontinuation57; therefore, it is impossible to make recommendations based on scientific evidence, that establish when the administration of the drug should be discontinued or what to expect after discontinuation. Nonetheless, recent publications support tapering the RTX dose in patients with RA, maintaining the efficacy and reducing the percentage of serious infections with such strategy.15
Additionally, non-radiographic spondyloarthritis was also considered, and the term was included in the search; however, we failed to identify any studies conducted in a population with this characteristic and which evaluated the effects from reducing of discontinuing biologic therapy.
The level of agreement established a priori to make any high level recommendation in this consensus was set at 8 or more, and this was achieved in 9 of the 13 recommendations.
One of the cases in which agreement was not reached was the one referring to NOT discontinuing the biologic therapy in patients with established RA. Despite the arguments herein described, some of the participants claim that in some patients with significant structural damage, to whom BT was indicated and the therapeutic goal is achieved, the biological therapy may be discontinued as long as the conventional DMARDs are maintained and strictly adhered to.
The same happened with the recommendation to NOT discontinue BT in patients with PsA after achieving the therapeutic goal. The participants in the consensus argue that the evidence so far is insufficient to strongly make this recommendation.
Another recommendation in which the level of agreement was not reached, was the suggestion to use an NSAID for 4 weeks in patients with AS, experiencing a mild relapse. The participants argue that if the patient is receiving BT is because he/she previously failed to respond to the use of NSAIDs, and therefore the approved standard dose of BT for this indication should be re-established.
Finally, no agreement was reached with regards to reducing the RTX dose in patients with RA, because of the scarce evidence to make such recommendation with hard outcomes such as radiological progression.
Reliable parameters are required to help to predict in clinical practice which patients may adequately keep the disease under control after reducing the BT dose. However, to date, neither the serum levels of TNF inhibitors (TNFi), nor the levels of drug antibodies, have been able to predict the successful reduction of the dose or the discontinuation of etanercept, infliximab or adalimumab in patients with RA who remained with a stable, low activity disease. Apparently, only the high levels of adalimumab could be predictors; therefore, using only these tests to adjust the dose is not recommended.59
Another study confirms this statement since it was not able to identify any predictors that could reliably ensure the reduction of the discontinuation of BT in patients with RA. Nevertheless, some factors have been identified that could be predictors; for instance: having high levels of the medication, lower Sharp/van der Heijde erosion score, and shorter duration of symptoms at the start of the BT.59
Over the past years, a series of studies have shown that joint swelling identified with ultrasound, and findings such as synovitis using Doppler, and to a lesser extent, synovial hypertrophy with the gray scale, are better predictors of relapse than the clinical measurements once the BT is reduced or discontinued.60–62 However, the added value of ultrasound to the clinical examination still requires more evidence.63 There are still missing data regarding the predictive ability of nuclear magnetic resonance after reducing or withdrawing biologic therapy; nonetheless, synovitis and bone marrow edema detected with MRI have shown to predict radiographic progression in patients with RA.64,65 Nuclear magnetic resonance is a reliable and amenable tool for multicenter studies (with central reading).
In general, patients with early RA or patients with a more profound or extended clinical remission, are less likely to experience a relapse of the disease upon reduction of the BT, but further studies are needed to confirm these results.7
Therefore, the unanswered questions regarding the reduction of the dose of BT include: which patients, or what disease characteristics are predictive of the risk of relapse and whether these characteristics could be used for designing an algorithm for patient selection. Moreover, the TNFi dose regimen following a relapse due to tapering of the dose, that provides the same control of the disease is yet unknown.66,67 Although the restart of the TNFi therapy has been successful to regain control of the disease in most patients (up to 100% of the patients in certain studies),27,61,66 not every study has shown such beneficial results and the number of patients was usually small (remission recovery in only 57% of the patients).68
Studies such as The imPact of Residual inflammation detected via imaging techniques, drug levels and patient characteristics on the outcome of dose taperIng of adalimumab in clinical remission rheumatoid arThritis (RA) study (PREDICTRA)69 and a few others with a similar design, will probably help us answer some of the many questions that with the available literature remain unanswered; however, in our clinical practice we still ask ourselves these questions when tapering or discontinuing biologic therapy in patients with these three diseases.
FinancingColombian Association of Rheumatology.
Conflict of interestAll of the authors stated their conflict of interests in writing, but none has any conflicts relating to this document.
Members of ASOREUMA that participated as interim reviewers: Dr. Juan Manuel Bello, Dr. Luis Cajas, Dr. Aura Domínguez, Dr. Adriana Rojas and Dr. Carlos Jaime Velásquez.
External reviewer: Dr. Isidoro González-Álvaro. Rheumatology Service, Instituto de Investigación Sanitaria La Princesa, Hospital Universitario de La Princesa, Madrid-Spain.
Please cite this article as: Edwin J, Wilson B, Adriana B, Oscar F, Andrés F, Daniel F, et al. Asociación Colombiana de Reumatología. Consenso sobre recomendaciones para disminución y descontinuación de la terapia biológica en pacientes con artritis reumatoide, espondilitis anquilosante y artritis psoriásica. Rev Colomb Reumatol. 2019;26:11–23.