ANCA-associated vasculitis may occur concomitantly with primary Sjögren's syndrome (SS) or arise during its evolution. We present the case of a patient who underwent dry symptoms, a positive Schirmer test and an SS-compatible autoimmunity profile and, simultaneously, deterioration of renal function, anaemia, and dyspnoea, requiring renal biopsy and fibrobronchoscopy. Complementary studies documented acute necrotizing glomerulonephritis with extracapillary proliferation, and membranoproliferative pattern with immune complex deposition. Bronchoalveolar lavage was compatible with alveolar haemorrhage. Kidney lung syndrome secondary to ANCA vasculitis was diagnosed and treatment with steroid and intravenous cyclophosphamide with clinical and paraclinical improvement was instituted. Mixed renal involvement found in this case is uncommon in patients with SS, and treatment changes significantly, hence the importance of differential diagnosis and reporting in the literature.
La vasculitis asociada con anticuerpos anticitoplasma de neutrófilos-ANCA puede presentarse concomitantemente con síndrome de Sjögren primario o surgir durante su evolución. Se presenta el caso de una paciente que cursó con síntomas secos, test de Schirmer positivo, perfil de autoinmunidad compatible con síndrome de Sjögren y, de forma simultánea, deterioro de la función renal, anemia y disnea, por lo que requirió biopsia renal y fibrobroncoscopia. Los estudios complementarios documentaron glomerulonefritis aguda necrosante con proliferación extracapilar y patrón membranoproliferativo con depósito de complejos inmunes. El lavado broncoalveolar fue compatible con hemorragia alveolar. Se hizo diagnóstico de síndrome de pulmón-riñón secundario a vasculitis ANCA y se instauró tratamiento con esteroide y ciclofosfamida intravenosa, con mejoría clínica y paraclínica. El compromiso renal mixto encontrado en este caso es infrecuente en pacientes con SS, y el tratamiento cambia ostensiblemente, de ahí la importancia del diagnóstico diferencial y el reporte en la literatura.
The joint presentation of 2 or more autoimmune diseases represents a diagnostic challenge in clinical practice, given the diversity of manifestations and the complexity of management. Within the set of polyautoimmunity syndromes, the concomitance of vasculitis and Sjögren’s syndrome (SS) is rare and is based on genetic and environmental mechanisms. The clinical picture is diverse, making it difficult to recognize one of them as a primary manifestation of the other. Among the most serious complications are diffuse alveolar hemorrhage and rapidly- progressive glomerulonephritis (which are more common in cases of pauci-immune vasculitis than in SS) and are associated with high mortality rates if not treated promptly. Additionally, the mixed renal involvement found in this case (acute necrotizing glomerulonephritis and membranoproliferative pattern) is unusual and has important implications for treatment.1–3
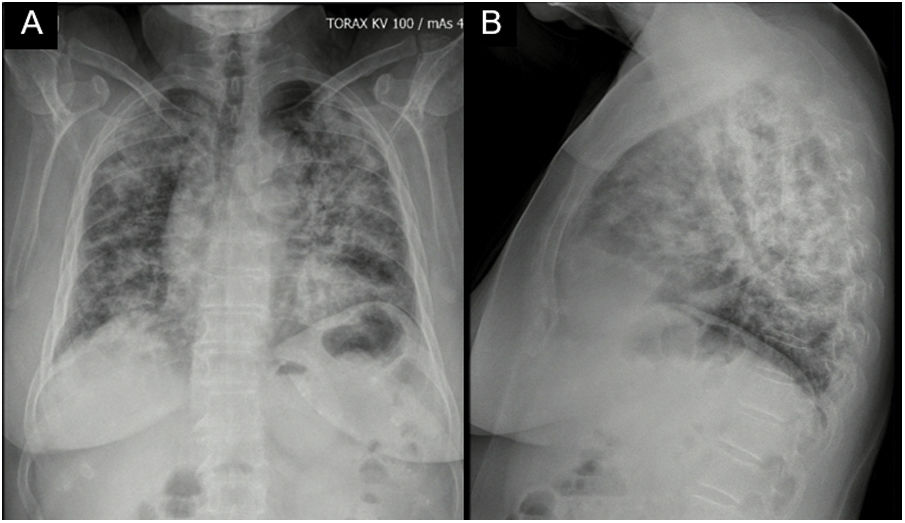
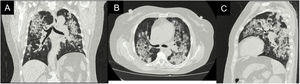
Case reportA 62-year-old female with a history of exposure to wood smoke for 13 years was admitted due to 6-month history of dry, intermittent cough, not associated with changes in temperature or exercise. In the last 15 days, it was accompanied by dyspnea on moderate exertion and odynophagia. She refers 2 years of xerophthalmia and xerostomia, without other stigmata of autoimmunity. She has no known family history of autoimmunity. She was admitted normotensive, without desaturation, tachycardic and afebrile, with fine rales predominantly in the lung bases. A chest X-ray was taken showing evidence of multilobar alveolar opacities (Fig. 1).
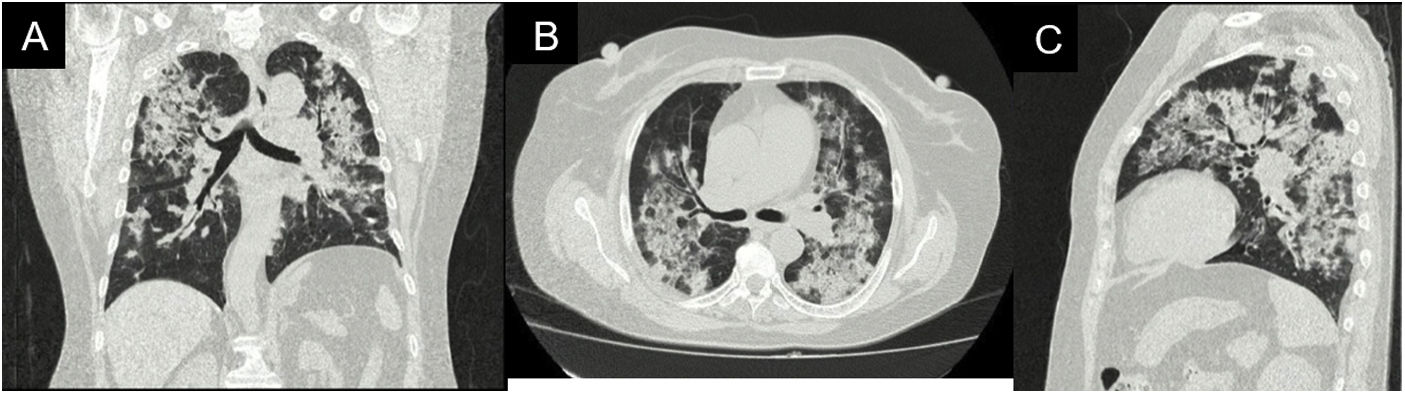
During her stay, the patient never presented fever, purulent expectoration, or symptoms suggestive of an infectious process. In the pandemic context, SARS-CoV-2 infection was ruled out, with a negative rtPCR test. Within the admission lab tests, anemia (hemoglobin 9.9 g/dl) of normal volumes, homogeneous and regenerative; thrombocytosis (platelets 507,900/mm3); elevated ESR and creatinine (2.29 mg/dl); urinalysis with active sediment and subnephrotic proteinuria were documented. An ultrasound showed preserved corticomedullary relationship and renal size. A high-resolution tomography of the chest was performed (Fig. 2), showing a pattern of organizing pneumonia, so fiberoptic bronchoscopy was performed to search for the etiology.
Studies were conducted to evaluate the etiology of the patient’s systemic involvement, with a negative infectious profile (HIV, hepatitis C, hepatitis B, RPR). Among the autoimmunity studies, normal complement, positive antinuclear antibodies (1/320 in a speckled pattern), anti-DNA 1/80, anti-Ro, and anti-La strongly positive were found (Table 1). The Schirmer test was positive, which configured a sicca syndrome associated with SS. The patient presented progressive anemia (up to hemoglobin of 7.8 g/dl), without overt bleeding and progression of kidney injury to creatinine of 3.4 mg/dl, without requiring renal replacement therapy. The findings in the fiberoptic bronchoscopy were compatible with alveolar hemorrhage (30% positive special iron stain for haemosiderophages).
Autoimmunity profile.
| Lab test | Result/Title | Reference range |
|---|---|---|
| ANCA by IIF | ||
| pANCA | Positive 1:640 | Negative |
| cANCA | Negative | Negative |
| ANCA by ELISA | ||
| Anti-PR3 | 2.7 U/mL | Negative: less than 5 U/mL |
| Anti-MPO | 46.1 U/mL | Negative: less than 5 U/mL |
| Antinuclear antibodies (Hep2000) | 1/320 dilutions | Negative: less than 1/80 dilution |
| Speckled pattern | ||
| Anti-La (SSB) | 87.96 U | Negative: 0−20 U |
| Anti-Ro (SSA) | 142.27 U | Negative: 0−20 U |
| Anti-RNP | 13.87 U | Negative: 0−20 U |
| Anti-Sm | 5.81 U | Negative: 0−20 U |
| Anti-DNA | Positive 1/80 dilutions | Negative |
| C3 | 115.69 mg/dl | 88−165 mg/dl |
| C4 | 56.39 mg/dl | 14−44 mg/dl |
ANCA: Anti-neutrophil cytoplasmic antibodies; IIF: Indirect immunofluorescence; ELISA: Enzyme-linked immunosorbent assay; PR3: Proteinase 3; MPO: Myeloperoxidase.
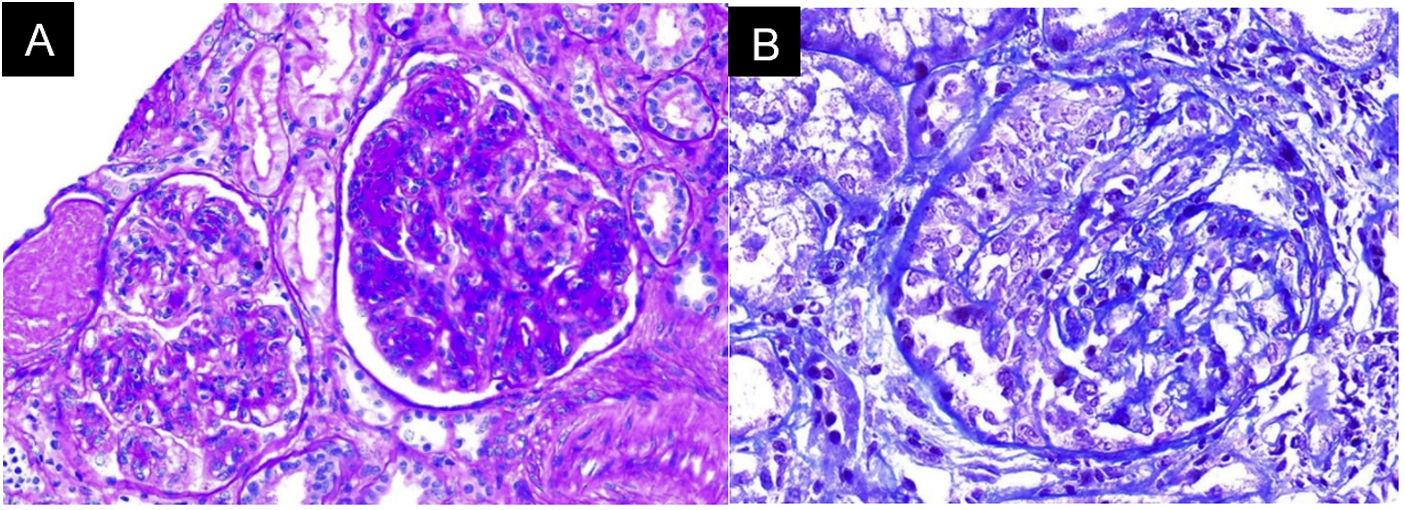
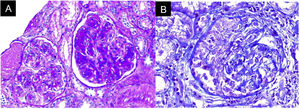
In the absence of other renal damage and given the evidence of active sediment with alveolar hemorrhage, glomerular involvement was suggested, probably secondary to rapidly progressive glomerulonephritis in the context of pulmonary-renal syndrome, for which antineutrophil cytoplasmic antibodies (ANCA) by ELISA and renal biopsy were requested (Fig. 3). The ANCA report was positive for antimyeloperoxidase antibodies (MPO) 46.1 U/mL (normal < 5 U/mL). Biopsy showed findings of acute necrotizing glomerulonephritis with cellular crescents, accompanied by mononuclear infiltrate in the interstitium, active tubular damage, and tubular atrophy with scant immune deposition and fibrinoid necrosis. Indirect immunofluorescence (IIF) showed scant mesangial deposits of IgA, IgM, and C3, with negative C1q and IgG. Due to the severe clinical involvement, a pauci-immune condition was considered predominant over the glomerular and interstitial involvement of the SS. Given the clinical presentation of the patient associated with the positivity for anti-MPO antibodies and the renal biopsy findings with necrotizing glomerulonephritis and extra capillary proliferation, the diagnosis of ANCA-associated vasculitis (AAV) was considered in addition to SS.
Pulses of methylprednisolone (prior deworming with ivermectin) and intravenous cyclophosphamide were prescribed based on the scheme used in the CYCLOPS4 study and low doses of steroids according to the PEXIVAS study.5 The clinical evolution was satisfactory, without respiratory deterioration and with stabilization of renal function and hemoglobin, for which the patient was discharged on day 20 of hospitalization. A check-up appointment was carried out 2 months after discharge, when the total cumulative cyclophosphamide dose was 2.25 g, with control creatinine at 1.15 mg/dl and hemoglobin at 11.3 g/dL.
DiscussionSS is an autoimmune disease that occurs in middle-aged women and affects exocrine glands but can occasionally spread to other organs such as kidneys, lungs, and nervous system.2 It can occur as primary SS (pSS) or in association with other connective tissue diseases.6 The most common renal lesion in SS is interstitial nephritis with lymphocytic infiltration, fibrosis, and tubular atrophy; in contrast, glomerulonephritis can occur but is rare.7
AAV can occur concomitantly with the diagnosis of pSS or arise during its evolution.7 In the current case, the evidence of positive anti-Ro and anti-La values and the Schirmer test, along with the presence of more than one domain in the Sjögren’s Syndrome Disease Activity Index (ESSDAI) scale scored in the classification criteria for Sjögren syndrome established by the European League Against Rheumatism (EULAR).8
The presence of anti-DNA in patients with SS is rare; in the studied cohorts, it varies from 2 to 10%.9–11 The meaning of positivity is associated with the probability of developing other autoimmune diseases subsequently. In this case, systemic lupus erythematosus was ruled out due to a discrepancy between activity markers and renal paraclinical and histopathological involvement12; however, it could not be ruled out that in the future this patient could present superimposed polyautoimmunity phenomenon, thus follow-up is mandatory.
At the time of diagnosis, it is common for patients with AAV concomitant with pSS to present elevated acute-phase reactants and marked anemia.13 Additionally, although rare, they may have AAV-associated renal involvement, as occurred in this case. In a study by Borg et al.,14 approximately one-third of the patients had anti-MPO AAV with limited kidney involvement. Font et al. demonstrated a significant association between ANCA positivity in pSS with Raynaud’s phenomenon, cutaneous vasculitis, and peripheral neuropathies15–17; however, this patient did not develop such manifestations, which makes us think that the necrotizing glomerulonephritis was secondary to an AAV involvement as a distinct entity from SS.
The combined lung and kidney affection by some of these diseases is also described as pulmonary-renal syndrome.18 When AAV is suspected in the context of SS, high-resolution tomography often determines the predominant pattern of injury. Of these, organizing pneumonia is the most seen.19 Another manifestation that can occur is alveolar hemorrhage, which can manifest as masses of variable size with or without the halo sign (perinodular hemorrhage).20
In 2015, Cornec et al.15 conducted a literature review searching for the coexistence between pSS and AAV; they identified 22 case reports; in all of them, the diagnosis of AAV was concomitant with or following that of pSS. In 5 cases, manifestations of pSS and AAV began simultaneously. Women represented 86.4% of the subjects. The mean age at diagnosis was 63 years. Of the 22 patients, 16 met pSS criteria proposed by the American European Consensus Group (AECG). Within the vasculitis tests, 76.2% of the patients were positive for pANCA and anti-MPO with a biopsy-proven typical histological pattern of AAV-renal involvement.3
Treatment of AAV and SS cases should be based on the severity of the disease and the organ involved. In this case, due to the presence of alveolar hemorrhage and impaired renal function in association with necrosis and extra capillary cell proliferation, the use of intravenous cyclophosphamide was chosen according to the CYCLOPS study scheme, due to its efficacy on remission-induction in severe forms of vasculitis, with considerable mortality reduction in studies published in the literature.21,22 Additionally, since there are observational studies that indicate that high-dose glucocorticoids are related to an increased risk of infections in patients with AAV, and glomerular involvement of SS is associated with raised morbidity and mortality due to infectious causes, it was decided to use the low-dose steroid scheme according to the PEXIVAS study.4,5
As the presence of ANCA in the context of pSS is uncommon and can progress to systemic vasculitis, patients should be monitored periodically, to carry out both clinical and paraclinical assessments; urinalysis, renal function, and C-reactive protein tests are recommended. In patients with pSS with signs of inflammatory response in lab tests, positron emission tomography with fluorodeoxyglucose could be considered to discriminate between activity due to pSS and associated vasculitis.23
As a strength of this case report, we highlight the description of an uncommon condition in clinical practice, contributing to enriching the current knowledge of the expression of these entities. We recognized the lack of a salivary gland biopsy as one limitation of this study, which could have corroborated typical findings of SS. A case of systemic lupus erythematosus is unlikely, due to the discrepancy between the activity markers (normal complement and low-positive anti-DNA) and the paraclinical and histopathological renal involvement evidenced in the patient.24 However, during the follow-up of similar patients as in this case, progress to other autoimmune diseases such as systemic lupus erythematosus and mixed connective tissue disease has been observed.25 For this reason, periodic surveillance of these patients is necessary.
ConclusionAlthough, rarely, these two disorders occur simultaneously (AAV and pSS), they must be suspected and diagnosed to treat them promptly, thus avoiding long-term complications that impair the quality of life and survival of patients who suffer from them.
Ethical ConsiderationsThis case report was approved by the institutional Ethics and Research Committee of the Hospital Universitario San Ignacio, Bogotá (Colombia). The patient gave informed consent for her case to be published.
Availability of informationThe information related to the case report is presented within the manuscript. Additional material will be available after the corresponding request to the author.
FinancingThis research has not received specific aid from public sector agencies, the commercial sector, or non-profit entities.
Conflict of interestsThe authors declare that they have no conflict of interest for the preparation of this manuscript.
To Samuel Morales MD, a pathologist at Hospital Universitario San Ignacio, Bogotá (Colombia), for the contribution of the images of the renal biopsy.