Clinically amyopathic dermatomyositis (CADM) is associated with antibodies directed against the protein encoded by the melanoma differentiation-associated gene 5 (MDA5). CADM patients have an increased risk of developing rapidly progressive interstitial lung disease (RP-ILD) and spontaneous pneumomediastinum. Two Peruvian cases of RP-ILD-associated CADM with spontaneous pneumomediastinum are presented, one of them was anti-MDA5 antibody positive. To our knowledge, this is the first report of anti-MDA5-associated CADM in the Peruvian population.
La dermatomiositis clínicamente amiopática (DMCA) se relaciona con anticuerpos dirigidos contra la proteína codificada por el gen asociado con la diferenciación del melanoma 5 (MDA5). Los pacientes con DMCA tienen un mayor riesgo de desarrollar enfermedad pulmonar intersticial rápidamente progresiva y neumomediastino espontáneo. Se presentan dos casos peruanos de DMCA asociada con enfermedad pulmonar intersticial rápidamente progresiva con neumomediastino espontáneo, uno de ellos positivo para anticuerpos anti-MDA5. Hasta nuestro conocimiento, este es el primer reporte de DMCA asociado con anti-MDA5 en la población peruana.
Clinically amyopathic/hypomyopathic dermatomyositis (CADM) is a rare disease which approximately represents 20% of dermatomyositis (DM) patients. Amyopathic is defined as cutaneous features of DM without muscle weakness, elevated serum muscle enzymes or abnormalities on muscle test as electromyography (EMG) or muscle biopsy. Hypomyopathic is an entity without objective weakness but may have subclinical muscle involvement on muscle enzymes, imaging, EMG or biopsy.1 Most patients with CADM have antibodies against the protein encoded by the melanoma differentiation-associated gene 5 (MDA5). This antibody increases the risk of patients developing a rapidly progressive interstitial lung disease (RP-ILD) and spontaneous pneumomediastinum in 26- and 15-fold, respectively; these risks are higher than in patients without anti-MDA5.2 Both aforementioned complications are associated with a poor prognosis3,4; moreover, survival rate in CADM patients is about 40.8–45.0% by six months.5 We are now reporting two cases of RP-ILD-associated CADM complicated with pneumomediastinum (one of them was anti-MDA5 antibody positive) from a Latin-American (Peruvian) center.
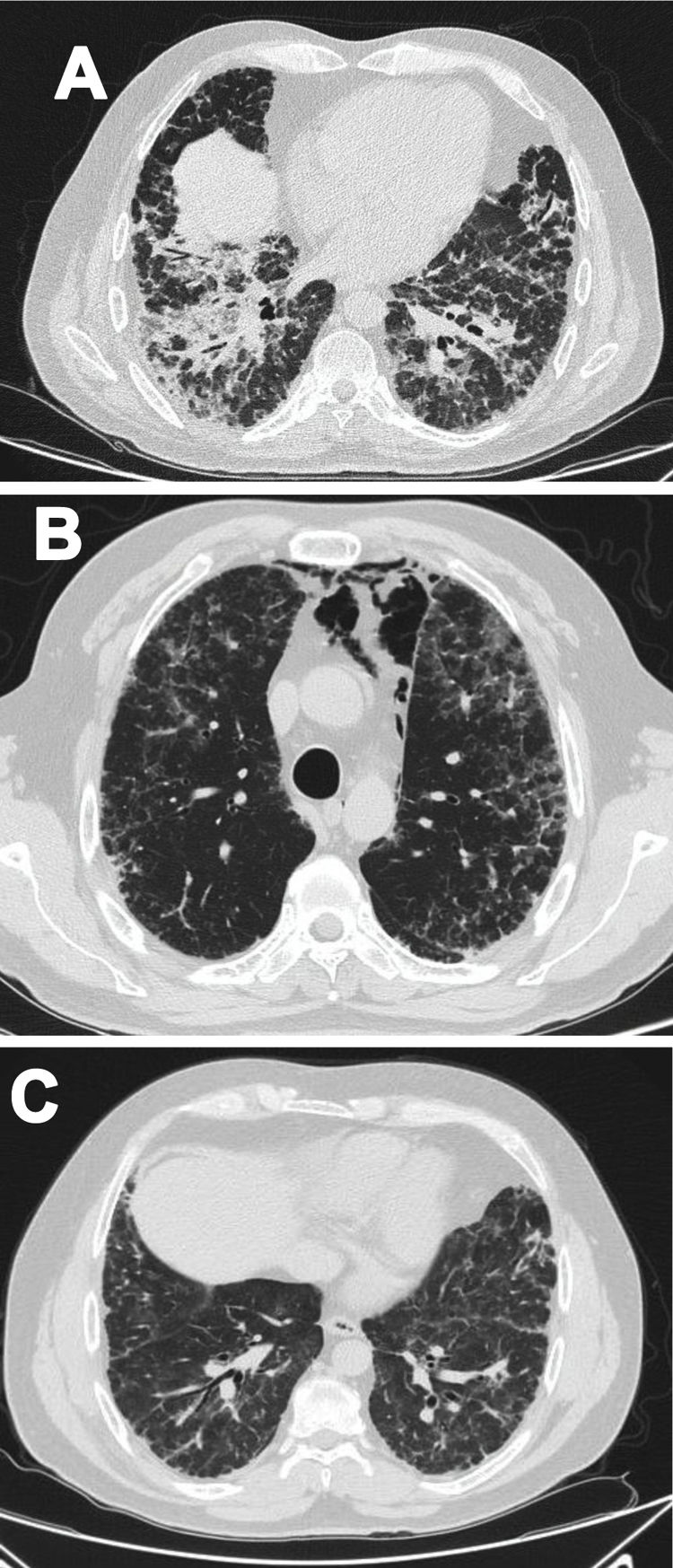
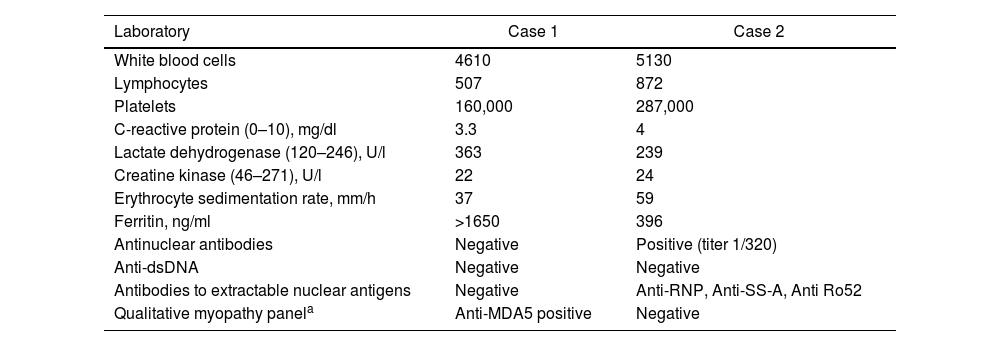
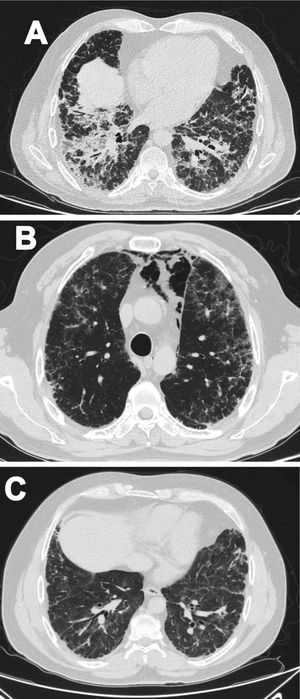
Case 1A 47-year-old male Mestizo patient, previously healthy, was admitted to the hospital with a three-month history of a 10kg weight loss, diffuse alopecia, decreased proximal muscle strength in upper limbs, myalgia, fever, progressive dyspnea, and Raynaud's phenomenon. On physical examination, he was on acute respiratory distress but hemodynamically stable. Vital signs were Blood Pressure (BP): 120/70mmHg, heart rate: 78bpm, breathing 22 breaths per minute. He exhibited Gottron's papules over the metacarpophalangeal joints (MCPs) and knees, and a non-suppurative superficial ulcerative Gottron's on elbow. Muscle strength was 4/5 proximally and 5/5 distally in the upper extremities, and 5/5 proximally and distally in the lower extremities His blood oxygen saturation (SpO2) was 92%. Baseline tests were obtained: white blood cells (WBC): 4610, lymphocytes: 507, platelets: 160,000/mm3, C-reactive protein (CRP): 3.3 (0–10) mg/l, lactate dehydrogenase (LDH): 363 (120–246) U/l, creatine kinase (CK): 22 (46–271) U/l, erythrocyte sedimentation rate (ESR): 37mm/h and ferritin levels>1650 (28–365) ng/ml. Antinuclear antibodies (ANA), anti-double stranded DNA (anti-dsDNA) and antibodies to extractable nuclear antigens were negative (Table 1). A spirometry was performed, and it showed obstruction with a possible restrictive pattern; a chest computed tomography (CT) showed signs of severe diffuse interstitial lung injury with non-specific interstitial pneumonia (NSIP) pattern (Fig. 1A). In addition, a bronchoscopy was performed which did not show any signs of an infection and the cultures obtained were all negative. Electromyography was performed and showed an incipient myopathic pattern in both thighs.
Laboratory findings from the two patients.
| Laboratory | Case 1 | Case 2 |
|---|---|---|
| White blood cells | 4610 | 5130 |
| Lymphocytes | 507 | 872 |
| Platelets | 160,000 | 287,000 |
| C-reactive protein (0–10), mg/dl | 3.3 | 4 |
| Lactate dehydrogenase (120–246), U/l | 363 | 239 |
| Creatine kinase (46–271), U/l | 22 | 24 |
| Erythrocyte sedimentation rate, mm/h | 37 | 59 |
| Ferritin, ng/ml | >1650 | 396 |
| Antinuclear antibodies | Negative | Positive (titer 1/320) |
| Anti-dsDNA | Negative | Negative |
| Antibodies to extractable nuclear antigens | Negative | Anti-RNP, Anti-SS-A, Anti Ro52 |
| Qualitative myopathy panela | Anti-MDA5 positive | Negative |
Panel include antibodies against: Mi-2 alpha, Mi-2 beta, transcription intermediary factor 1 (TIF1) gamma, melanoma differentiation-associated protein 5 (MDA5), nuclear matrix protein 2 (NXP2), SUMO activating enzyme E1 (SAE1), Ku, PM-Scl100, PM-Scl75, Jo-1, signal recognition particle (SRP), threonyl-tRNA synthetase (PL-7), alanyl-tRNA Synthetase (PL-12), glycyl-tRNA synthetase (EJ), isoleucyl-tRNA synthetase (OJ) and Ro-52.
Due to these findings, the patient was diagnosed as having ILD-associated DM; therefore, a qualitative myopathy panel was performed and anti-MDA5 antibodies were found to be positive (one band corresponds to MDA5). Methylprednisolone (MTP) pulses 1g/d for two days then prednisone 70mg/d were started; MTP was held due to increased dyspnea (SpO2 88–89%) and antibiotic therapy was initiated due to a suspected pneumonia. Despite this, dyspnea persisted, and a follow up chest CT was performed which showed pneumomediastinum (Fig. 1B). In view of these findings, intravenous immunoglobulin (IVIG) 120g (total dose of 2g/kg) and then intravenous (IV) cyclophosphamide (CYC) 800mg (dose of 500mg/m2) were started. Patient had progressive and gradual improvement completing six-monthly doses of CYC. Furthermore, prednisone was tapered to 5mg/d. Tacrolimus 2mg/d was started as maintenance therapy. Sixteen months after disease onset the patient remains clinically stable, has not needed supplemental oxygen and there has been significant imaging improvement per CT done one year after treatment initiation (Fig. 1C).
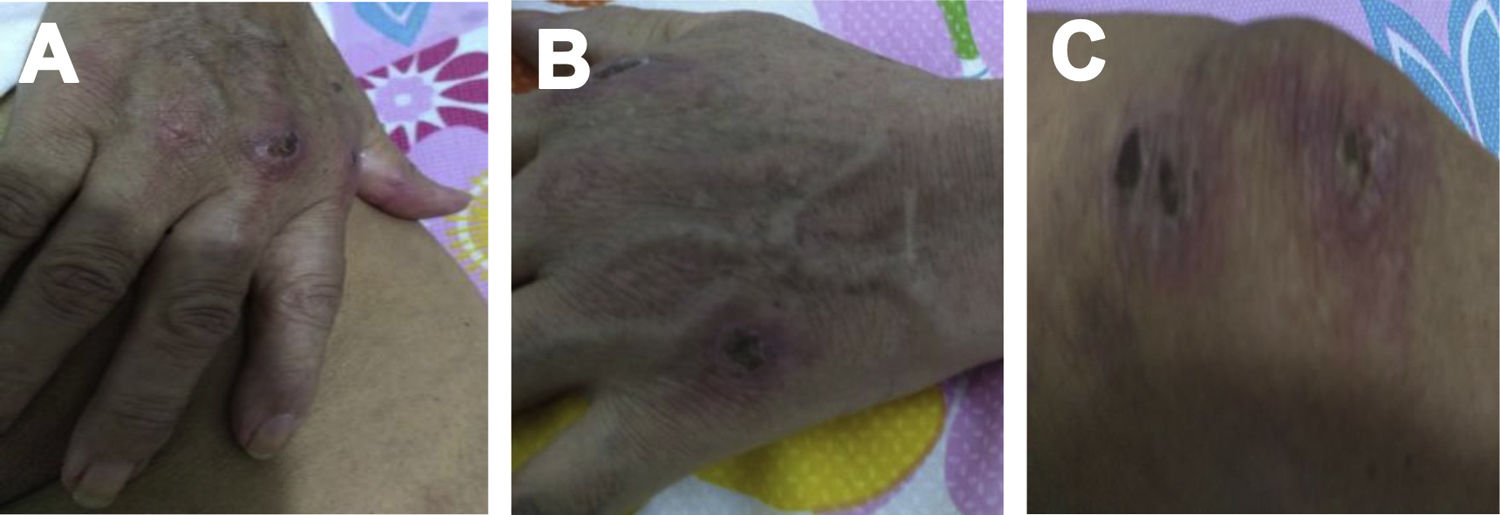
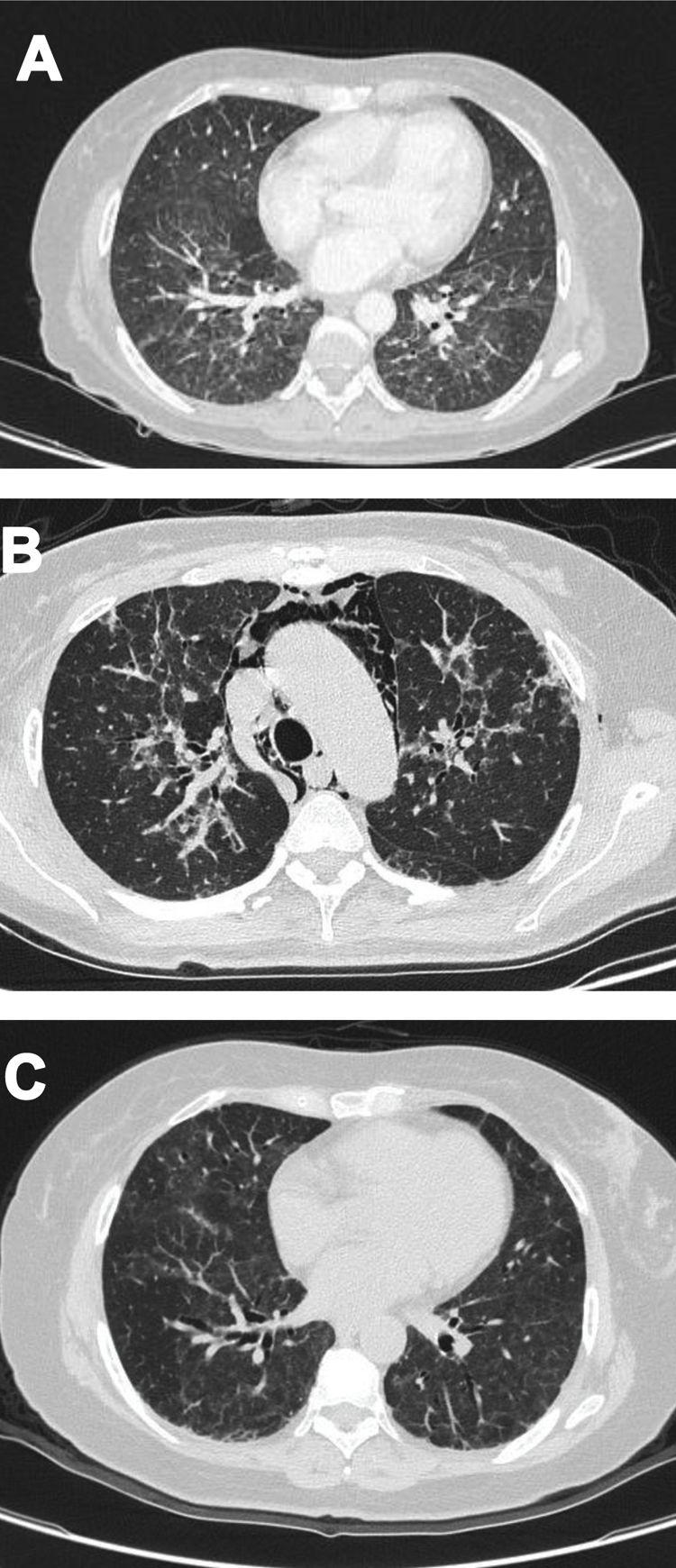
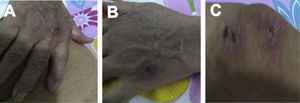
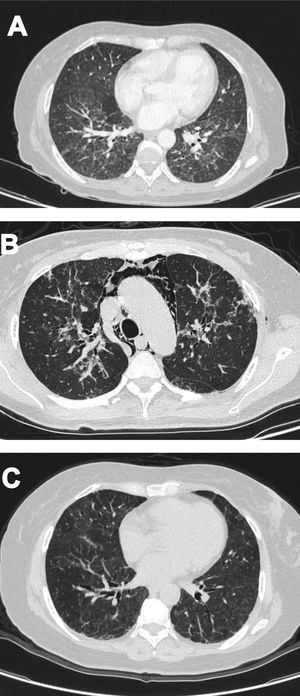
Case 2A 61-year-old woman with a past medical history of hypertension and hypothyroidism, was admitted to the hospital with a four-month history of malaise, dyspnea, fatigue, 8kg weight loss and fever. She was on acute respiratory distress but hemodynamically stable. Vital signs were BP: 110/70mmHg, heart rate: 130bpm, breathing 26 breaths per minute. Physical examination revealed livedo reticularis in lower limbs; non-suppurative superficial ulcerative Gottron's papules over the MCPs, elbows and knees, the ulcerations size was around 5mm (Fig. 2). A chest CT showed interstitial lung disease with NSIP pattern (Fig. 3A). Her ANA was positive (titer 1/320 with anti-centromere and cytoplasmic patterns) with positive profile for anti-nuclear ribonucleoproteins (RNP), anti-Sjögren's-syndrome-related antigen A (SS-A) and anti-Ro52; however, anti-dsDNA, antiphospholipid antibodies and Coombs test were negative, and the complement levels were normal. Hydroxychloroquine 200mg/d, prednisone 20mg/d and azathioprine 50mg/d were started. One month later, decreased of proximal muscular strength of upper and lower limbs was evident; however, muscle enzymes levels (LDH: 239U/l, CK: 24U/l) and electromyography were normal. Baseline tests revealed: WBC: 5130, lymphocytes: 872, platelets: 287,000/mm3, CRP: 4.0mg/l, ESR: 59mm/h and ferritin levels 396ng/ml (Table 1). Mycophenolate 2g/d and prednisone 25mg/d (0.5mg/kg) were started; however, after three months, dyspnea progressed to the point that minimal efforts precipitated it. A follow up chest CT was performed and showed subcutaneous emphysema and pneumomediastinum with progression of lung infiltrates (Fig. 3B). Due to these findings, anti-MDA5-associated CADM was suspected but myopathy panel was not performed because the patient could not afford it. Monthly IV-CYC 500mg/m2 to complete six months was started along with two doses of Rituximab 1g fortnightly. During the first two months of treatment, patient used supplemental oxygen at home on a prn basis. The patient gradually improved, prednisone was tapered to 7.5mg/d and tacrolimus 2mg/d was started as maintenance therapy by month 15th of her illness. Anti-MDA5 was assessed after one year of treatment and it was negative then. Currently, the patient is clinically stable, without need for supplemental oxygen; there has been imaging improvement after one year of treatment (Fig. 3C).
The authors have obtained written informed consent of the patients included in this study. Also, the information on the patients as noted in the manuscript does not allow their identification and is protected by confidentiality. Approval by the Ethics Committee was not required given that no experimental intervention took place.
DiscussionWe report two Peruvian patients with RP-ILD-associated CADM with pneumomediastinum, one of them was associated to anti-MDA5 antibody and the other one was highly suspicious of this association due to her clinical course but could not be proven since the test was not done. To our knowledge, these are the first cases published from our country and two of the few cases published from Latin-America.6–9 This might be explained since very few centers can perform the myopathy panel which includes the anti-MDA5 antibody and even if available it is exceedingly expensive and may not be covered by third party payors. In our country, no hospital performs this panel and its price in the private sector is about $800.00; for example, the case of a patient with overlap Systemic Lupus Erythematosus and Dermatomyositis syndrome who developed pneumomediastinum has been reported but unfortunately, like with our second patient, anti-MDA5 antibody could not be obtained.10
The pathogenesis of anti-MDA5-associated CADM is unknown. Recently, a linking with endothelial dysfunction has been reported, where anti-MDA5 DM patients have a higher levels of soluble intercellular adhesion molecule-1 (sICAM-1), soluble vascular cell adhesion molecule-1 (sVCAM-1), endotheline-1 (ET-1) and von Willebrand factor (vWF) than polymyositis or control patients.11 On the other hand, there is a possible role for lymphocytes; a study demonstrated a decreased count of lymphocytes in Anti-MDA5 DM patients which could be explained by the transferring of lymphocytes to the lungs,12 there is also a possible participation of activated monocytes/macrophages during the cytokine storm activation.13 A relationship between infection, genetic and environmental factors have also been proposed.14 Viral infection is believed to interacts with MDA5, therefore there is an increase of cytokines and activation of macrophages and helper T cells. Then, this would entail cell lysis with release of MDA5. This would result in antibody production against MDA5. Genetic factors, including the role of HLA-II has been supported in a Chinese population. Environmental factors have been studied in patients from China and Japan; an association between this disease and inhabiting rural areas around South East Asia rivers has been reported. These environmental factors may trigger a cytokine storm with predominant type I interferon signature.
An uncommon complication in RP-ILD-associated CADM patients is pneumomediastinum which can be explained by two possible mechanisms. The first one suggests that pneumomediastinum might be caused by an increase of the intra-alveolar pressure in the presence of ILD resulting in the air dissecting the perivascular sheaths and into the mediastinum.15 The second mechanism suggests that pneumomediastinum might be caused by necrosis of the bronchial wall due to co-existent vasculopathy; this finding is supported by the high frequency of cutaneous vasculopathy in CADM patients.16,17
As it has already been noted, the presence of anti-MDA5 in CADM patients is associated with higher risk of RP-ILD up to 16 times and with a tendency to deterioration despite immunosuppressive treatment.18 Risk factors associated with the development of RP-ILD include high ferritin levels (>450ng/mL), alveolar-arterial oxygen gradient levels greater than 30mmHg, a ground-glass opacity score ≥2 at the level of the right middle lobe19 and the presence of anti-Ro52 antibodies.17,20 Furthermore, partial pressure of arterial O2 around 62% at the first visit has been associated with poor prognosis.21 As to our patients, the first one had a high ferritin level and the second one had anti-Ro52 antibodies, which implies the presence of RP-ILD and worsened interstitial lung disease despite immunosuppressive treatment. It is worth noting that anti-Ro52 could be involved in the pathogenesis of this condition; in fact, as it has been proposed that the interaction between MDA5 and Ro52 induces the formation of molecular complexes and subsequently increased immunogenicity.22
Currently, the guidelines’ level evidence of about anti-MDA5-associated CADM treatment is limited to medical records review studies which have shown that combined therapy with corticosteroids plus CYC and/or a calcineurin inhibitor have the best outcomes.23 In our cases, IVIG and CYC were chosen which have shown to work very well in refractory cases as well.24 However, triple therapy with prednisolone, a calcineurin inhibitor and CYC as initial treatment has shown to improve survival.21 On the other hand, it has been reported that rituximab could be useful in the treatment of these patients25; moreover, it is possible to test negative for the anti-MDA5 antibody after receiving this treatment.26 This last assertion might explain why our second patient was anti-MDA5 negative as this antibody was assessed after treatment with CYC and rituximab have been ongoing for 12 months.
In conclusion, we report two cases of RP-ILD-associated CADM complicated with pneumomediastinum. One of these cases, to our knowledge, is the first reported of anti-MDA5-associated CADM in the Peruvian population and it is one of the few published cases from Latin-America.
Conflict of interestsThe authors declare that they have no conflict of interest.