Pompe disease, or glycogen storage disease type II, is an autosomal recessive disorder due to the deficiency of lysosomal acid α-glucosidase, the enzyme responsible for degrading glycogen to glucose. The adult-onset form is rare and is characterized, primarily by accumulation of glycogen in striated, cardiac, and smooth muscle tissue. It causes muscle weakness of proximal predominance, so it can be confused with an inflammatory myopathy. The case is presented of a 60 year-old adult with a previous diagnosis of polymyositis in whom Pompe disease was confirmed with a demonstration of the enzymatic deficit in a biological substrate and a genetic identification was obtained.
La enfermedad de Pompe o glucogenosis tipo II es un trastorno autosómico recesivo, debido a la deficiencia de la enzima lisosomal α-glucosidasa ácida encargada de degradar glucógeno a glucosa. La forma de inicio en el adulto es rara y se caracteriza fundamentalmente por acumulación de glucógeno en tejido muscular estriado, cardiaco y liso. Causa debilidad muscular de predominio proximal, por lo que se puede confundir con una miopatía inflamatoria. Se presenta el caso de un adulto de 60 años con diagnóstico previo de polimiositis, en quien se confirmó una enfermedad de Pompe con demostración del déficit enzimático en sustrato biológico y se logró realizar una identificación genética.
Pompe disease (glycogen storage disease type II, OMIM 232300) is a rare disease of autosomal recessive inheritance, initially described in 1932 by the Dutch pathologist Johannes C. Pompe.1,2 It is caused by the genetic deficiency of the lysosomal enzyme acid α-glucosidase (AAG), which leads to abnormal accumulation of glycogen in the cells, with dysfunction of multiple organs, but mainly the muscles.3,4 The accumulation of glycogen in the striated muscle causes muscle dysfunction in patients, which is typically manifested by muscle weakness of proximal predominance, being a differential diagnosis of other myopathies. The muscle commitment can extend to the respiratory muscles generating diaphragmatic paralysis, alveolar hypoventilation and, in some cases, respiratory failure and death.3,5,6 Cardiac accumulation with manifestations such as cardiac hypertrophy and cardiac rhythm disorders has also been documented.3,7,8 The disease has an infantile presentation but there is also a late presentation (after one year of age), which is a diagnostic challenge.4,7,8 In patients with the classical infantile form the disease manifests itself in the first months of life with hypertrophic cardiomyopathy, significant hypotonia and skeletal muscle weakness and, if they are not treated, they typically die from heart failure during the first year of life.6,8 Patients with the late-onset form have a milder phenotype, which typically consists of proximal skeletal muscle weakness with slow progressive myopathy, but they rarely have cardiac affectation.4,6,7,9 The early onset of the disease results from a complete or almost complete deficiency of the functional AAG protein, while late-onset patients maintain some residual enzyme activity.2 The late form, and especially when it occurs after the fifth decade, is not common.6
Clinical caseA 60-year-old male patient, who had an established diagnosis of an autoimmune myopathy of polymyositis type and met 3 of 4 criteria of Bohan and Peter, supported by progressive muscle weakness, electromyographic alterations with a myopathic pattern and elevation of muscle enzymes, was managed with prednisolone 30mg daily, azathioprine 75mg daily and physical therapy. The patient showed a poor response to the treatment established, and therefore he decided to consult.
The clinical examination showed significant atrophy in the anterior and posterior muscles of the arms, as well as in the thighs and hips. The muscle strength in the upper limbs was decreased: proximal 3/5 and distal 4/5. Weakness was also observed in the lower limbs: 2/5 proximal and 2/5 distal, in addition to patellar hyporreflexia. There was no sensory deficit. The Gowers’ sign was positive. The rest of the clinical examination and the vital parameters were normal.
Due to the patient's poor response to the treatment received, it was decided to reconsider the diagnosis and perform new studies (Table 1), which included a metabolic profile that was normal, an electromyography of the four extremities and a muscle biopsy. The electromyography was abnormal, showing alterations compatible with progressive muscular dystrophy and there were abundant signs of hyperexcitability of the cell membrane, characteristic of inflammatory myopathies. The muscle biopsy of the distal third of the biceps of the left arm showed loss of muscle fibers with replacement by adipose tissue. When immunohistochemical studies were performed, no inflammation was observed and infiltrates indicating an inflammatory myopathy were not found. Periodic acid Schiff (PAS) stains were not performed.
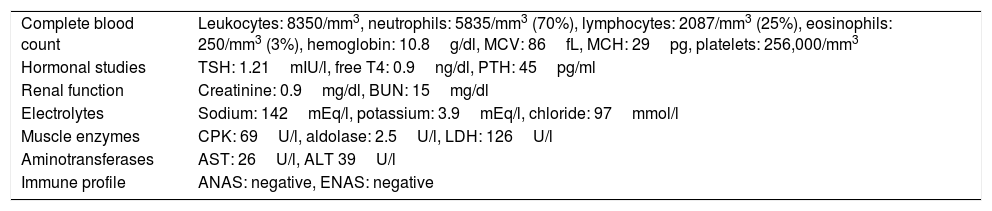
Results of the general laboratory tests.
| Complete blood count | Leukocytes: 8350/mm3, neutrophils: 5835/mm3 (70%), lymphocytes: 2087/mm3 (25%), eosinophils: 250/mm3 (3%), hemoglobin: 10.8g/dl, MCV: 86fL, MCH: 29pg, platelets: 256,000/mm3 |
| Hormonal studies | TSH: 1.21mIU/l, free T4: 0.9ng/dl, PTH: 45pg/ml |
| Renal function | Creatinine: 0.9mg/dl, BUN: 15mg/dl |
| Electrolytes | Sodium: 142mEq/l, potassium: 3.9mEq/l, chloride: 97mmol/l |
| Muscle enzymes | CPK: 69U/l, aldolase: 2.5U/l, LDH: 126U/l |
| Aminotransferases | AST: 26U/l, ALT 39U/l |
| Immune profile | ANAS: negative, ENAS: negative |
ALT: alanine transaminase; ANAS: antinuclear antibodies; AST: aspartate aminotransferase; CPK: creatine phosphokinase; ENAS: extractable nuclear antigen antibodies; LDH: lactic dehydrogenase; PTH: parathyroid hormone; TSH: thyroid stimulating hormone.
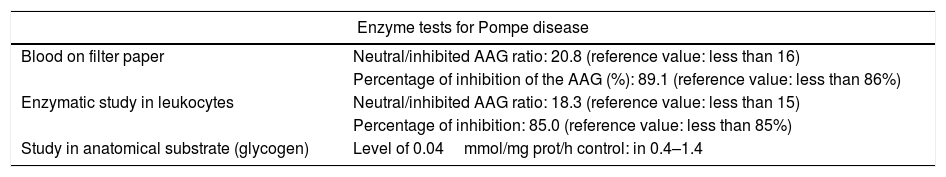
Given the diagnostic uncertainty, a screening for Pompe disease was carried out with a blood spot on filter paper, which was altered, observing a high neutral/inhibited α-glucosidase ratio and a high percentage of inhibition of the AAG. Taking into account the positive screening test, an enzymatic study on leukocytes was carried out, with a neutral/inhibited ratio that was also altered, thus confirming an alteration of the AAG. (Table 2).
Studies conducted in the patient to confirm Pompe disease.
| Enzyme tests for Pompe disease | |
|---|---|
| Blood on filter paper | Neutral/inhibited AAG ratio: 20.8 (reference value: less than 16) |
| Percentage of inhibition of the AAG (%): 89.1 (reference value: less than 86%) | |
| Enzymatic study in leukocytes | Neutral/inhibited AAG ratio: 18.3 (reference value: less than 15) |
| Percentage of inhibition: 85.0 (reference value: less than 85%) | |
| Study in anatomical substrate (glycogen) | Level of 0.04mmol/mg prot/h control: in 0.4–1.4 |
| Study of DNA and detection of mutation for Pompe disease | |
|---|---|
| Cytogenetic location | 17q25.2–q25.3 |
| Exon/intron | Intron 1 |
| Nucleotide change | c.-32-12T>G |
| Zygosity | Homozygous |
| Database and number | HGMD number: CS941489 |
AAG: acid α-glucosidase; HGMD: Human Gene Mutation Database.
Finally, it was decided to perform a study on anatomical substrate (glycogen), which showed an abnormally low level, with which the diagnosis of adult-onset Pompe disease was confirmed. In addition, it was carried out a genetic study that showed c.-32-12T>G alteration in the homozygous state of the AAG gene intron 1 (Table 2). After the diagnosis, management with enzyme replacement was carried out with recombinant human α-glucosidase, with doses of 20mg/kg by slow intravenous perfusion every 15 days. After the enzyme replacement therapy was started, not only stability of the disease was observed, but also improvement of weakness at 3 months of follow-up, showing strength of 3/5 bilaterally in the lower limbs. The patient continues on clinical follow-up by rheumatology.
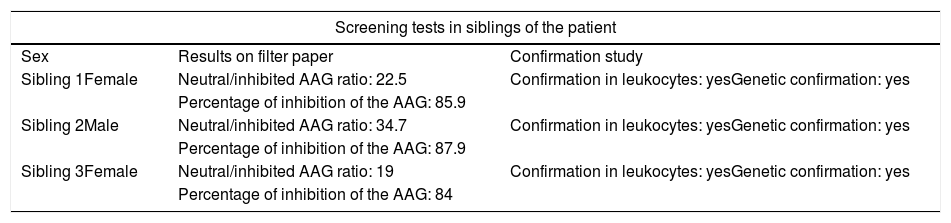
Taking into account the genetic nature of the disease, screening of 10 siblings of the patient was done, being able to detect alteration in the enzymatic function in 3 of them (Table 3). A confirmatory study with a leukocyte test and a genetic study which confirmed the alteration and the mutation of the AAG were also carried out; however, at the time of the exam none of them showed symptoms.
Results of screening tests in siblings of the patient.
| Screening tests in siblings of the patient | ||
|---|---|---|
| Sex | Results on filter paper | Confirmation study |
| Sibling 1Female | Neutral/inhibited AAG ratio: 22.5 | Confirmation in leukocytes: yesGenetic confirmation: yes |
| Percentage of inhibition of the AAG: 85.9 | ||
| Sibling 2Male | Neutral/inhibited AAG ratio: 34.7 | Confirmation in leukocytes: yesGenetic confirmation: yes |
| Percentage of inhibition of the AAG: 87.9 | ||
| Sibling 3Female | Neutral/inhibited AAG ratio: 19 | Confirmation in leukocytes: yesGenetic confirmation: yes |
| Percentage of inhibition of the AAG: 84 | ||
Neutral/inhibited AAG ratio with reference value less than 16.
Percentage of inhibition of the AAG with reference value less than 86%.
Pompe disease is considered a rare condition, with an incidence between 1:14,000 and 1:300,000, depending on the geographic area studied, being the highest incidence of the infantile form among Chinese people, while the adult late-onset form has the highest incidence in the Netherlands.7,10 The diagnosis is difficult and is usually delayed between 4 and 30 years after the onset of the symptoms.3 The age of onset can range from <1 to 52 years and the age of diagnosis can vary from <1 to 78 years.9 In the case presented, the patient was diagnosed with a polymyositis, but he did not had a good response to the management established, and for this reason, diagnostic alternatives were sought, which included: congenital, inflammatory (other than polymyositis, inclusion bodies, dermatomyositis), endocrine, mitochondrial, and metabolic (McArdle disease, carnitine palmitoyltransferase type II deficiency, Pompe disease) myopathies, muscular dystrophies (mainly Duchenne dystrophy, Becker dystrophy and limb-girdle dystrophy).8,11 Other differential diagnoses that can be included are the upper and lower motor neuron diseases, which may also occur with muscle atrophy and weakness.12 After a positive screening test with blood spot on filter paper, it was possible to focus on a deficit of AAG.
The clinical manifestations of adult-onset Pompe disease are varied, being the most frequent proximal muscle weakness, trunk muscle weakness, intolerance to exercise, difficulty breathing due to respiratory muscle weakness (in up to 80%), ineffective cough, difficulty walking and difficulty in getting up from a squatting position (known as Gowers’ sign). However, it can have manifestations in multiple organs including the heart (hypertrophic cardiomyopathy, supraventricular tachycardia and Wolff-Parkinson-White syndrome), the nervous system (small fiber neuropathy, hearing loss), the vascular system (cerebral aneurysms, basilar dolichoectasia), the osseous system (osteopenia, osteoporosis, scoliosis) and the gastrointestinal system (chronic diarrhea, fecal incontinence).2,3,6,7,11 From the biochemical point of view, a significant percentage of patients can present with elevation of creatine phosphokinase (CPK) and other muscle enzymes, which masks the diagnosis and patients can be misdiagnosed with inflammatory myopathies for several years.13
The diagnostic standard for Pompe disease is the demonstration of the deficiency of the enzymatic activity of the AAG.4,7,8,14 The biochemical measurement can be performed on samples of cultured skin fibroblasts, muscle tissue biopsy or blood samples on filter paper, purified lymphocytes and mixed leukocytes4,7,10,15 Other forms of diagnosis that can be established are through a muscle biopsy with positive PAS staining, taking into account that the muscle glycogen content increases up to 10-fold above the normal value in the infantile Pompe disease and to a lesser extent in late-onset patients.4,6,7
The gene that encodes the AAG is located on chromosome 17q25.3 and its mutation is associated with the autosomal recessive Pompe disease.2,6,8,9 More than 350 mutations of the disease have been described.2,9,16 Among the recurrent mutations in the cases of infantile onset there is a deletion of Δ525T, which is observed in 9% of cases in the United States and in 34% of the Dutch cases, and the mutation of the splice site of IVS1 (-13T->G) represents approximately 50% of the cases of late-onset in Caucasian patients.7,8 In our case, the intronic mutation c.-32-12T>G in the homozygous state of the AAG gene was documented, which corresponds to the most frequent pathogenic mutation for late-onset Pompe disease. Although this mutation was documented for the first time many years ago (in 1994 by Huie et al.), the mechanism by which it affects the encoding of the AAG is not clear so far.17
Once a patient is diagnosed with Pompe disease, it is ideal to have a basic study of the cardiopulmonary structure and function, being indicated in the management guidelines the performance of a chest X-ray, an electrocardiogram and an echocardiogram at the first visit. In the adult forms it is ideally recommended to carry out pulmonary function tests with spirometry, lung volumes with measurement of the maximum expiratory pressure and the maximum inspiratory pressure. It is also important to provide patients with an integral rehabilitation plan that allows to preserve the motor an physiological function, prevent or minimize the secondary complications and maximize the benefits of the enzyme replacement therapy (ERT).6,7,11
ERT was approved in 2007 for patients with early-onset Pompe disease and in 2010 for the late presentation.8,11 This therapy has shown benefits, especially when it is started early.2,4,10,18 The therapy with the recombinant human AAG enzyme (Myozyme®) has a standard dose of 15–20mg/kg every 15 days in continuous infusion for 4h.2,11
Since it is an autosomal recessive disease, the probability of transmission of the disease from a parent to a child is approximately 25%, therefore, genetic counseling should be carried out.7,8,11 The active search for the disease among siblings is also justified, as in this case, where it was possible to determine the enzymatic alteration in 3 of 10 siblings; however, they may be asymptomatic or slightly symptomatic due to intrafamilial clinical variability.8,11 Currently, the evidence is insufficient to treat the presymptomatic disease or for the management of the mutation carriers.14
ConclusionLate-onset Pompe disease is a rare condition, with low suspicion, that may go unnoticed if it is not taken into account within the differential diagnoses of inflammatory myopathies, since it may present similar clinical manifestations with proximal muscle weakness and elevated muscle enzymes. Its early detection is important to initiate an ERT that allows to stop the clinical deterioration and to avoid the derived complications improving the quality of life of the patients.
Conflict of interestThere is no conflict of interest.
Please cite this article as: Lemus-Barrios GA, Saldarriaga-Rivera LM. Enfermedad de Pompe del adulto: reporte de un caso como diagnóstico diferencial de una miopatía inflamatoria. Rev Colomb Reumatol. 2019;26:58–62.