Turner's syndrome (TS) is one of the most common sex chromosome disorders caused by numeric or structural abnormalities of the X chromosome. A case of TS and Systemic Sclerosis (SSc) is reported, along with a summary of all associated TS/autoimmune diseases described in English literature from 1948 to 2020, using a search in MEDLINE (Pubmed). A 32-year-old woman affected by TS was seen due to inflammatory arthralgia in small joints and dysphagia, as well as a two-year history of Raynaud's phenomenon and puffy hands. Biohumoural laboratory tests and severity scales revealed changes that allowed us to diagnose SSc. This case report emphasises the role played by sex hormones and chromosomal abnormalities in the pathogenesis of autoimmune disorders, and to our knowledge, this is the only case described in literature of a TS patient who developed SSc.
El síndrome de Turner (TS) es uno de los trastornos cromosómicos sexuales más comunes causados por anomalías numéricas o estructurales del cromosoma X. En este documento informamos de un caso de TS y esclerosis sistémica (SSc) y resumimos toda la asociación de TS/enfermedades autoinmunes descrita en la literatura inglesa de 1948 a 2020, encontrada buscando en MEDLINE (PubMed). Una mujer de 32 años afectada por TS acudió a nuestra observación debido a la artralgia inflamatoria en pequeñas articulaciones y disfagia y 2 años de historia del fenómeno de Raynaud y las manos hinchadas. El laboratorio biohumoral y las pruebas instrumentales revelaron alteraciones que nos permitieron diagnosticar SSc. Nuestro informe de caso hace hincapié en el papel desempeñado por las hormonas sexuales y las anomalías cromosómicas en la patogénesis del trastorno autoinmune; y hasta nuestro conocimiento, este es el único caso descrito en la literatura de un paciente TS que desarrolló SSc.
Turner's syndrome (TS) is one of the most common sex chromosome disorders caused by numeric or structural abnormalities of the X chromosome. The minimal diagnostic criterion for TS is an abnormal karyotype in which a portion or the entire one of the two X chromosomes is missing.1
Phenotype includes female gender, short stature, primary ovarian failure, physical features deriving from fetal lymphedema and skeletal abnormalities and gonadal dysgenesis. Other clinical features observed in TS are represented by congenital cardiovascular defects, atherosclerosis, osteoporosis and fractures, endocrine and metabolic disorders, hearing loss and specific cognitive deficits that contribute to increased morbidity and mortality and decreased life expectancy in this syndrome.2 The karyotypes are non-mosaic or mosaic, including 45,X, 46,X,del(Xp), 46,X,i(Xq), 45,X/46,XX, 45,X/46,XrX, 45,X/46,XY, 45X/47XXX. 45,X is a classical type.3
The possible association between TS and autoimmune diseases was hypothesized several decades ago.4
Nowadays it is clear that the risk of autoimmune diseases in patients with TS is approximately twice as high as in the general female population.5 The occurrence of various autoimmune diseases in TS patients has been reported, including, Hashimoto's thyroiditis, type 1 diabetes (T1DM), inflammatory bowel diseases (IBD), Addison's disease, psoriasis, celiac disease, juvenile idiopathic arthritis, systemic lupus erythematosus and some cutaneous disorders as vitiligo, Halo nevus and alopecia areata.5–7
However, except for the thyroid autoimmunity there seems to be no clear agreement about the actual risk of other autoimmune diseases, neither of the overall burden of autoimmunity in TS. In addition, the reason why TS predisposes to autoimmunity remains unclear.
In this paper, we report a case of TS and Systemic Sclerosis (SSc) and summarize all TS/autoimmune diseases association described in English literature.
Our case report emphasizes the role played by sex hormones and chromosomal abnormalities in the pathogenesis of autoimmune disorder, and to our knowledge, this is an uncommon case of a patient with TS who developed SSc.
Ethical considerationsThe patient gave her consent for information about herself and relative to appear in the Journal and associated publications.
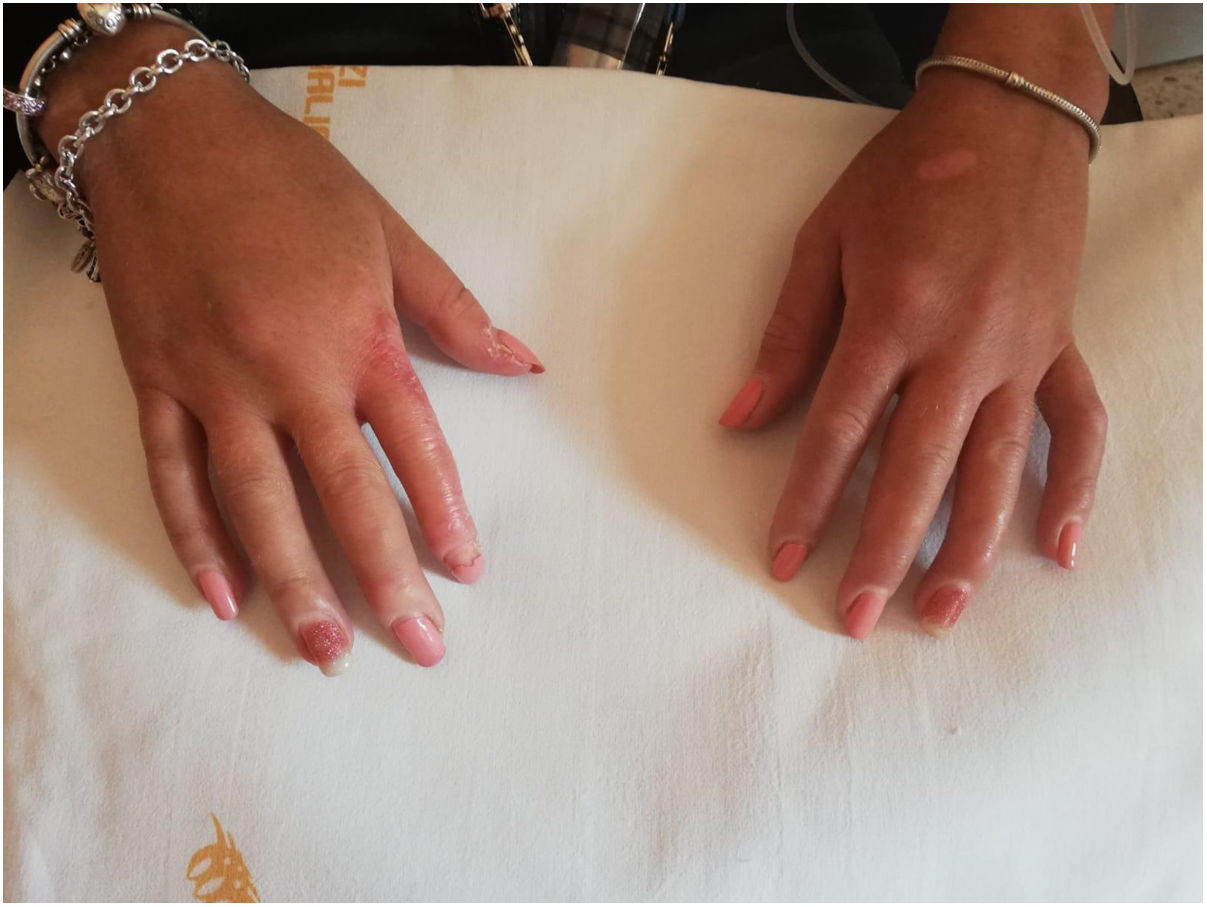
Case reportA 32-year-old Caucasian woman comes to our observation owing to inflammatory arthralgia in small joints and dysphagia occurred a few months ago and two years history of Raynaud's phenomenon and puffy hands (Fig. 1). She was a housewife. She had never come into contact with skin irritants. She was diagnosed with TS at birth owing to the presence of length less than the 10th percentile for gestational age, dysmorphic notes (low hairline, ear pads, and pterigium colli). Chromosomal abnormality was confirmed by cytogenetic analysis (karyotype 45X). Moreover at age of 18 years she was diagnosed with Hashimoto's thyroiditis and osteoporosis. She was being treated with oral estroprogestinic and levothyroxine. The sole rheumatological familiarity was the paternal grandma with rheumatoid arthritis.
On physical examination: short stature (135cm in height) and juvenile female external genitalia, sclerodactyly with skin thickening of the fingers of both hands extending distal to metacarpal-phalangeal joint, microcheilia, telangiectases, Modified Rodnan skin score 6/51. There are not fingertip lesions; but she described some alterations occurred in the winter months of the previous two years described as fingertip pitted scars. Mild intellectual disability. Biohumoral Laboratory tests revealed ANA (Antinuclear Antibody) 1:1280 speckled pattern, ENAs are absent. Esophageal scintigraphy highlighted areas of stasis along the esophageal tract. Periungueal video-capillaroscopy showed a scleroderma late pattern. Cardiological and pneumological instrumental examinations (ECG, echocardiogram, chest X-Ray, global spirometry, 6MWT) showed no abnormalities. She did not complain of dyspnoea, but cardio-pulmonary examinations were performed in order to exclude abnormalities secondary to TS too. Patient underwent echocardiography as routine basal screening to assess the presence of direct and indirect signs of pulmonary arterial hypertension (PAH) and the possible presence of pericardial effusion. DETECT algorithm did not suggest referral to (right heart catheterization) RHC and was subsequently used in the patient's follow-up. Actually, patients underwent lung HRTC, which according to pulmonary function tests and chest X-Ray showed no parenchyma alterations consistent with interstitial lung disease.
She was diagnosed with Systemic sclerosis, according to ACR/EULAR 2013.8 She was started methotrexate 10mg/w and hydroxycloroquine 200mg/day.
DiscussionTS is a genetic condition in which a female is partly or completely missing an X chromosome. Typically, they develop menstrual periods and breasts only with hormone treatment, and are unable to have children without reproductive technology. Heart defects, diabetes, and low thyroid hormone occur more frequently. No cure for Turner syndrome is known. Treatment may help with symptoms. Human growth hormone injections during childhood may increase adult height. Estrogen replacement therapy can promote development of the breasts and hips. Medical care is often required to manage other health problems with which TS is associated.
An electronic literature search on TS and autoimmune disorders was conducted using MEDLINE PubMed. Case reports published from 1948 to 2020 were evaluated.
Autoimmune thyroid diseases and thyroid autoimmunityThe relationship between thyroid disease and TS was first suggested by Atria et al. in 1948 when they reported the postmortem findings of a small thyroid gland with lymphocytic infiltration in a young TS woman.7
Antithyroglobulin antibodies and hypothyroidism can be seen in association with any karyotype, but patients with an isochromosome X appear to be at the highest risk for acute Hashimoto's thyroiditis and Graves’ disease.4,9
Pai and associates found elevated thyroid antibody titers in 9 of 15 of their patients over 10 years of age (60 percent).10
A prevalence of hypothyroidism in one of every five TS patients in a Danish cohort confirms such disease as a significant contributor to the increased morbidity in both paediatric and adult TS populations with a prevalence of autoimmune disease increasing with age.11 In this study anti-TPO did not present before the age of 12 years. Here, in fact, despite the relatively low number of paediatric TS subjects, these findings may support earlier findings of thyroiditis in TS as emerging in the beginning of the second decade and remaining a significant issue in adulthood.11,12
Finally it may also be that cases of hypothyroidism are under-reported because of subclinical presentation which is a biochemical condition characterized by serum TSH above the upper limit of the reference range and serum FT4 levels within the reference range and an associated diagnostic delay.13
Type 1 diabetes mellitusThe increased incidence of DM in TS patients was first reported almost 50 years ago by Ann Forbes and Eric Engel.14
Diabetes mellitus occurs more frequently in women with TS (2 percent to 15 percent) as compared with age-matched controls,4 but the first study of patients with TS 25 percent to 60 percent have diabetic levels on oral glucose tolerance tests without frank diabetes (range that are also changed in the years), moreover antibodies to islet cells have not been measured in diabetic patients with TS.15
Abnormal glucose metabolism is found in >70% of adults affected by TS (12, 13); the abnormalities include impaired glucose tolerance (IGT), hyperinsulinaemia, and reduced insulin sensitivity.16
An epidemiologic study in Denmark showed that the incidence of type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) in TS patients is 11 times and 3–4 times greater than that in healthy people, respectively.17 However, clinical endocrinal studies in adult populations suggest that the phenotype of gradually progressive, adult-onset glucose intolerance is more likely to be T2DM. Indeed, the link between TS and T1DM is not well characterized.16
It is hypothesized that occurrence of DM in TS is linked to insulin resistance or impaired β-cell function, with deranged insulin secretion by mechanisms that are not entirely clear.18
A prospective cross-sectional study of Danish TS patients (n=107, median age 36.7 years, range: 6–60 years) showed glutamic-acid-decarboxylase 65 autoantibodies (anti-GAD-65) was present in 4%, which is somewhat above the general population prevalence of 1.1% as reported across adult age-groups.11 Among four patients with anti-GAD-65 none had T1DM, but two were classified as having T2DM. Overall, three patients had been diagnosed as diabetics, of whom two were classified as T2DM (both anti-GAD-65-positive) and one T1DM (anti-GAD-65-negative). Although a relatively small cohort, these findings point towards an increased risk of diabetes in TS, as shown previously,17 and it may be speculated that the non-diabetic anti-GAD-65-positive patient might eventually develop diabetes. Further, the presence of anti-GAD-65 in four patients could be interpreted as indicative of more patients than anticipated previously suffering from T1DM misclassified as T2DM. It is therefore proposed that all patients with TS and newly developed diabetes are tested for GAD-65 antibodies.
Anti-GAD-65 was increased in isochromosomal karyotypes (3/23 versus 1/84, p=0.008) with no other association found between autoantibodies and karyotype.11
Inflammatory bowel diseases, coeliac disease and other gastrointestinal autoimmune diseasesInflammatory bowel diseases (IBD) is comprised of two major disorders, Crohn's disease (CD) and ulcerative colitis (UC). Association between TS and IBD has been described the first time in 1979.19
The incidence of TS with IBD is rare,17 and the pathogenic mechanism linking TS with IBD has not been elucidated, but hormone therapy often used to treat these patients, seems to be a susceptibility factor to IBD occurrence.19,20
Since the 1970s, several reports have indicated an association between TS and CD.21 However, most data have been limited to case series at specialist centers for TS and have yielded inconsistent estimates for CD prevalence in TS (range, 2–9%).22,23 Often is accompanied with other autoimmune disorders. The available data and publications indicate that screening for CD should be performed in patients with TS, even before clinical symptoms emerge to avoid diagnostic delay, and intestinal biopsy should be carried out in patients with positive results.24
An association of TS with autoimmune cholestatic liver disease is not well understood, but some cases are described.25–27
Skin manifestationTS has been associated with several cutaneous autoimmune abnormalities including psoriasis, alopecia areata, vitiligo, Halo nevus and lichen planus.3,5–7,28
The relative risk of developing psoriasis in TS patients is 2.25 fold higher than in the general population.6 Some authors explain this elevated risk with the fact that psoriasis and TS probably have common pathogenetic factors; both these conditions, for example, share the association with multiple cardiovascular risks and comorbidities, including metabolic syndrome and T2DM especially in adults.
Alopecia areata is reported to affect TS patients 3 times more frequently than the general population.6
An increased frequency of pigmented Nevi is described, but few reports consider nevi in detail. Halo nevus (HN) is clinically defined as a melanocytic nevus surrounded by a halo of depigmentation.
Vitiligo, a dermatologic disorder characterized by the presence of depigmented patches on the skin, has been described in the list of cutaneous findings associated with TS. In contrary to the common belief, Halo nevus, rather than vitiligo, is the typical dermatologic finding of TS.29
In the end Chakhtoura et al. reported lichen sclerosus in 17.3% of their TS patients30 and Cadoret F report an uncommon case of a pregnant woman with TS whose pregnancy (with favorable outcome) was complicated by a pemphigoid gestationis in third trimester.31 Pemphigoid gestationis or gestational pemphigoid is a rare pregnancy-associated autoimmune skin disorder that is immunologically and clinically similar to the pemphigoid group of autoimmune blistering skin disorders. The pathogenesis is not yet fully established, but it belongs to the group of autoimmune skin disorders characterized by an immune response directed against different hemidesmosomal proteins affecting the adherence between the dermis and epidermis causing blistering of the skin and mucosal membranes.
Rheumatic diseases and other immune-related conditionsRecent reports have described an association with juvenile idiopathic arthritis (JIA) and psoriatic arthritis (linked to the presence of psoriasis). JIA is an autoimmune condition that might be associated with TS. The prevalence seems to be at least six times greater than would be expected if the two conditions were only randomly associated.32 Investigators claim that suspicion of an underlying inflammatory arthritis is warranted in search for radiological findings consistent with JIA in girls with TS and joint symptoms; conversely believe that it is important to consider the diagnosis of TS in girls with JIA, recognizing that characteristic radiographic findings, such as metacarpal shortening, are usually present in these patients.33 We remember the commonly reported X Rays changes in TS patients: in the hands, drumstick distal phalanges, short fourth metacarpals, carpal sign: change in angulation of the carpal bones, shortening of all hand bones, madelung's deformity; in feet, similar to hands, pes cavus; lateral dislocation of the patellae, hypoplastic patellae, irregularity of tibial metaphysis and epiphysis, “Mushroom” projections, medial surface of the proximal tibial metaphyses (medial tibial condyle), scoliosis, lack of lumbar lordosis, Schmorl's nodes, hypoplasia of the arch of the atlas, shortening of anteroposterior diameter of vertebral bodies; thin ribs, developmental abnormalities; in pelvis, android configuration (50 percent), occasional widening of symphysis pubis; in Skull midfacial hypoplasia deepening of posterior cranial fossa and widely spaced mandibular rami.4 However there are scarcity of literature addressing JIA-TS association and the reports are relatively old.
Several ocular diseases have been associated with TS in the past, including one case only of proven iridocyclitis. Thus, uveitis should be included in the list of ocular manifestations in TS. It may tend to become chronic and may be found especially in those patients presenting other associated autoimmune systemic disease.34
Other manifestation of the known tendency for Turner patients to develop immunologic disease are glomerulonepritis.35–37
Although rare, few cases of LES are described in a TS patient.38 It is documented that male patients with Klinefelter's syndrome (XXY) have similar risk to develop SLE compared to females (XX). This is consistent with the low prevalence of SLE in TS, characterized by an abnormal or missing X chromosome.
In the end in the literature are also described others autoimmune conditions in TS, such as myasthenia Gravis.39,40
Moreover, frequently observed autoimmune diseases in TS are also seen in the autoimmune polyendocrine syndrome type I (APS I), of which Addison disease is a key component, so much that an overlapping antibody profile between TS and APS I could be considered.41
To our knowledge our case reposrt is the first description of a TS patients that develops SSc. The fact that SSc affects women more frequently than men is consistent with the low prevalence of SSc in TS, characterized by an abnormal or missing X chromosome.42,43 Unlike, there are described Klinefelter's syndrome (XXY) associated with SSc.42
Another point to consider is that the diagnosis of SSc in our patient occurred after hormonal therapy. The impact of the estrogenic therapy on immune system cells and on pro-inflammatory cytokine regulation as a factor for a higher tendency of developing autoimmune diseases in women is being investigated.44
Some limitations of our clinical case must be taken into account: we have not information about alterations of cytokine and immunological aberrations of the patient.
Even though the pathogenesis of these associations is unknown, possible factors explaining higher autoimmune dysregulation in TS include X-chromosome genes haploinsufficiency, X chromosome origin, excessive production of proinflammatory cytokines, hypogonadism and estrogenic therapy.6,44
ConclusionThe presented clinical case shows a higher incidence of autoimmune diseases in TS, in line with the literature. The presence of a high risk of developing autoimmune diseases, including SSc, in patients with TS requires careful clinical evaluation aimed at this in all patients.
Particular attention must also be paid to the use of estrogen therapy as it can detect immune-based pathologies such as SSc. The pathogenetic mechanisms of SSc in TS is not clear. Further researchers are needed to shed light on the relationship between TS and SSc.
FinancingThere is not financial support.
All authors are responsible for the entire content of the letter.
PresentationThe content of the manuscript has never been presented in professional meetings.
Conflicts of interestNone.