Optic neuritis is a rare ocular manifestation of inflammatory bowel diseases and spondyloarthritis. The use of tumour necrosis alpha inhibitors to treat these conditions remains possible. We report a case of a 62-year-old-woman with a 17 year-history of axial and peripheral spondyloarthritis associated with Crohn's disease, treated with tumour necrosis alpha inhibitors, who developed an asymmetric retrobulbar optic neuritis that promptly responded to a high dose of steroids.
La neuritis óptica es una manifestación ocular poco común de las enfermedades inflamatorias intestinales y de la espondiloartritis. El uso de fármacos contra el factor de necrosis tumoral (anti-TNF) para tratar estas patologías sigue siendo posible. Se presenta el caso de una mujer de 62 años con un historial médico de espondiloartritis axial y periférica asociada con la enfermedad de Crohn, tratada con anti-TNF y que padece una neuritis óptica asimétrica. La paciente alcanza una respuesta favorable a la terapia basada en los esteroides.
Retrobulbar optic neuritis (RBON) is an inflammatory condition of the optic nerve in which the disease process occurs behind the lamina cribrosa.1 It is frequently associated with demyelinating diseases as Multiple Sclerosis (MS),2 autoimmune disorders and infections.3 However, many other rare causes have been identified. We report the case of a patient with enteropathic spondyloarthritis treated with tumor necrosis factor alpha inhibitors (TNF alpha inhibitors) presenting a RBON.
Case presentationOur patient is a 62-year-old-woman with a family medical history of MS, a 17 year-history of axial and peripheral spondyloarthritis associated with Crohn's disease (CD), with no previous neurologic or ocular manifestation. She was initially treated with a 20mg/week of oral Methotrexate for two years, which failed to bring her symptoms under control. Then, she received Infliximab (5mg/kg). She became asymptomatic after Infliximab-induction therapy (weeks 0, 2, and 6). The treatment was discontinued after the eightieth dose for secondary non-response. Then, she was treated with Etanercept (50mg/week), discontinued after 4 years for lymphopenia, and lastly with 40mg/2 weeks of Adalimumab. One year after the switch to Adalimumab, anti-nuclear, anti-double stranded, and anti-histone antibodies were positive, consistent with a drug induced lupus. Adalimumab was discontinued for 6 months then reintroduced after an immunologic remission.
Two years later she presented with an occipital headache, central blind spots in both eyes, and pain with her left eye movement too. At the time of presentation, the neurologic physical examination found a quadripyramidal syndrome, a 10° convergent strabismus in the left eye and a normal pupillary reflex. Extra-ocular movements were full with no overreactions. The visual acuity was 20/20 in both eyes. Anterior segment examination was unremarkable and no optic disc swelling was observed. Formal visual field showed the presence of caecocentral scotoma in both eyes.
Laboratory tests demonstrated normal levels for complete blood count and no vitamin B12 deficiency was detected. The Neuromyelitis optica (NMO) antibodies were negative. A visual evoked potential showed prolonged latency consistent with bilateral demyelinating RBON. MRI of the brain was otherwise normal.
The diagnosis of an asymmetric bilateral RBON was made. Both anti-TNF therapy and inflammatory diseases (CD and spondyloarthritis) were incriminated. The patient received 1g intravenous Methylprednisolone per day on 3 successive days with a dramatic improvement of blind spots and eye pain. A registration of the case was recorded in the national pharmacovigilance database under the number 482/2012 on April 29, 2019. According to the French method,4 the imputability score was C2S1 I1. The decision was to discontinue Adalimumab.
An ophthalmic and a neurologic follow-up were recommended. No signs of relapse were noted during follow-up visits.
DiscussionThe frequency of ophthalmic involvement in CD is 6.8%.5,6 It is more frequently seen in the first follow-up year, during activity of the bowel disease, in colonic localisation and in the case of coexistence of articular manifestations (entheropathic arthritis).7
Episcleritis and acute anterior uveitis are the most frequent ocular complications.7 The latter is also reported as the most common extra-articular manifestation of Spondyloarthritis, affecting more than 20% of the patients.8 Many other ophthalmic manifestations have been described in relation to inflammatory bowel disease (IBD) such as glaucoma, keratitis and dry eyes.7 However, the reported incidence of posterior segment involvement ranges between less than 1% and 30%, depending upon the series.8,9 Optic neuritis (ON), which is an inflammation or demyelination or a degeneration of the optic nerve, may be present in up to 4% of adult IBD patients.9,10 Recently, a Taiwanese population-based study aimed to evaluate possible factors associated with ON in patients with IBD11: IBD patients who were not with concomitant ON on the index date were included. All the patients were followed up until the development of ON or the end of the study period (the mean follow-up time was of 7 years). A four-fold matched group was selected using age, sex and year of index date for comparison. At the study period conclusion, eight (0.18%) and five (0.003%) patients with and without IBD, respectively, had developed ON (p=0.001). Among the eight IBD patients who developed ON only one patient was diagnosed with Crohn's disease, and the male gender was slightly dominant. Adjusted HRs showed that patients aged between 30 and 39 years, with comorbidities including neuromyelitis optica, acute disseminated encephalomyelitis systemic lupus erythematosus and with a higher Charlson Comorbidity Index, had a significantly higher risk of developing ON (all p<0.005).11
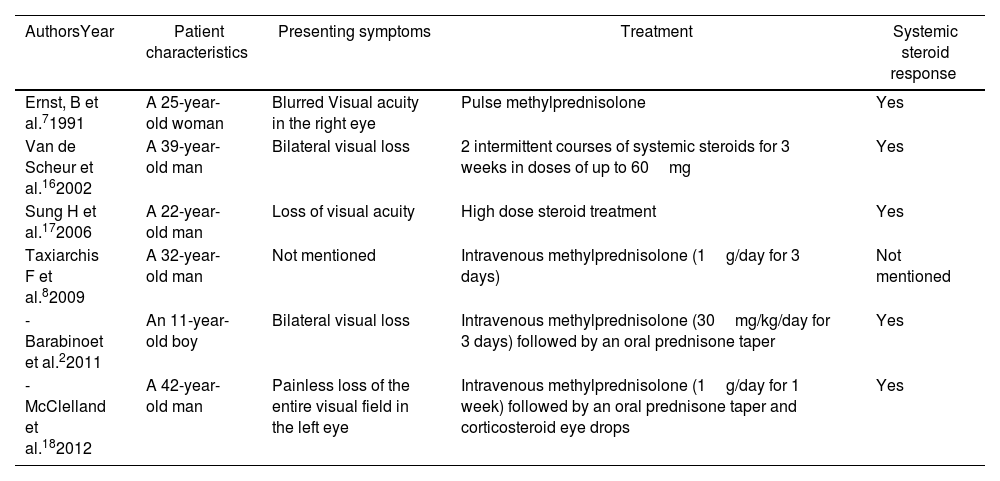
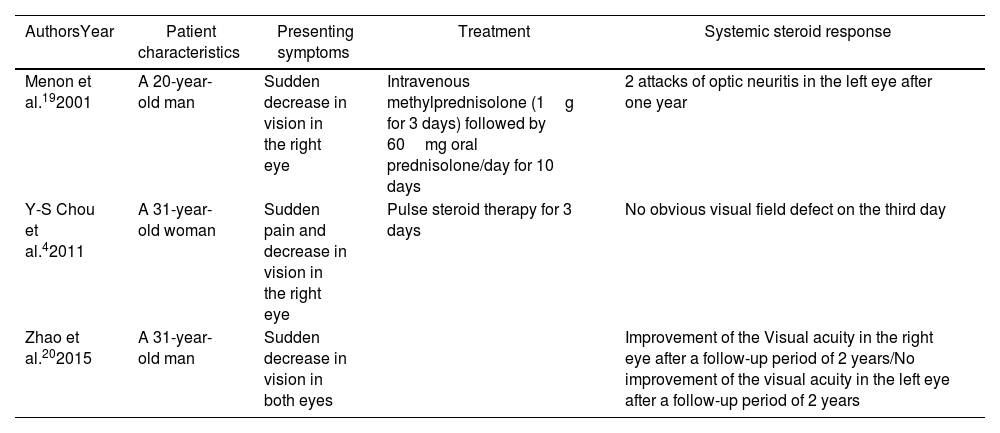
To our knowledge, only 6 documented cases of ON with CD were reported in the literature (Table 1).12 However, the co-existence of ankylosing spondylitis (AS) and ON were only reported in three cases (Table 2).13
Cases of co-existence of Crohn's disease and optic neuritis in the absence of anti-TNF therapy reported in the literature.
| AuthorsYear | Patient characteristics | Presenting symptoms | Treatment | Systemic steroid response |
|---|---|---|---|---|
| Ernst, B et al.71991 | A 25-year-old woman | Blurred Visual acuity in the right eye | Pulse methylprednisolone | Yes |
| Van de Scheur et al.162002 | A 39-year-old man | Bilateral visual loss | 2 intermittent courses of systemic steroids for 3 weeks in doses of up to 60mg | Yes |
| Sung H et al.172006 | A 22-year-old man | Loss of visual acuity | High dose steroid treatment | Yes |
| Taxiarchis F et al.82009 | A 32-year-old man | Not mentioned | Intravenous methylprednisolone (1g/day for 3 days) | Not mentioned |
| -Barabinoet et al.22011 | An 11-year-old boy | Bilateral visual loss | Intravenous methylprednisolone (30mg/kg/day for 3 days) followed by an oral prednisone taper | Yes |
| -McClelland et al.182012 | A 42-year-old man | Painless loss of the entire visual field in the left eye | Intravenous methylprednisolone (1g/day for 1 week) followed by an oral prednisone taper and corticosteroid eye drops | Yes |
Cases of co-existence of ankylosing spondylitis and optic neuritis in the literature.
| AuthorsYear | Patient characteristics | Presenting symptoms | Treatment | Systemic steroid response |
|---|---|---|---|---|
| Menon et al.192001 | A 20-year-old man | Sudden decrease in vision in the right eye | Intravenous methylprednisolone (1g for 3 days) followed by 60mg oral prednisolone/day for 10 days | 2 attacks of optic neuritis in the left eye after one year |
| Y-S Chou et al.42011 | A 31-year-old woman | Sudden pain and decrease in vision in the right eye | Pulse steroid therapy for 3 days | No obvious visual field defect on the third day |
| Zhao et al.202015 | A 31-year-old man | Sudden decrease in vision in both eyes | Improvement of the Visual acuity in the right eye after a follow-up period of 2 years/No improvement of the visual acuity in the left eye after a follow-up period of 2 years |
On the other hand, a possible association between IBD and MS was hypothesized and an approximately 3-fold increased risk of MS in IBD patients was suggested.13 A retrospective study performed to examine the relation between the two diseases in the era before TNF alpha inhibitors showed a small but significant association.12 Patients with CD and ulcerative colitis were 54% and 75% more likely than community controls to have been diagnosed with MS, ON or other demyelinating conditions.14 ON was recorded in 6 out of 7988 CD patients (0.08%) and in 17 out of 12,185 ulcerative colitis patients (0.14%), in comparison with 50 out of 80,666 controls (0.06%).12
TNF alpha inhibitors which are commonly used for the treatment of IBD and refractory rheumatic diseases are incriminated in increasing the risk of developing MS and ON. By 2001, the Food and Drug Administration had received more than 20 reports of MS or other demyelinating conditions in patients treated with these medications.12 In 2004, clinician and patient warnings were updated on 3 major anti-TNF α therapies: Etanercept, Adalimumab, and Infliximab.12 The mechanism of this complication is not yet well elucidated. A hypothesis suggests that systemically administered anti-TNF agents may inhibit the apoptosis of self-reactive T cells but fail to penetrate and reach the central nervous system, inducing an autoimmune demyelinating process.15 The other hypothesis suggests an activation of infecting microorganisms which may result in a demyelinating process.15
In our case, the incrimination of anti-TNF α therapy remains possible, but it is less likely to be the sole cause of the RBON, since one of the most common criterion for causality, which is the absence of alternate cause, is lacking.4
ConclusionON is rarely the ocular manifestation of AS or CD. To date, the reported cases of demyelination after anti-TNF therapy suggest a possible causal relationship. However, ON can also be coincidental. Further research should be conducted to elucidate the mechanism of this complication which is a serious adverse event.
Ethical considerationsThe institution ‘s ethics committee was not obtained since this article does not contain personal information that allows to identify the patients. The research complies with the current regulations on bioethical research.
Conflict of interestNone.
None.